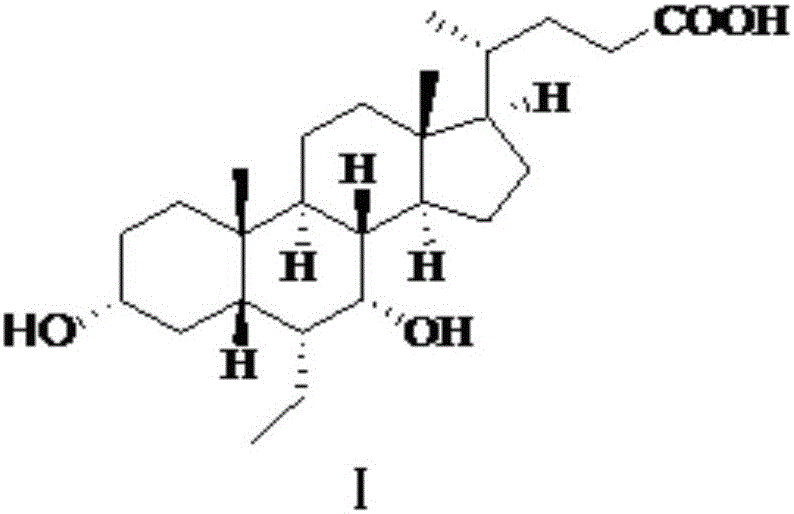
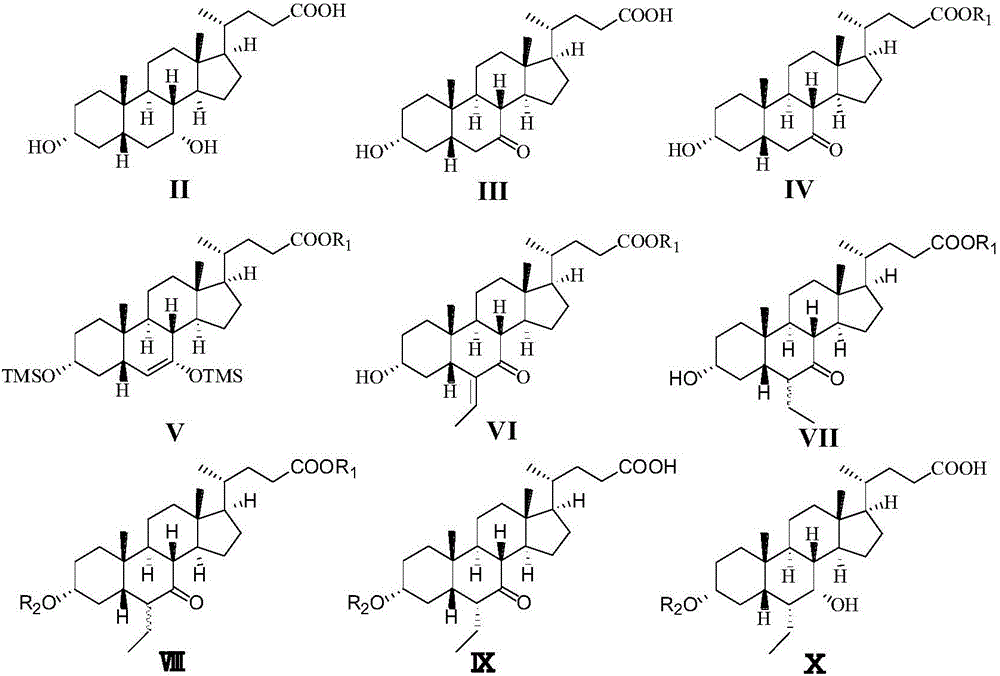
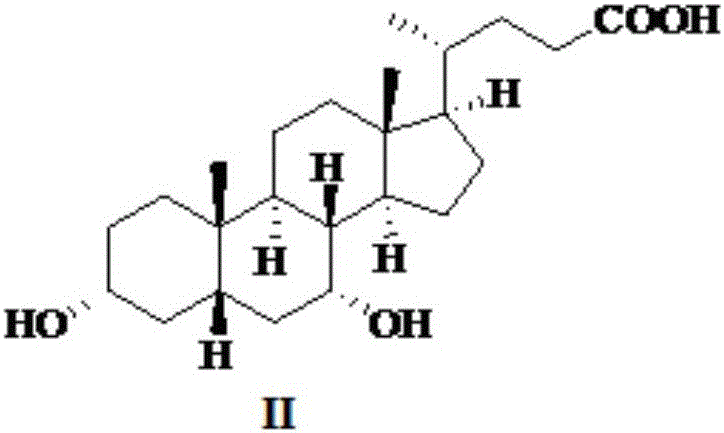
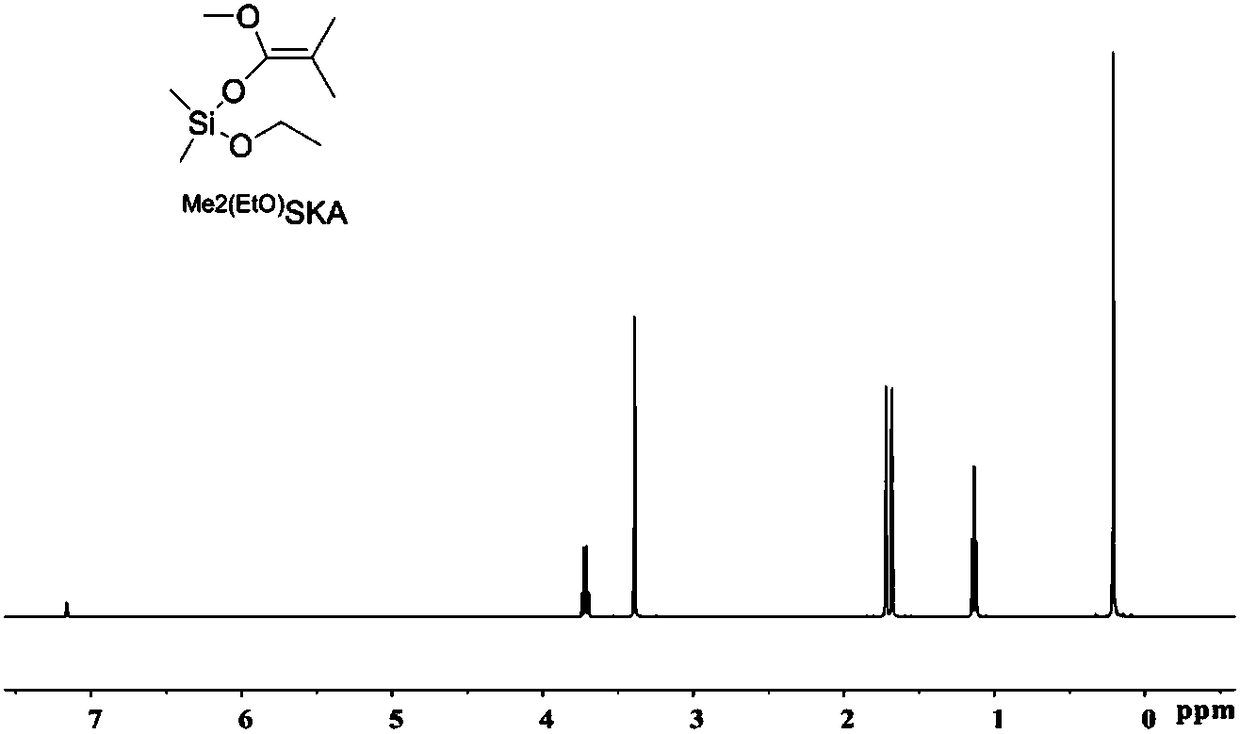
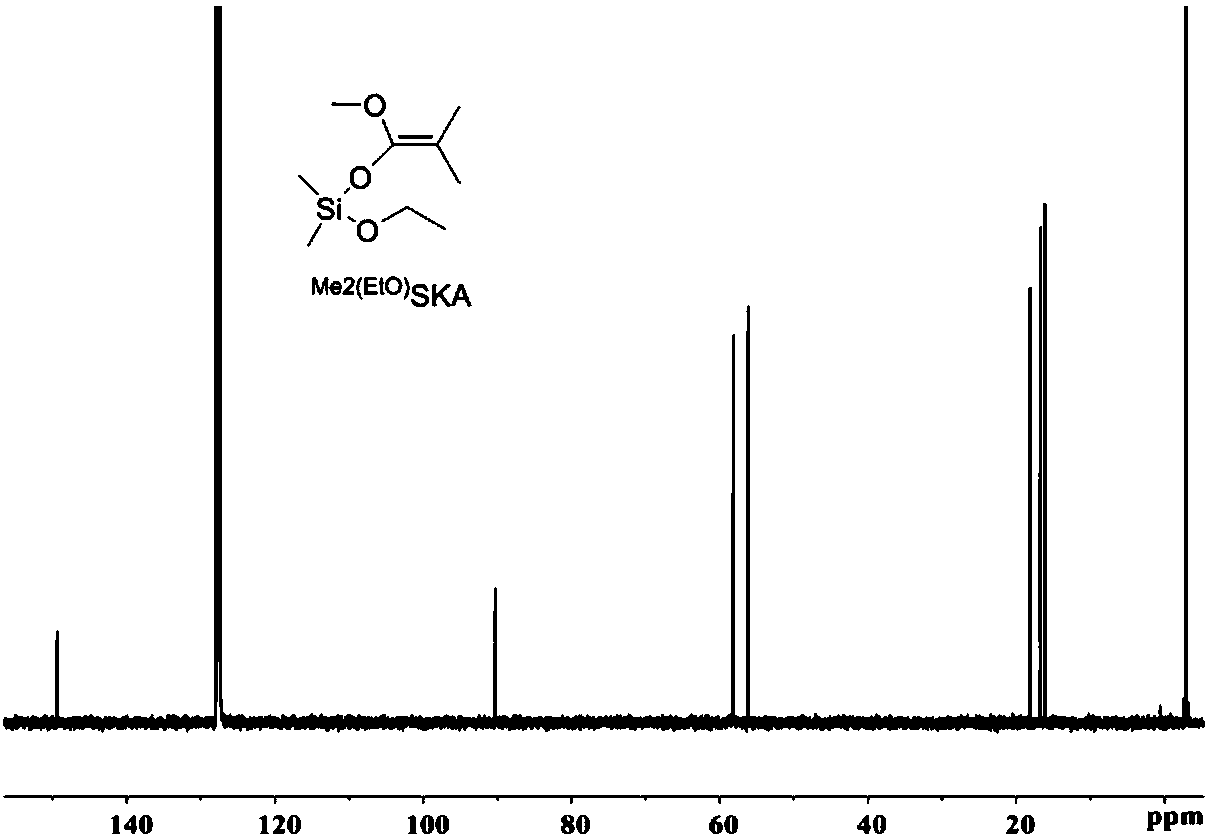
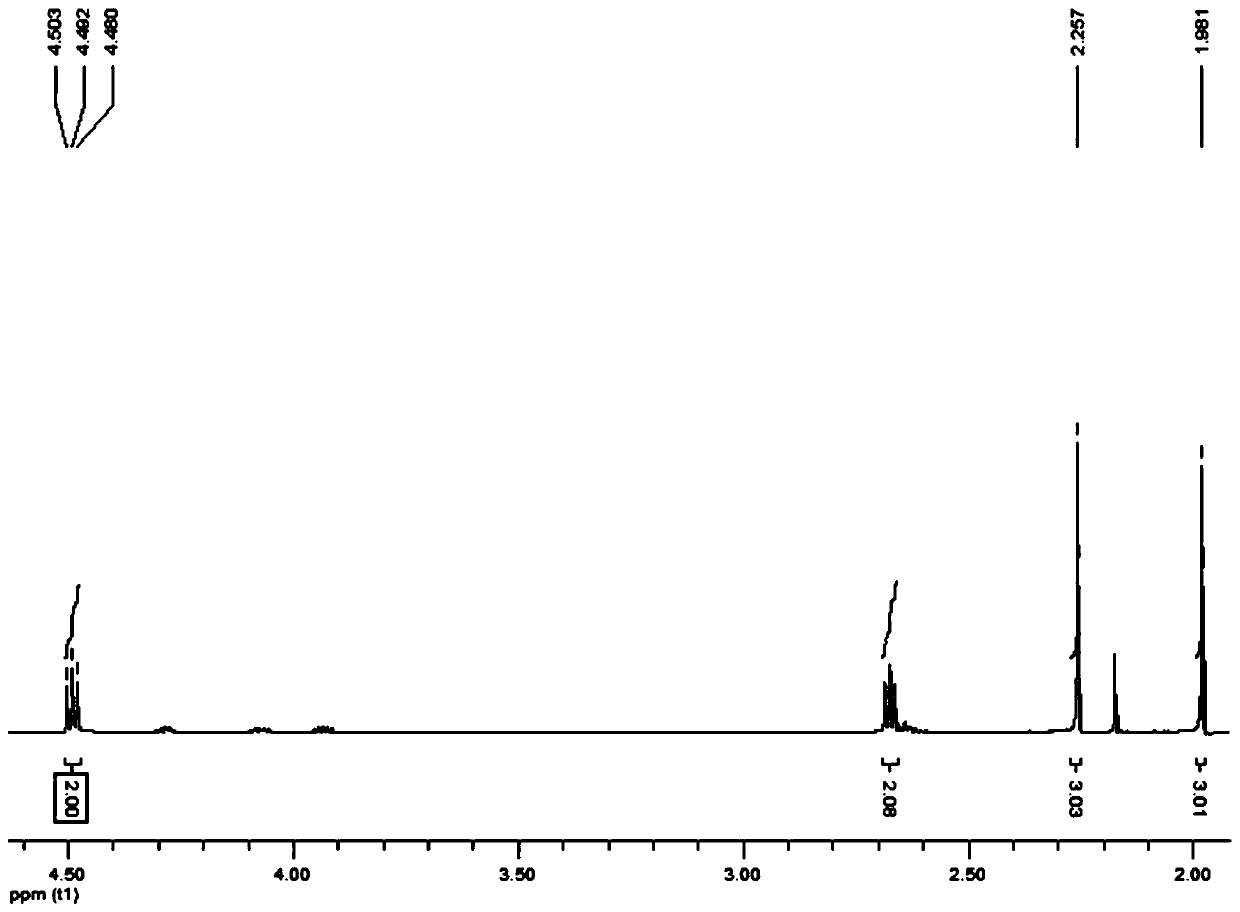
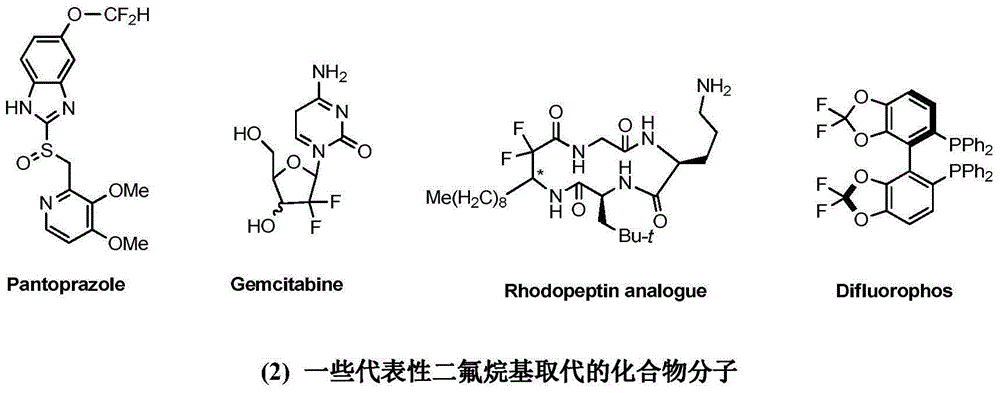
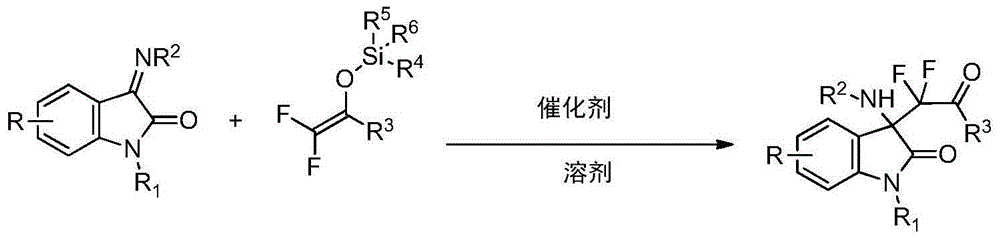
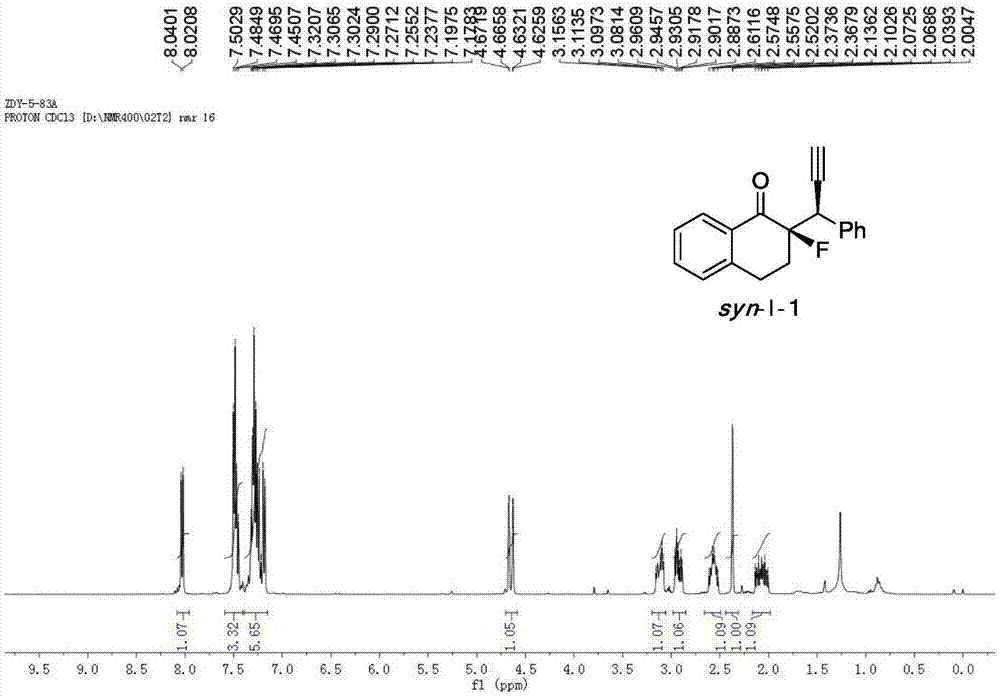
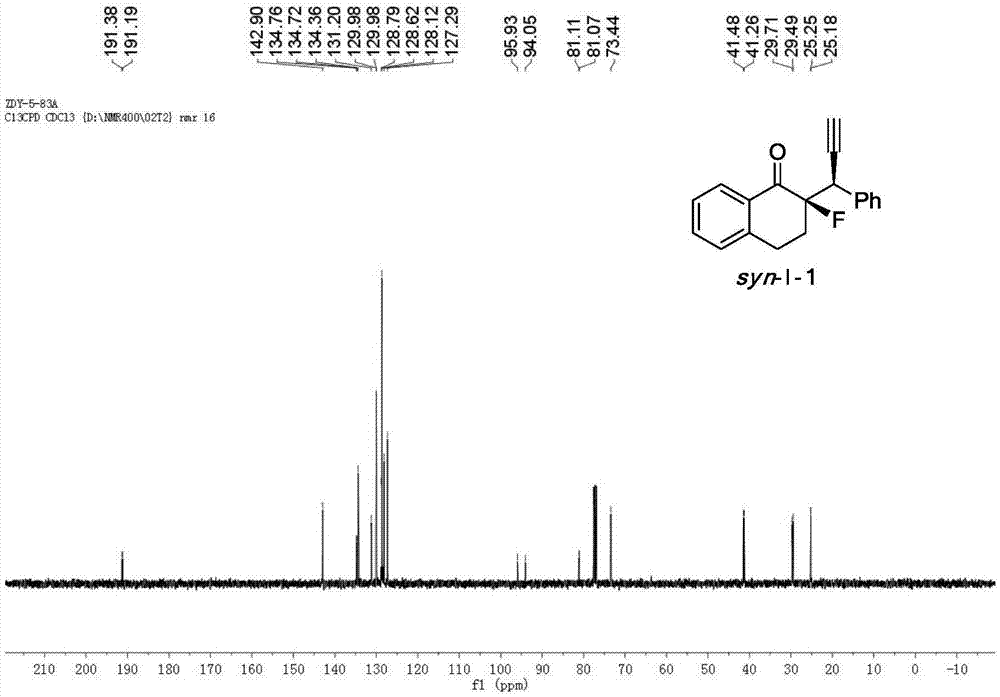
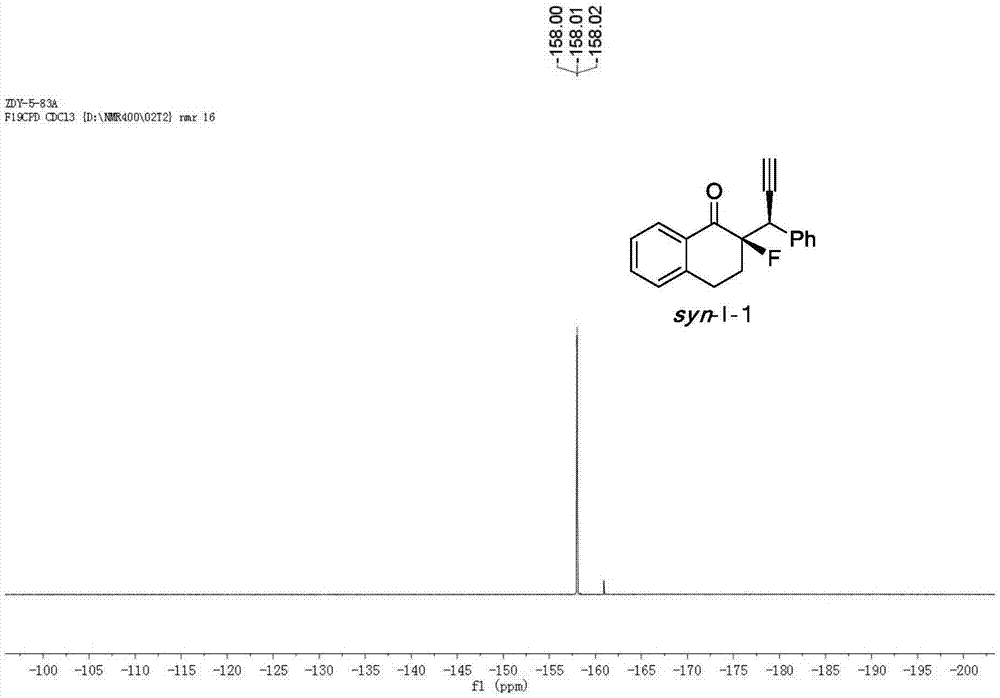
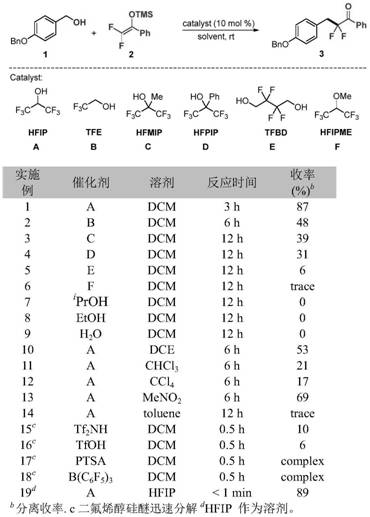
