Synthetic method of L-glufosinate intermediate
A synthesis method and glufosinate-ammonium technology are applied in the field of synthesis of L-glufosinate-ammonium intermediates, which can solve the problems of increasing the total cost of the process, high process risk, complicated process operation, etc., and achieve improved safety, reaction rate, The effect of reducing costs and avoiding racemization problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
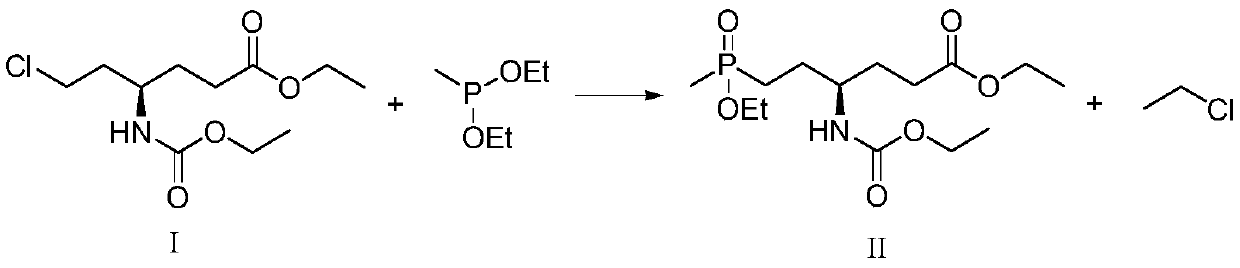
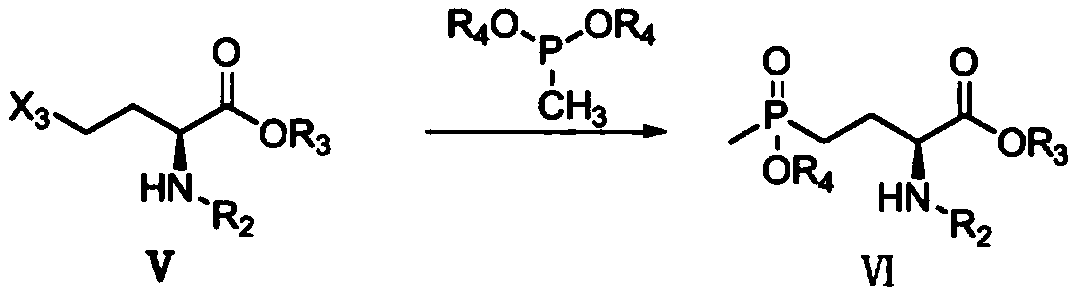
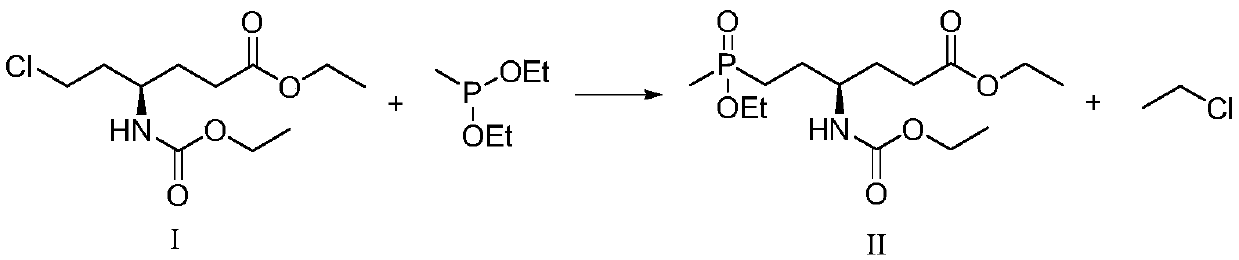
[0020] Concrete, the synthetic method of L-glufosinate-ammonium intermediate shown in formula II comprises the following steps:
[0021]
[0022] Under the protective gas atmosphere, react the compound of formula I with diethyl methyl phosphinate in the presence of a catalyst to obtain the L-glufosinate-ammonium intermediate shown in formula II; the catalyst is MgBr 2 +PEG-400, LiBr+PEG-400, KCl+PEG-400, MgBr 2 +TBAB, MgCl 2 , MgBr 2 , TMSBr+TBAB, LaCl 3 +TBAB or CaCl 2 .
[0023] The inventor of the present invention has carried out a large amount of screenings to catalyst, finds that by adding MgBr 2 +PEG-400, LiBr+PEG-400, KCl+PEG-400, MgBr 2 +TBAB, MgCl 2 , MgBr 2 , TMSBr+TBAB, LaCl 3 +TBAB or CaCl 2 The consumption of diethyl methylphosphonite can be greatly reduced, and the safety and reaction rate of the process are improved. At the same time in the catalyst MgBr 2 +PEG-400, LiBr+PEG-400, KCl+PEG-400, MgBr 2 +TBAB, MgCl 2 Or under the action of MgBr, th...
Embodiment 1
[0041] In a 250mL four-necked flask, 4.88g of the compound of formula I (1eq), 3.51g of diethyl methylphosphonite (1.2eq), 0.16g of PEG-400 (0.02eq) and 0.147g of MgBr 2 (0.04eq), then replace the system with nitrogen three times, then heat to 120°C for 17 hours, after the reaction, the reaction system is down to room temperature, and the reaction situation is detected by sampling. The ee value of the compound of formula II is 99.0%, and the absolute yield is 79.3%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com