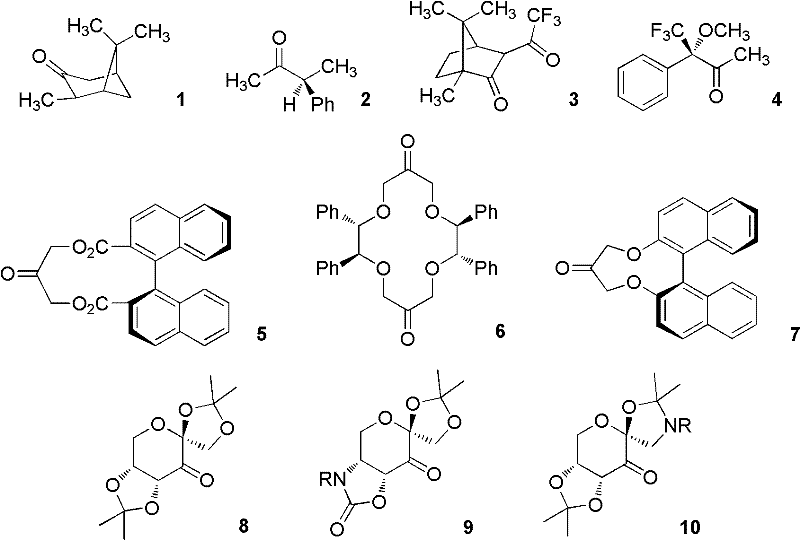
Preparation method of load type chiral ketone catalyst and application thereof
A technology of chiral ketones and catalysts, applied in the field of preparation of supported chiral ketones, can solve the problems of unpublished experimental results, no practical application value, low ee value, etc., and achieve low price, high ee value, and easy preparation process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
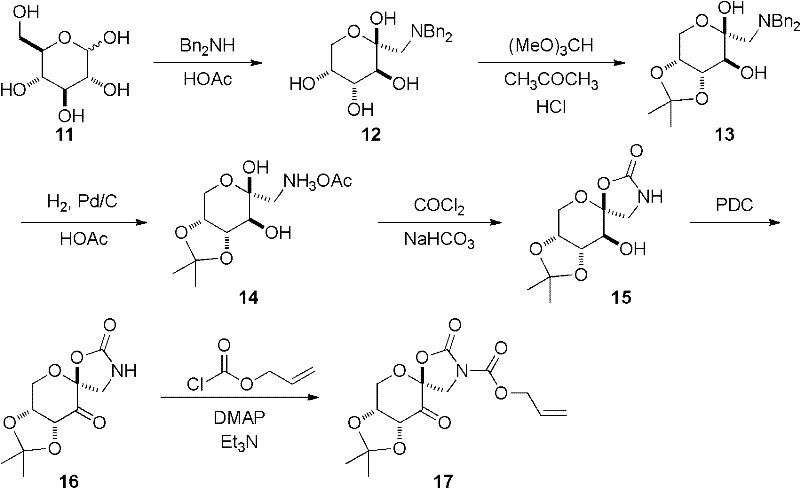
[0044] Embodiment 1, a kind of preparation method of supported chiral ketone catalyst is carried out in the following steps successively:
[0045] 1), preparation of chiral ketone:
[0046] Add D-glucose (11) 59.8g (332.0mmol) and Bn in 350mL ethanol 2 NH 64.0mL (332.0mmol), then add acetic acid 57.0mL (995.7mmol), reflux reaction for 3h, the reaction solution turns orange-yellow, and the viscosity increases, keep stirring continuously for 3 hours under heating and reflux; and the temperature of the oil bath should not be high At 90°C (the reaction is heated by an oil bath, in order to prevent the occurrence of side reactions, the temperature of the oil bath needs to be controlled below 90°C). After the reaction solution was cooled to 0°C, it was suction-filtered under reduced pressure, and the filter cake was continuously washed with ice ethanol until white, and dried in vacuo to obtain 95.5 g of white solid 1-dibenzylamino-1-deoxy-D-fructose (12). The yield was 80.5%.
[...
Embodiment 2
[0058] The epoxidation of embodiment 2, styrene
[0059] Add 52mg (0.5mmol) of styrene in the reaction flask, 50mg (0.025mmol, 5%mol) of the supported chiral ketone catalyst after activation, solvent DME-DMM (volume ratio 3: 1) 8mL, buffer solution 5mL (0.2 MK 2 CO 3 -AcOH, with 4×10 -4 M Na 2 EDTA aqueous solution, pH = 8.0), Bu 4 NHSO 4 (0.0075g, 0.02mmol), after the above reaction mixture was cooled to -10°C, Oxone solution (concentration of Oxone was 0.21M, mixed with 4×10 -4 M Na 2 EDTA aqueous solution) 4.2mL (that is, containing Oxone 0.548g, 0.882mmol) and K 2 CO 3 Solution (K 2 CO 3 The concentration is 0.48M, with 4×10 -4 M Na 2 EDTA aqueous solution) 4.2mL (that is, containing K 2 CO 3 0.278g, 2.01mmol) was added dropwise to the reaction system within 3h with a plunger pump. After completion of the dropwise addition, filter, and the obtained catalyst solid can be used for recycling, and the filtrate is extracted with n-pentane (5mL×3), and the organic...
Embodiment 3
[0060] The epoxidation of embodiment 3,2-chlorostyrene
[0061] Add 69.3 mg (0.5 mmol) of 2-chlorostyrene into the reaction flask, 50 mg (0.025 mmol, 5% mol) of the activated chiral ketone catalyst, 8 mL of solvent DME-DMM (volume ratio 3:1), buffer solution 5mL (0.2MK 2 CO 3 -AcOH, with 4×10 -4 M Na 2 EDTA aqueous solution, pH = 8.0), Bu 4 NHSO 4 (0.0075g, 0.02mmol), after the above reaction mixture was cooled to -10°C, Oxone solution (0.21M, with 4×10 -4 M Na 2 EDTA aqueous solution) 4.2mL (containing Oxone 0.548g, 0.89mmol) and K 2 CO 3 solution (0.48M, with 4×10 -4 MNa 2 EDTA aqueous solution) 4.2mL (contains K 2 CO 3 0.278g, 2.01mmol) was added dropwise to the reaction system within 3h with a plunger pump. After the addition was complete, it was extracted with n-pentane (5mL×3), and the organic phase was washed with brine and washed with anhydrous Na 2 SO 4 Drying, concentration, purification with flash column chromatography, specifically as follows: 5g 200...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com