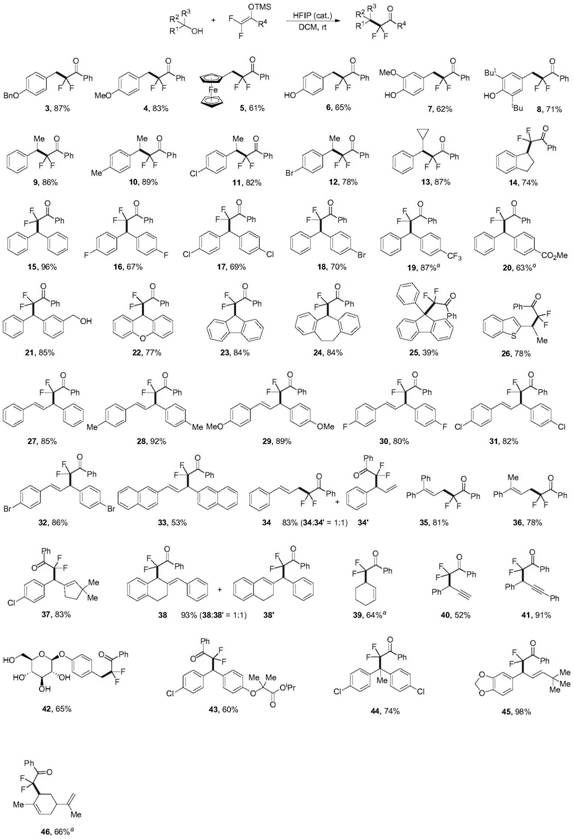
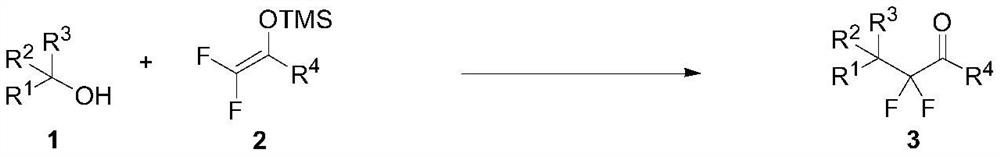
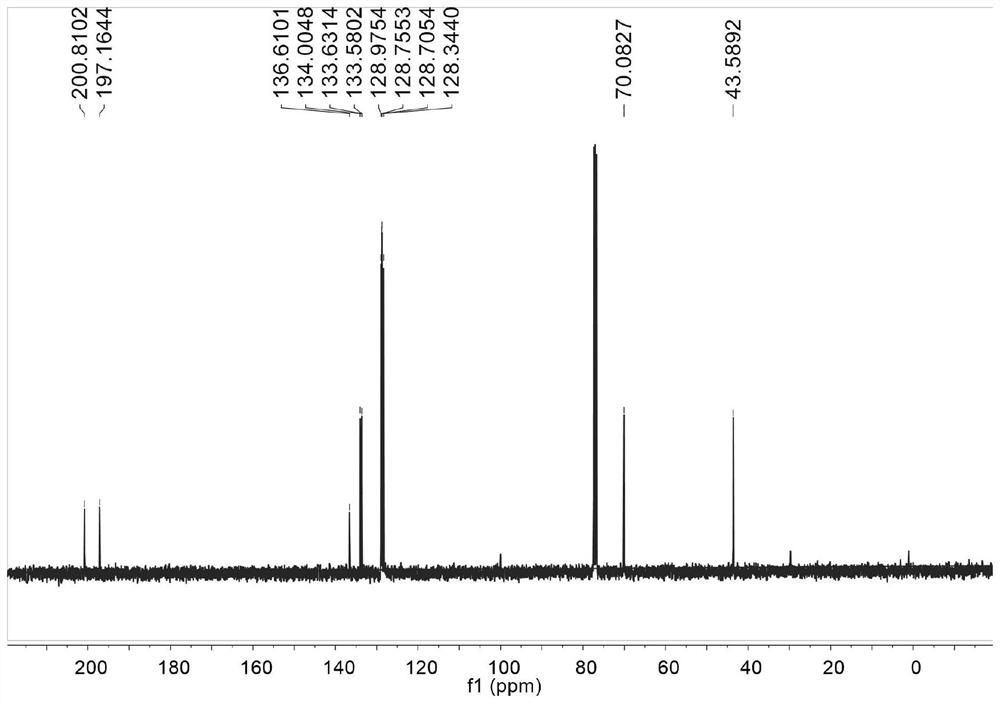
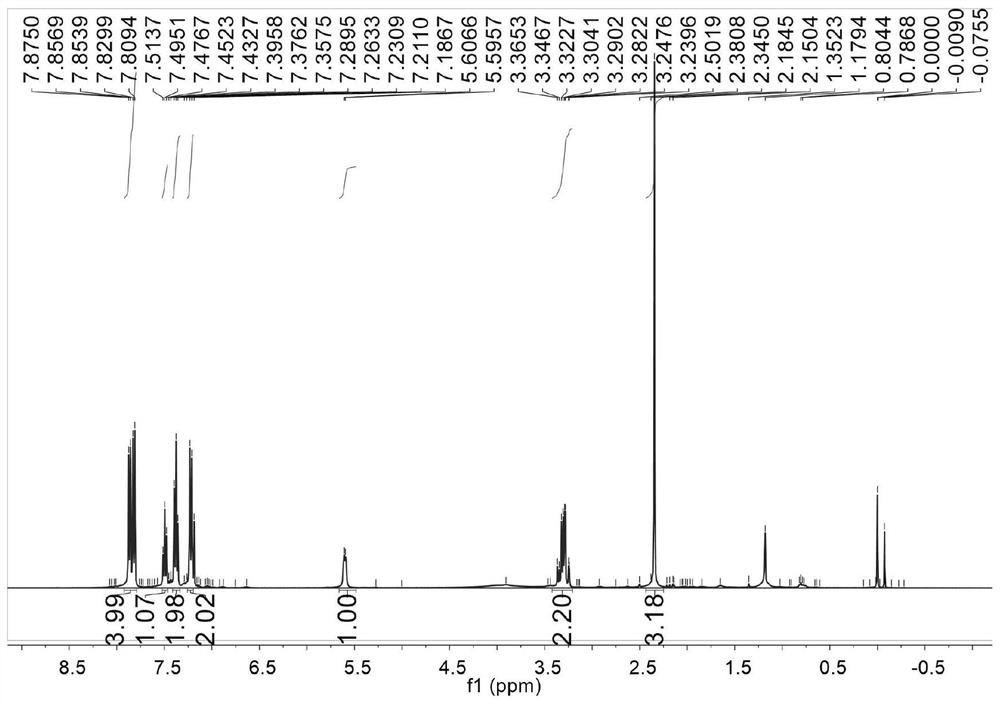
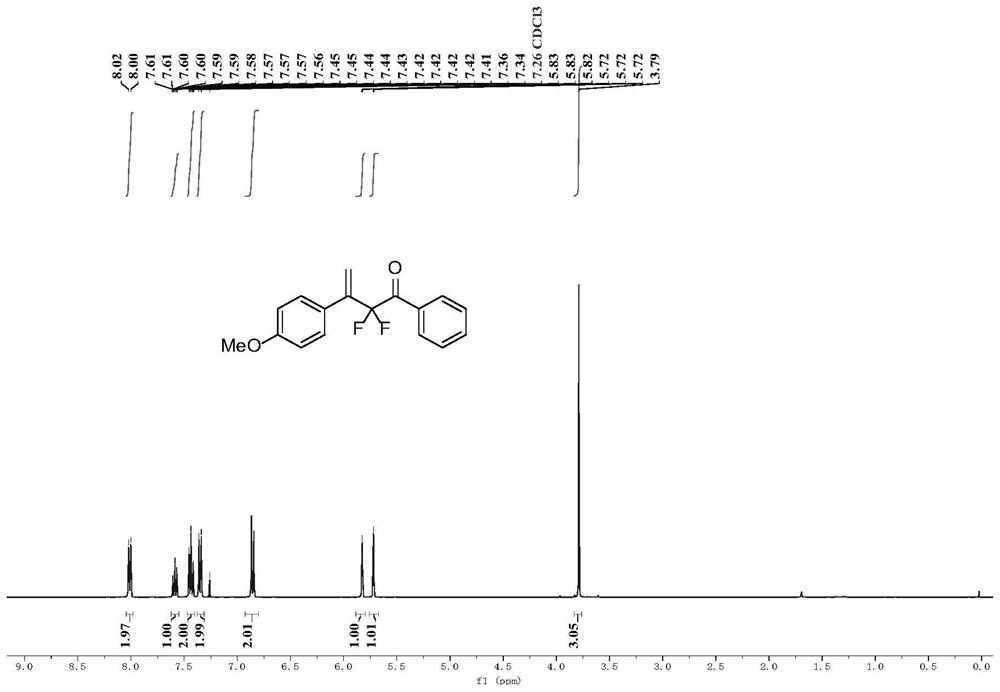
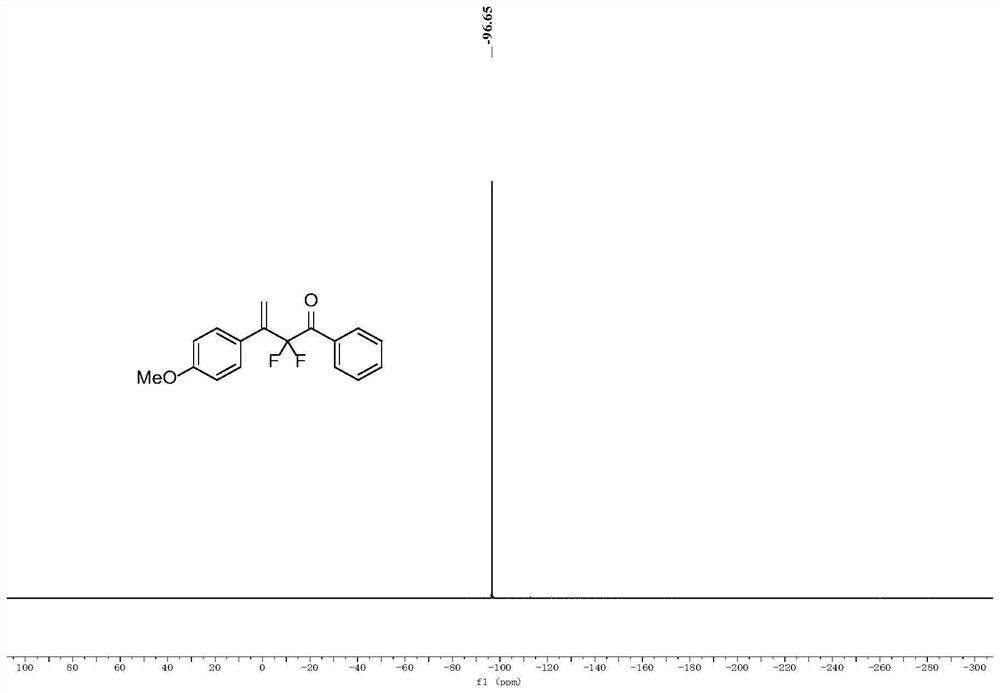
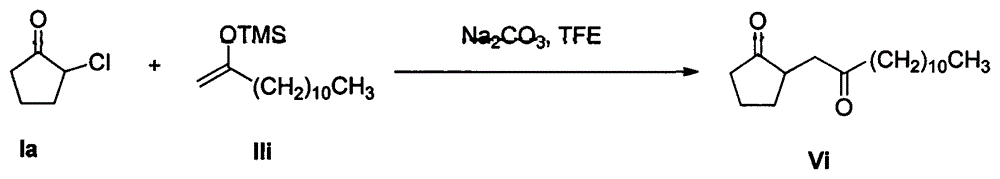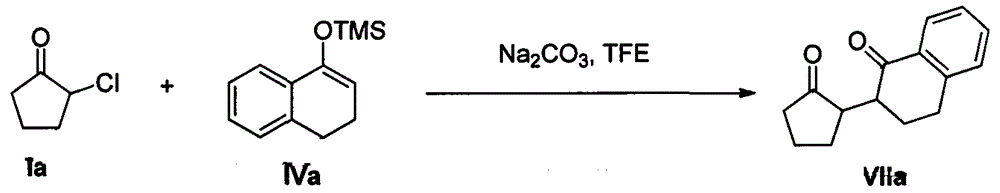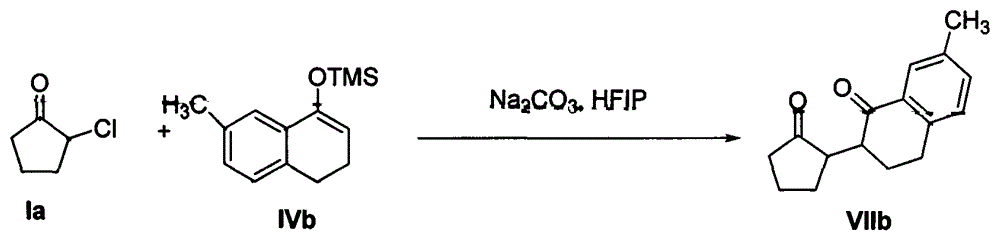Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "Silyl enol ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
