Method for synthesizing chiral bisaryl alcohol compound
A technology of biaryl alcohol and compound, applied in the field of synthesis of chiral biaryl alcohol compound, can solve the problems of low efficiency, unreported species source, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1, the preparation of the engineering bacteria of carbonyl reductase SSCR and alcohol dehydrogenase TbSADH gene or its mutant
[0058] 1. Preparation of wild-type genetically engineered strains of carbonyl reductase SSCR and alcohol dehydrogenase TbSADH genes
[0059] The carbonyl reductase SSCR gene derived from Sporobolomyces Salmonicolor and the thermoanaerobacter brockii alcohol dehydrogenase TbSADH were codon-optimized using Escherichia coli as the host cell, and optimized by Twist Bioscience (USA ) or GenScript (Nanjing) whole gene synthesis, the optimized sequences are SEQ ID No.1 and SEQ ID No.3 respectively.
[0060] The two target genes were respectively connected into the pET22b(+) plasmid through NdeI and HindIII restriction sites to obtain a recombinant vector. Electrotransform the recombinant vector pET22b-SSCR or pET22b-TbSADH into E.coli BL21(DE3) competent cells, culture them upside down on LB solid plates containing ampicillin (Amp) resista...
Embodiment 2
[0085] Example 2, Expression of carbonyl reductase SSCR and alcohol dehydrogenase TbSADH genes or their mutants and preparation of crude enzyme powder
[0086] Pick the transformants of recombinant bacteria E.coli BL21 (recombinant plasmids of carbonyl reductase SSCR or alcohol dehydrogenase TbSADH genes or their mutants) into 5mL LB liquid medium containing 50μg / mL ampicillin, shake at 37°C and 220rpm Overnight for 12-16 hours, according to the inoculation amount of 1% (volume percentage content), inoculate them into TB liquid medium containing 50 μg / mL ampicillin, and culture at 37°C until OD 600 When it was 0.7, add IPTG with a final concentration of 0.1mmol / L, induce expression at 20°C and 220rpm for 18h, then centrifuge at 4°C and 4000rpm for 10min to collect the bacteria. The collected bacteria were resuspended with potassium phosphate buffer (100 mM, pH 7.0) for use. Cells were sonicated in an ice bath. Centrifuge the sonicated sample at 4°C, 12000 rpm, for 10 min, an...
Embodiment 3
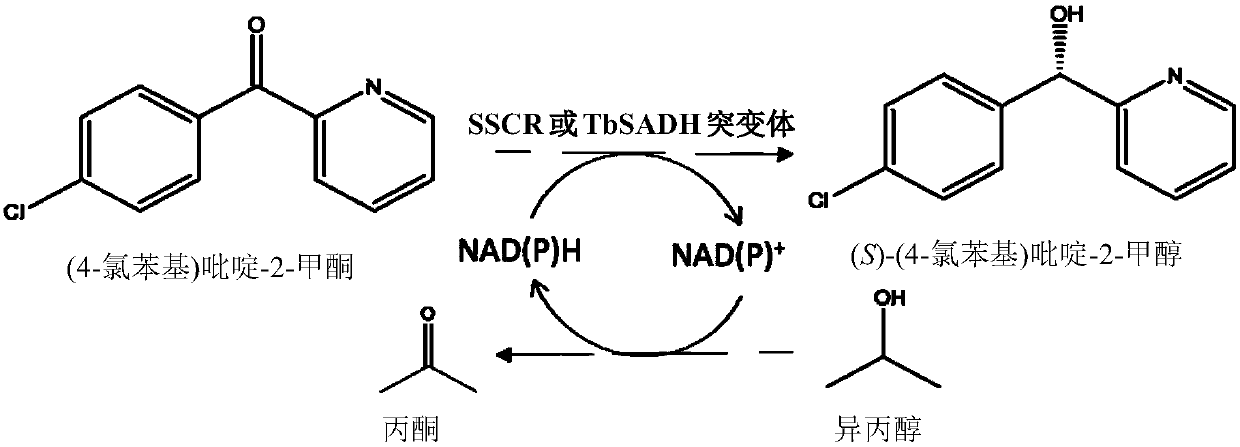
[0087] Example 3. Carbonyl reductase SSCR / alcohol dehydrogenase TbSADH and its mutants catalyze (4-chlorophenyl)pyridine-2-methanone to generate (S)-(4-chlorophenyl)pyridine-2-methanol
[0088] Carbonyl reductase SSCR / alcohol dehydrogenase TbSADH catalyzes the reduction of (4-chlorophenyl)pyridine-2-methanone to generate (S)-(4-chlorophenyl)pyridine-2-methanol. figure 1 shown.
[0089] Determination of the catalytic effect of carbonyl reductase SSCR or alcohol dehydrogenase TbSADH asymmetric reduction of prochiral bisaryl ketone (4-chlorophenyl) pyridine-2-methanone, wherein the substrate concentration is 10mmol / L, recombinant carbonyl reduction The dosage of enzyme SSCR or alcohol dehydrogenase TbSADH is 10g / L (crude enzyme powder), the dosage of isopropanol is 10% of the total volume, NAD(P) +The dosage is 1 mmol / L, the concentration of the phosphate buffer is 100 mmol / L, and the pH is 7.0. The temperature of the asymmetric reduction reaction was 30° C., and the reaction w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com