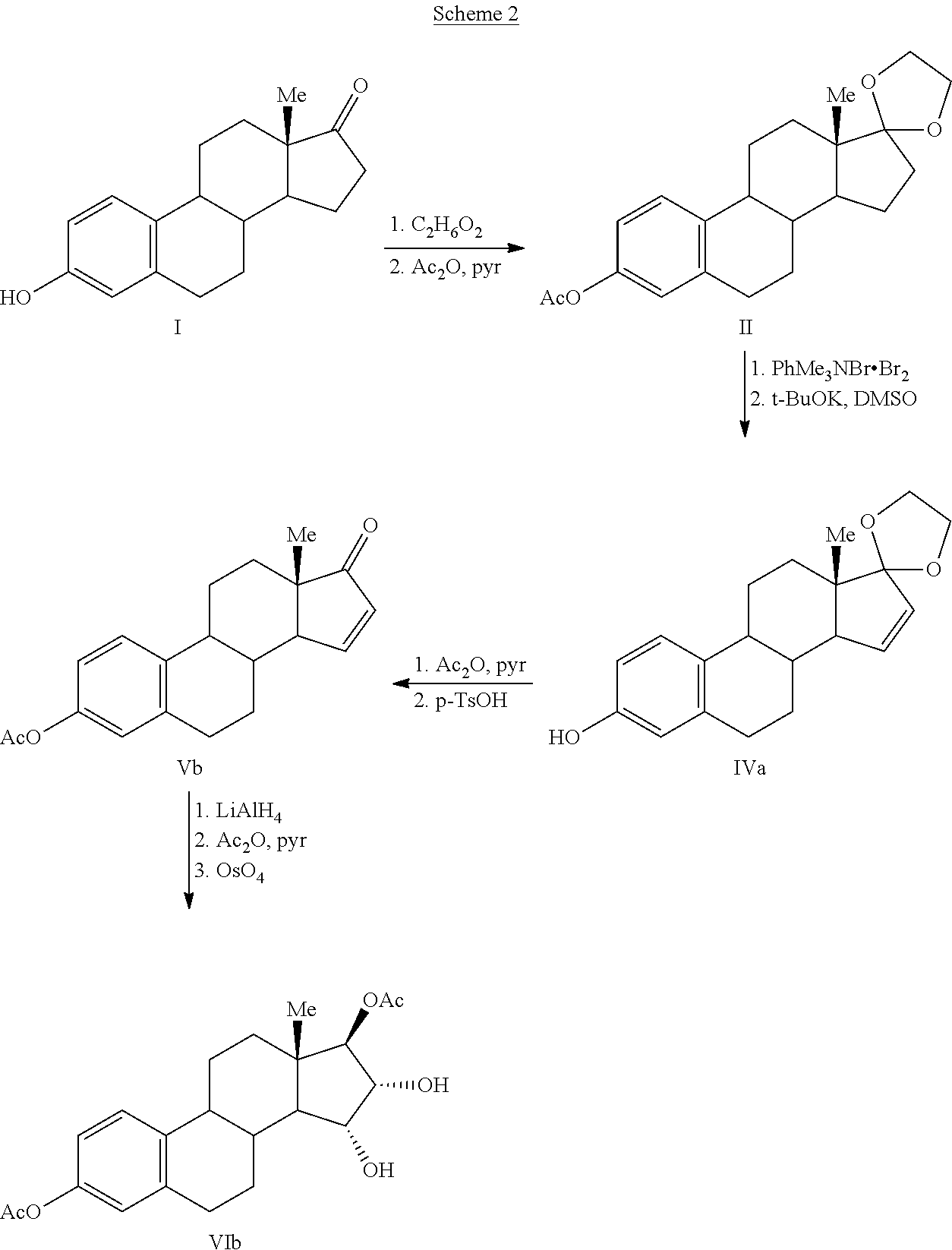
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis.
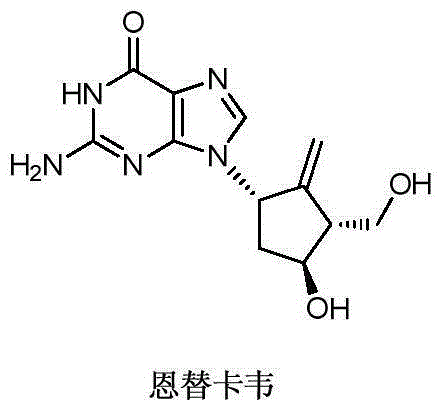
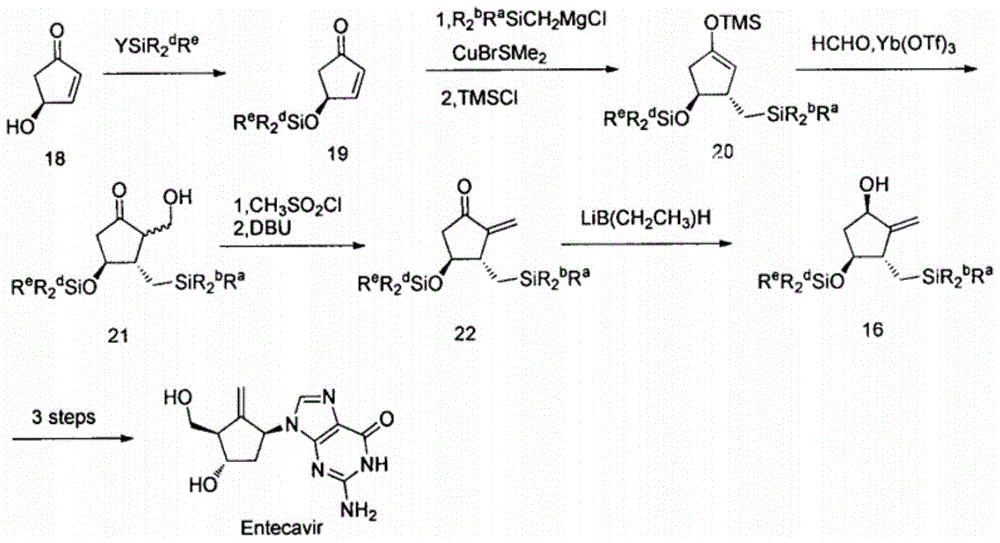
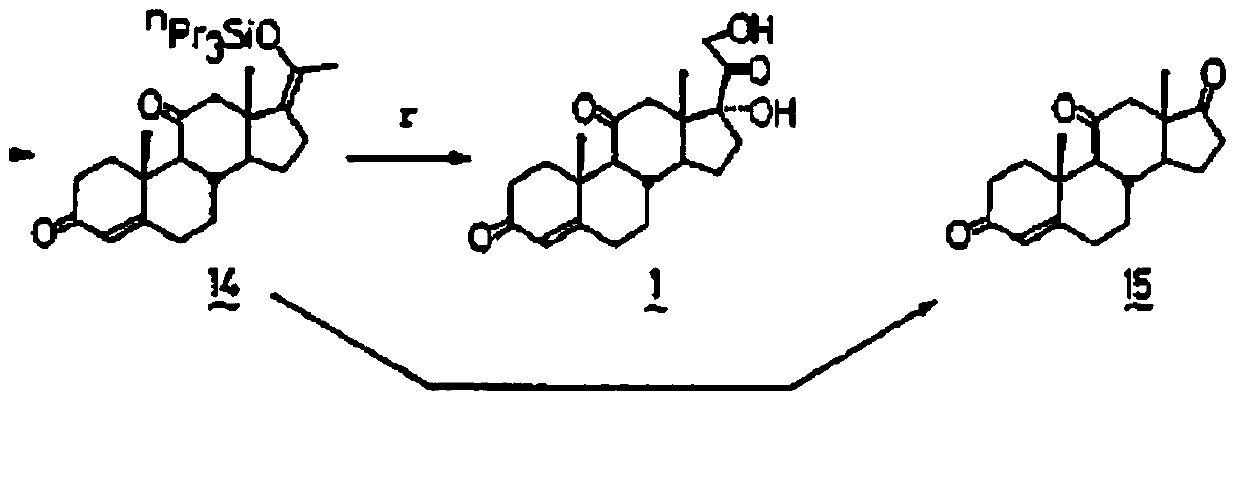
Novel synthetic method for entecavir compound
ActiveCN105037363ASolve rare problemsDosage controllableGroup 4/14 element organic compoundsKetoneAdipate
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
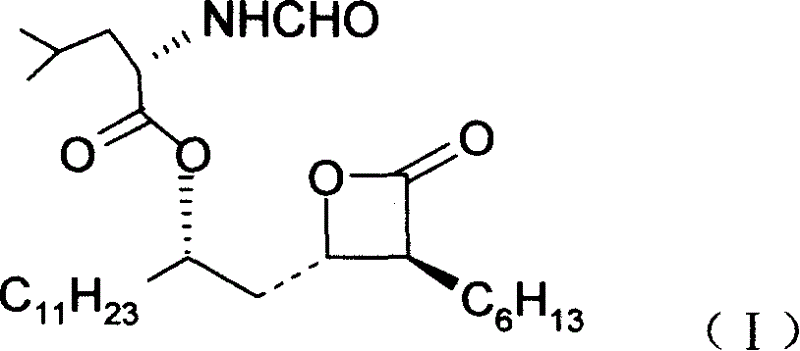
Orlistat preparation method
InactiveCN1765892AEasy to industrializeHigh utility valueOrganic chemistryPtru catalystCombinatorial chemistry
The invention relates to a preparation method for oleanstada, which comprises: on catalyst action, using enolsiliconether and lauraldehyde to condense directly and obtain chiral hydroxyl hexadeketo ester; after eight steps contained protecting hydroxyl group, reducing and cyclization, obtaining the objective product with chemical formula as (I) for efficient fat-reducing. This method needs low cost.
Owner:ARGUS PHARMA
Synthesis method of obeticholic acid
InactiveCN106279336AHigh yieldLess impuritiesSteroidsBulk chemical productionSynthesis methodsCarboxylic acid
The invention discloses a synthesis method of obeticholic acid. The synthesis method takes 3alpha,7alpha-dihydroxyl-5beta-cholestane-24-acid as a starting material and comprises the following steps: carrying out hydroxyl oxidation and carboxylic acid ethyl esterification, and reacting with trimethylsilyl chloride to synthesize silyl enol ether; then enabling the silyl enol ether and acetaldehyde to subject to Mukaiyama hydroxyaldehyde condensation to obtain 6-ethylidene-3alpha-hydroxyl-7-one-5beta-cholestane-24-ethyl; carrying out catalytic hydrogenation, hydroxyl protection and ester group hydrolysis; carrying out selective reduction through sodium borohydride; finally, carrying out de-protection to obtain the obeticholic acid. By optimizing synthesis steps and selecting different protection reagents to protect hydroxyl and carboxyl for a plurality of times, and adopting a selective hydrogenation reduction reaction, the problems in a synthesis reaction of the obeticholic acid that more impurities are caused, a structure is easy to overturn, the yield in a 6alpha-ethylation process is low, purification is difficult to realize and the like are effectively solved; the total yield of an obeticholic acid product is greatly improved; the synthesis method has good economical efficiency and is suitable for industrial production.
Owner:合肥诺瑞吉医药科技有限公司
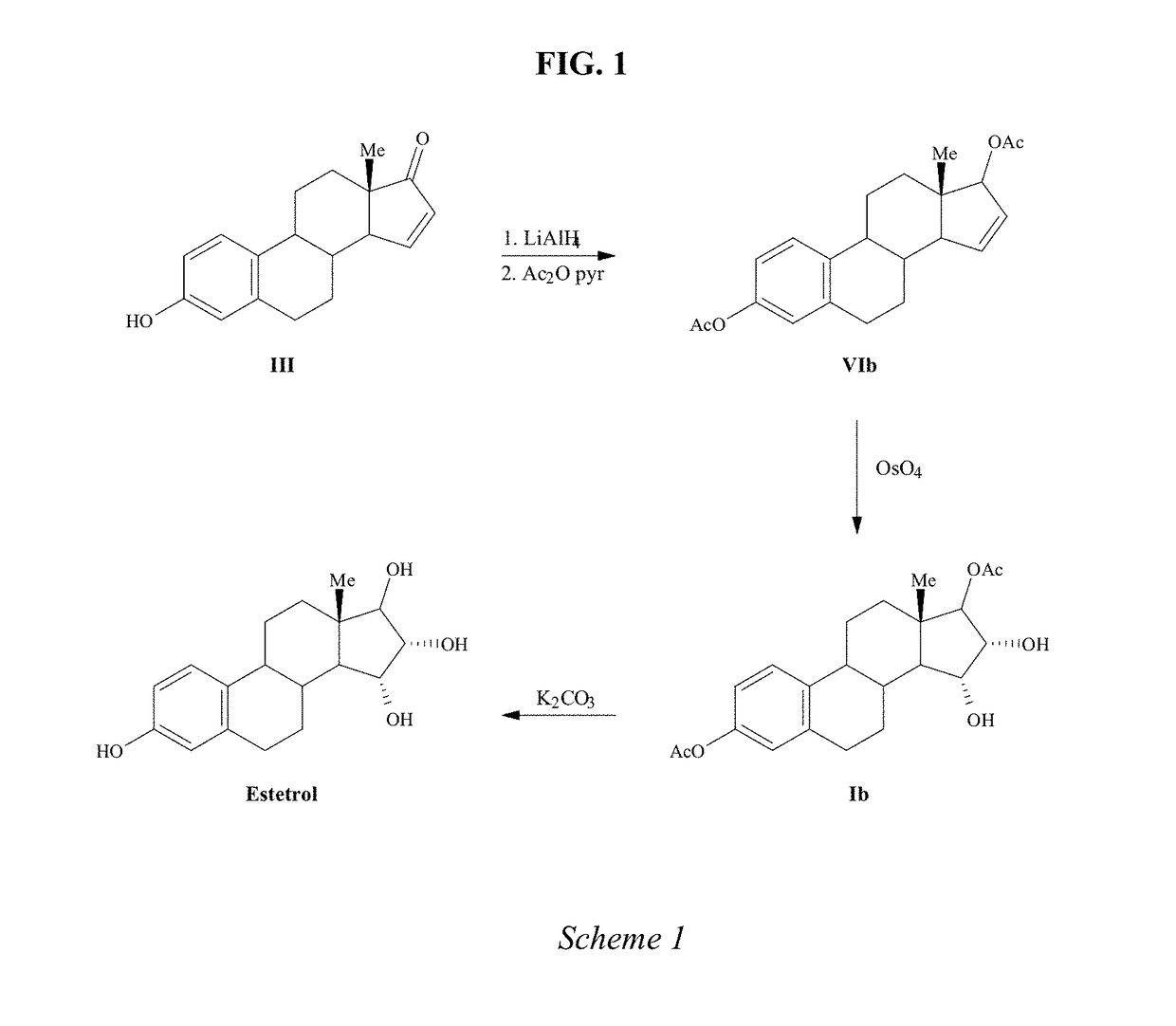
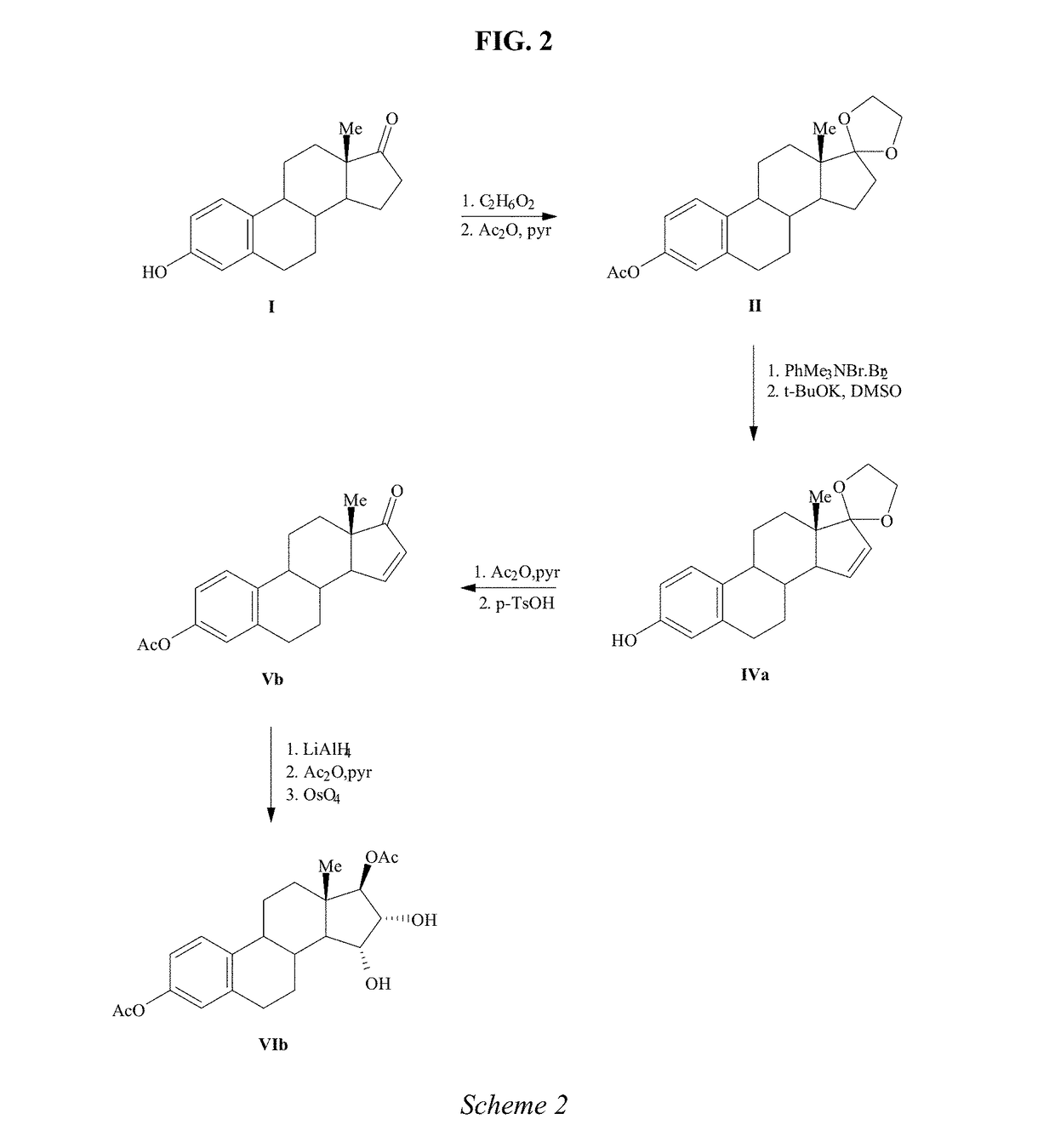
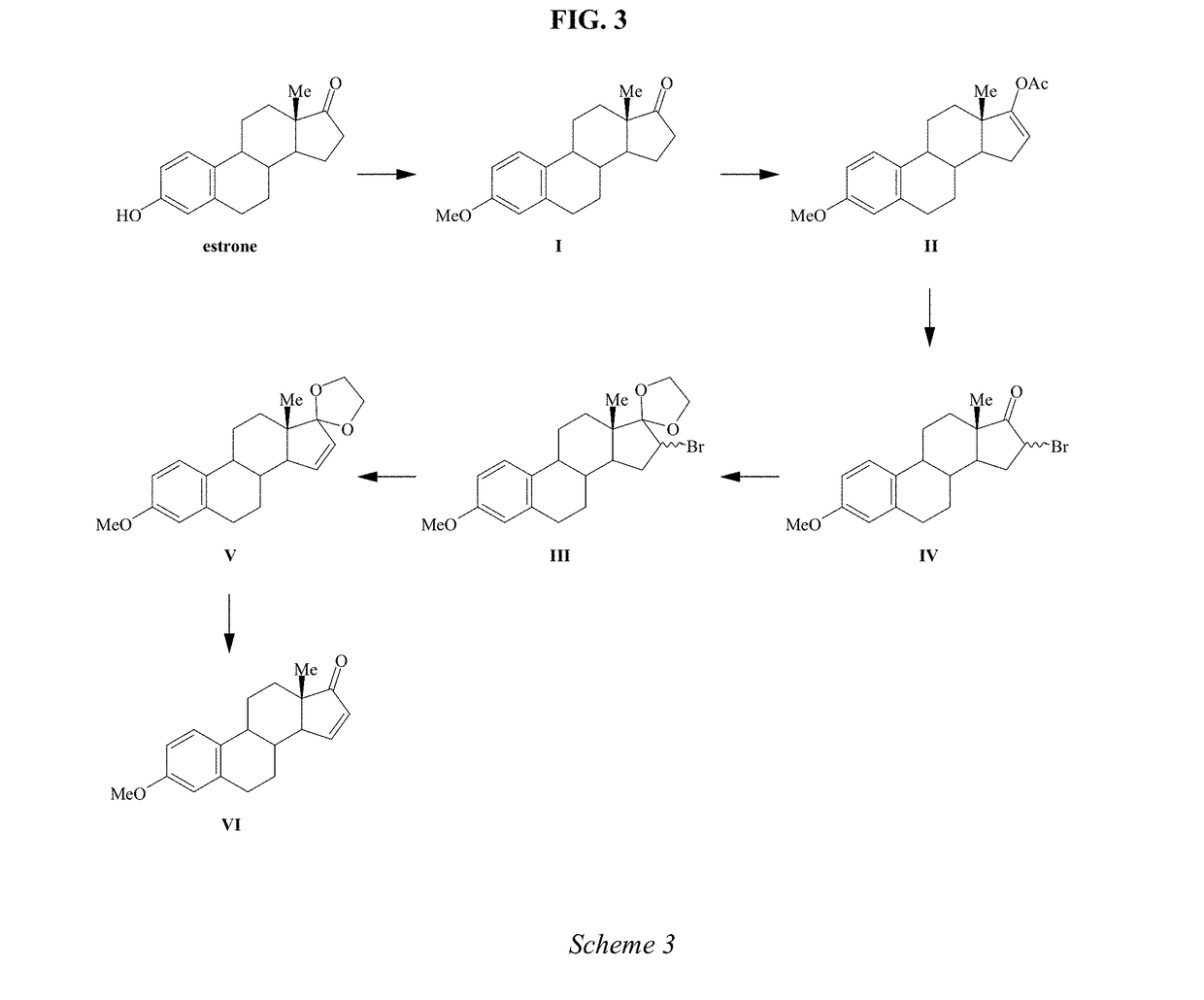
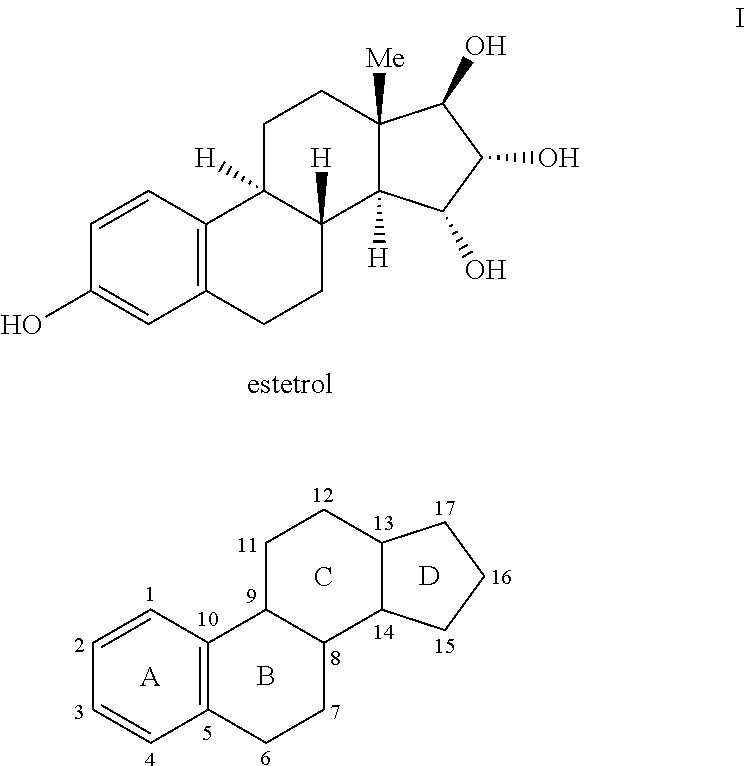
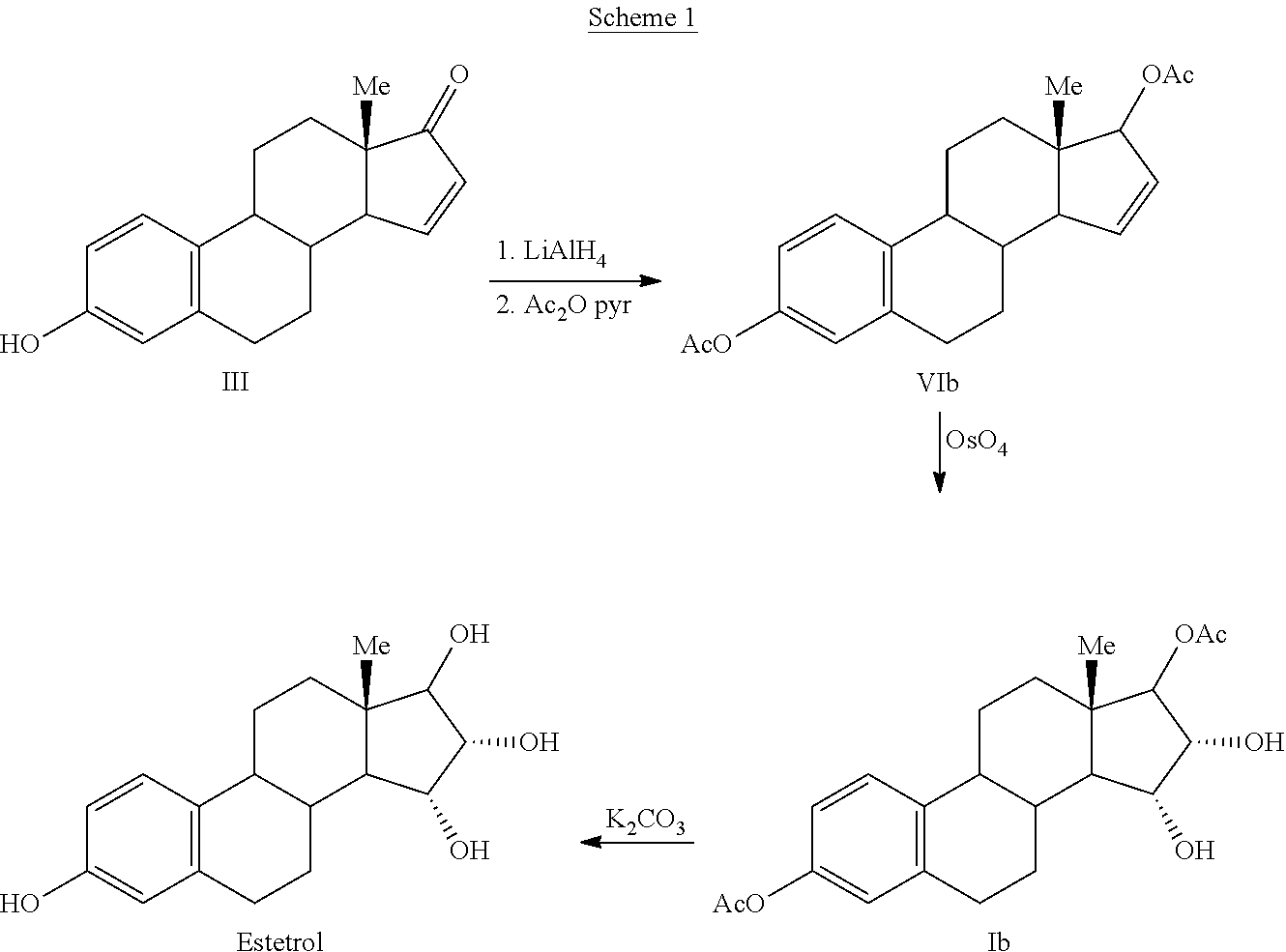
Process for the preparation of estetrol
Owner:MITHRA R&D SA +1
Process for the preparation of estetrol
The present invention relates to a process for the preparation of estra-1,3,5(10)-trien-3,15α,16α,17β-tetraol (estetrol), via a silyl enol ether derivative 17-B-oxy-3-A-oxy-estra-1,3,5(10),16-tetraene, wherein A is a protecting group and B is —Si(R2)3. The invention further relates to a process for the synthesis of 3-A-oxy-estra-1,3,5(10),15-tetraen-17-one, wherein A is a protecting group, via said silyl enol ether derivative.
Owner:ESTETRA S P R L
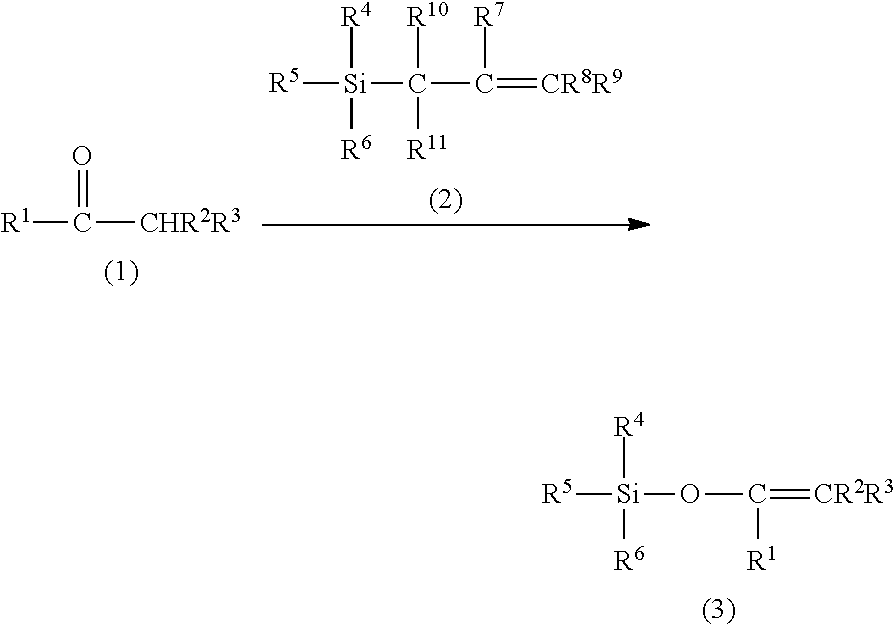
Method for producing silylenol ethers
InactiveUS20130053567A1Reduce wasteImprove purification effectSilicon organic compoundsSteroidsSilane compoundsSilylene
The invention relates to a method for producing silyl enol ether compound (3) by reacting ketone or aldehyde compound (1) with allylsilane compound (2) in the presence of a base and 0.00001 to 0.5 equivalents of an acid catalyst relative to ketone or aldehyde compound (1).
Owner:KYOTO UNIV
Method for synthesizing 1, 4-diketone compound without catalyst
InactiveCN104844401AMild reaction conditionsFew reaction stepsCarbonyl group formation/introductionCarbonyl compound preparation by condensationDiketoneAfter treatment
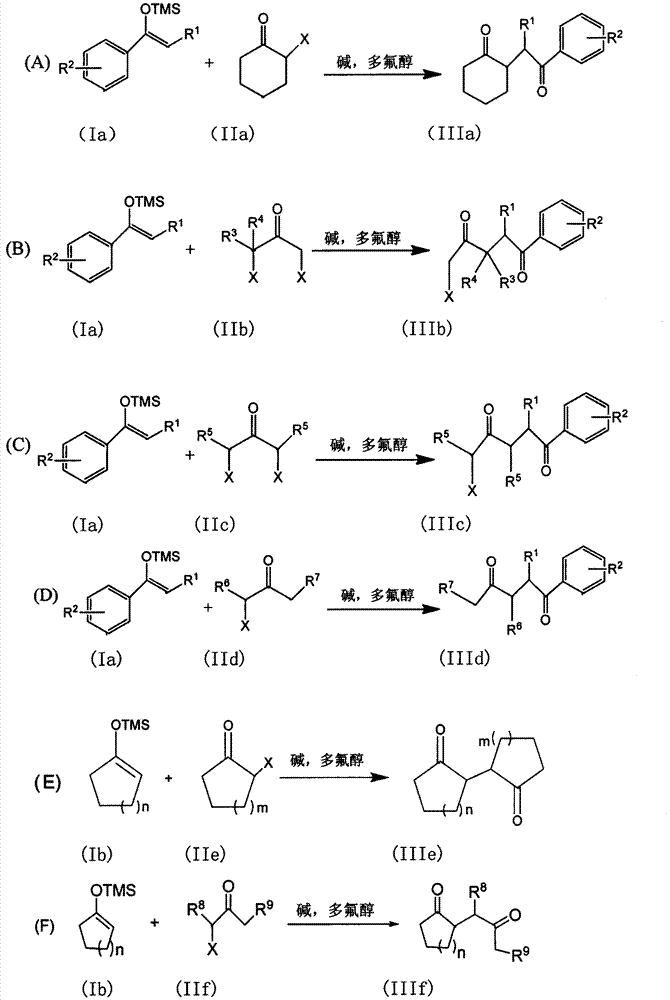
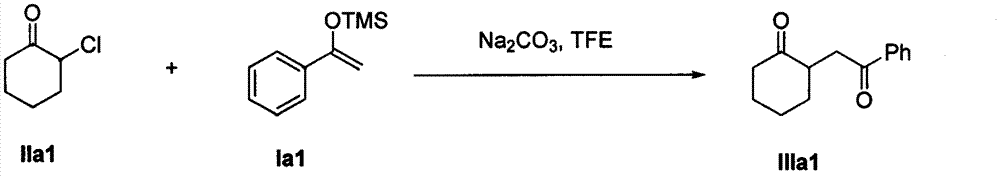
The invention discloses a method for synthesizing a 1, 4-diketone compound without a catalyst. In the existence of alkali, the corresponding 1, 4-diketone compound is obtained by reacting silyl enol ether and alpha-haloketone by taking perfluoroalkyl alcohol as a solvent. By adopting the method for synthesizing the 1, 4-diketone compound, the raw materials are easily obtained, the cost is low, the reaction conditions are mild, the operation is simple and easy to control, the side reactions are few, the after-treatment is simple, the product yield is relatively high, and the solvent can be recovered and recycled, so that the production cost is greatly saved; the method has good environment protection benefit and economical benefit and is suitable for industrial large-scale production.
Owner:CHONGQING MEDICAL UNIVERSITY
Halogen-free flame-retardant electronic material and preparation method thereof
The invention provides a halogen-free flame-retardant electronic material and a preparation method thereof. The preparation method comprises the following steps: adding a fullerene derivative into a cyanate chlorobenzene solution, adding diethylbenzene phosphate and enol silyl ether, carrying out reflux reaction for 80 minutes, adding dicarboxyl phthalimide, reacting for 75 minutes, and simultaneously carrying out rotary evaporation and drying to remove a solvent, so as to obtain a cyanate prepolymer; mixing the cyanate prepolymer with N-4-hydroxyphenyl maleic anhydride alkylamine, naphthol phenolic resin and tetraglycidyl diaminomethylene, stirring at 120 DEG C for 45 minutes, adding silicon carbide short fibers and 1,8-octane dithiol, continuing to stir for 20 minutes, and naturally cooling, so as to obtain a cyanate modified matter; crushing the cyanate modified matter, adding the crushed cyanate modified matter, polyphenylene sulfide and hollow aluminum oxide into an extruder, carrying out extrusion at 155 DEG C, so as to obtain halogen-free flame-retardant particles; and carrying out hot-pressing on the halogen-free flame-retardant particles, so as to obtain the halogen-free flame-retardant electronic material. The prepared halogen-free flame-retardant electronic material has excellent flame retardance and very good dielectric property and thermal performance.
Owner:SUZHOU YIKETAI ELECTRONICS MATERIAL
A kind of new synthetic method of entecavir compound
ActiveCN105037363BSolve rare problemsDosage controllableGroup 4/14 element organic compoundsWittig reactionDrugs synthesis
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +1
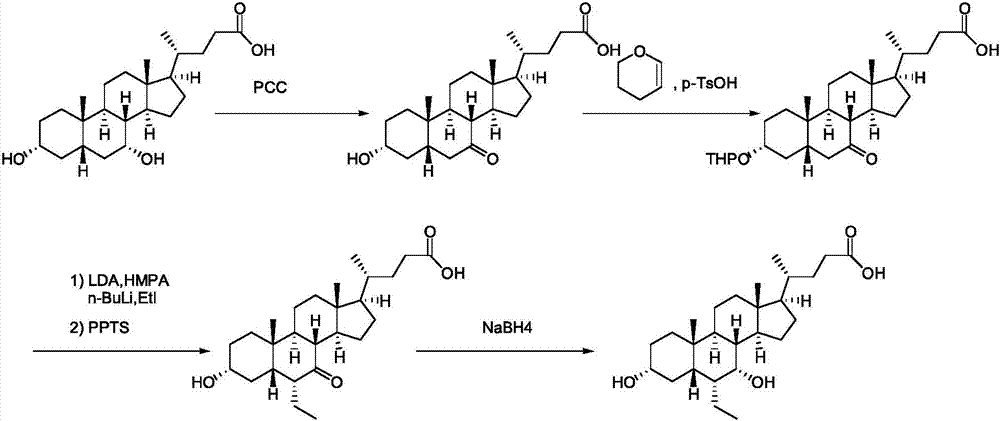
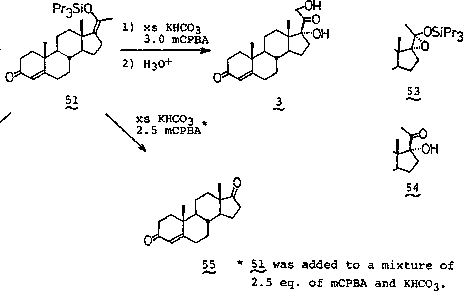
Obeticholic acid preparation method
The invention discloses an obeticholic acid preparation method, which comprises that (1) hyodeoxycholic acid II reacts with an alcohol compound III under the action of a catalyst to generate an ester compound IV; (2) the ester compound IV is subjected to PDC oxidation in dichloromethane to generate a compound V; (3) the compound V and trimethyl chlorosilane are subjected to a reaction at a temperature of -70 to -20 DEG C in tetrahydrofuran by using lithium diisopropylamide as an alkali to generate a silyl enol ether compound VI; (4) the silyl enol ether compound VI is subjected to m-chloroperoxybenzoic acid oxidation and deprotection in dichloromethane to generate a compound VII: (5) the compound VII and Yield generated from ethyltriphenylphosphonium bromide under the action of a strong alkali are subjected to a Wittig alkenylation reaction at a temperature of 0-70 DEG C to convert the ketone into the vinyl so as to generate a compound VIII; (6) the double bond of the compound VIII is subjected to catalytic hydrogenation reduction in a mixing solvent to generate a compound IX; and (7) the compound IX is hydrolyzed under an alkaline condition to generate the obeticholic acid.
Owner:XIAMEN HALOSYNTECH CO LTD
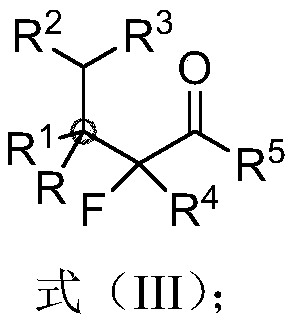
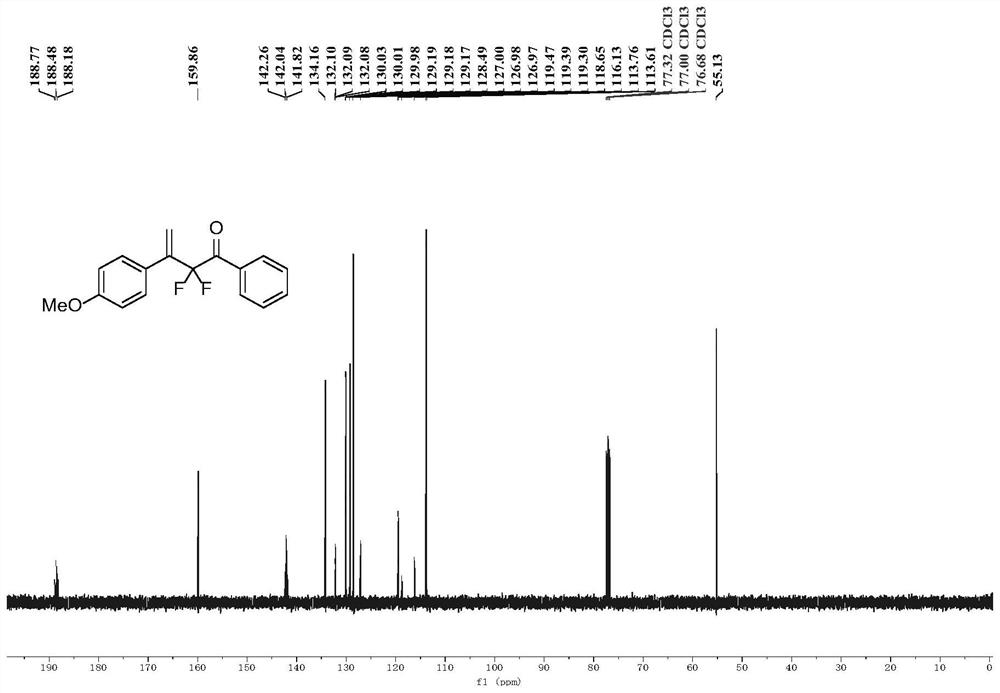
Beta-all carbon quaternary carbon-alpha-fluoroalkyl ketone compound and synthesis method and application thereof
InactiveCN110372481ARaw materials are cheap and easy to getMild reaction conditionsSugar derivativesOrganic compound preparationSilanesSynthesis methods
The invention discloses a beta-all carbon quaternary carbon-alpha-fluoroalkyl ketone compound and a synthesis method and application thereof. The compound has the structure shown in the following equation, has potential biological activity and pharmacodynamic activity, provides a strong technical support for development of new drugs, and has high practical value; and the synthesis method starts from olefin and the nucleophile agent fluoroenol silane, and efficiently synthesizes the beta-all carbon quaternary carbon-alpha-fluoroalkyl ketone compound in one step through a simple addition reaction. The beta-all carbon quaternary carbon-alpha-fluoroalkyl ketone compound and the synthesis method and application thereof have the characteristics that raw materials are cheap and readily available;the reaction condition is mild and operation is simple; and the application range of substrates is wide, and the higher yield can be obtained.
Owner:EAST CHINA NORMAL UNIV
Dicarboxy Boc-L drivative, its preparing method and use
This invention relates to a double hydroxy, high proline ramification and the preparation method and application. The invention utilize 5 - nitrine - 5 - deoxypentose chirality structure to prepare 5 - nitrine methyl - 3, 4 -double shield hydroxy nitrone, furthermore utilize addition reaction of nitrone and ester enol siloxane type nucleophilic reagent, to prepare double hydroxy high proline ramification. This invention has good regioselectivity, high stereoselectivity, be able to proceeding bulk preparation.
Owner:INST OF CHEM CHINESE ACAD OF SCI
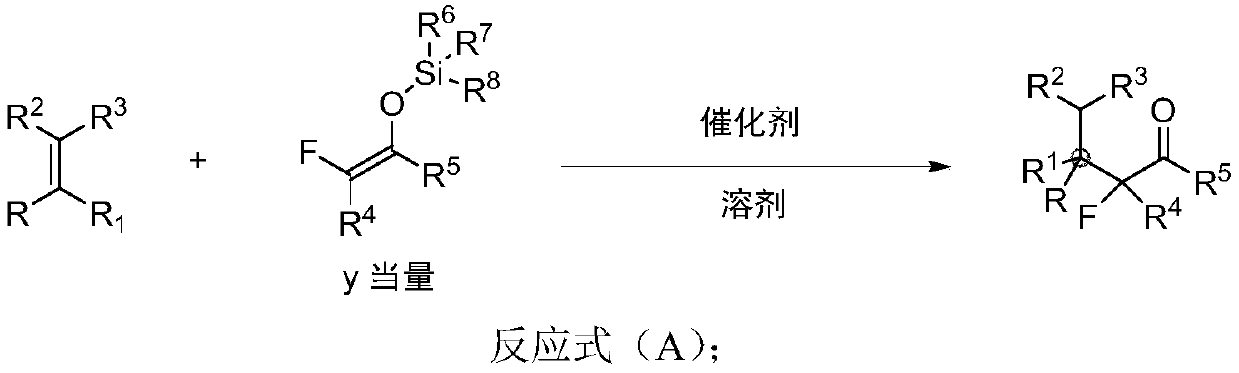
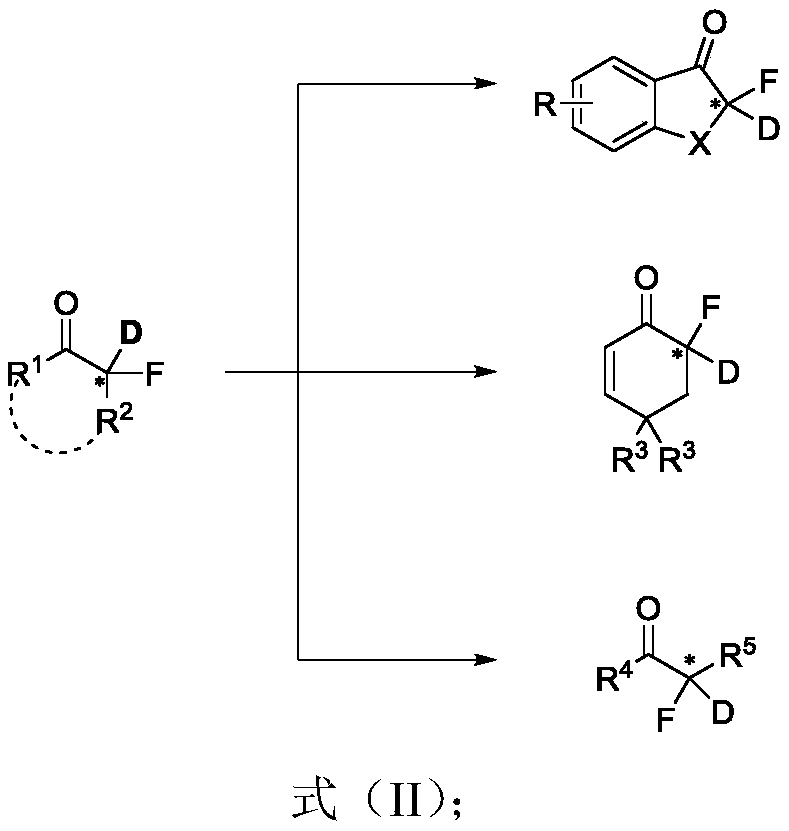
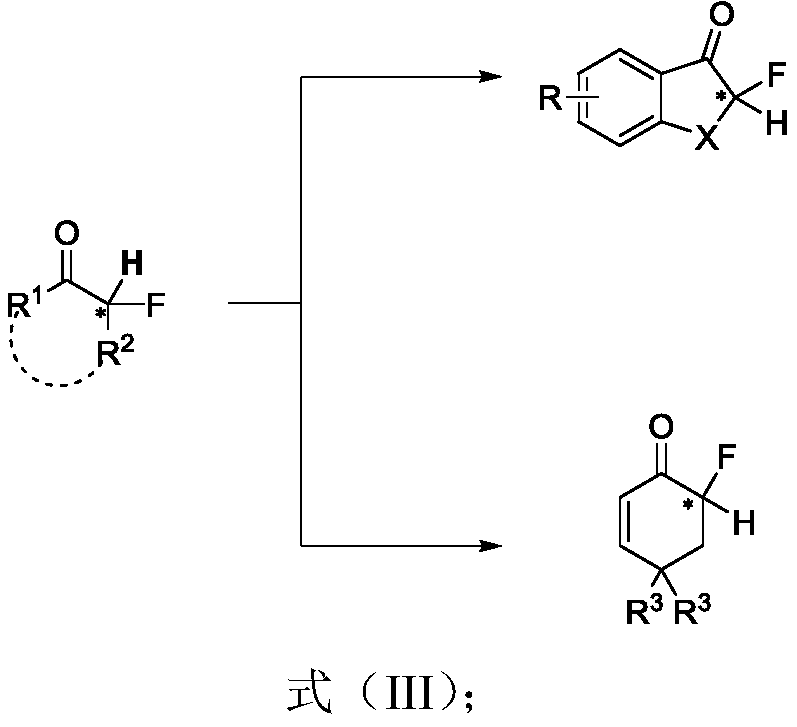
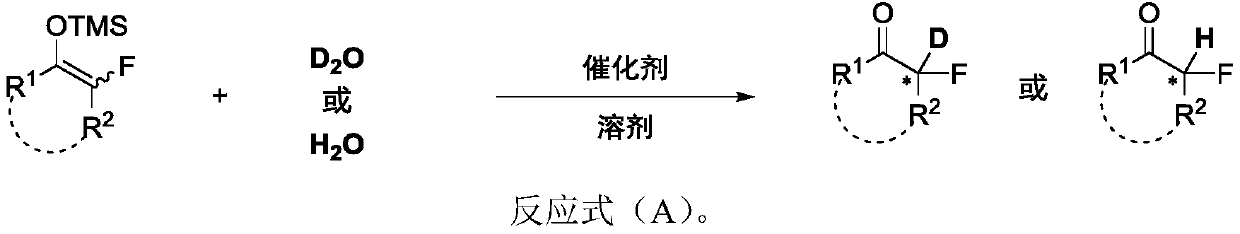
Chiral alpha-deuterium (hydrogen) alpha-fluoroketone compound and asymmetric catalytic synthesis method thereof
InactiveCN110183316ARaw materials are cheap and easy to getMild reaction conditionsIsotope introduction to heterocyclic compoundsOrganic compound preparationSynthesis methodsDrug biological activity
The invention discloses a chiral alpha-deuterium (hydrogen) alpha-fluoroketone compound and an asymmetric catalytic synthesis method thereof. The compound has the structure represented by the following formula (I), has potential biological activity and pharmacodynamic activity, provides a strong technical support for the development of new drugs, and has high practical value. The synthesis methodstarts from using distilled water or deuterium water and a nucleophilic reagent monofluoroenol silicon ether as raw materials, the chiral alpha-deuterium (hydrogen) alpha-fluoroketone compound is efficiently synthesized in one step by an asymmetric deuterium (proton) lysis reaction catalyzed by a chiral amine-hydrogen bond donor bifunctional catalyst, and the specific reaction process is shown inthe reaction formula (A). The method has the characteristics that the raw materials are cheap and easy to obtain; the reaction conditions are mild, and the operation is simple and convenient; and thesubstrate structure is rich and diverse, the good-to-excellent yield and enantioselectivity can be obtained, and the like.
Owner:EAST CHINA NORMAL UNIV
Halogen-free electronic material with high dielectric performance and preparation method of material
The invention provides a halogen-free electronic material with high dielectric performance and a preparation method of the material. A fullerene derivative is added to a chlorobenzene cyanate solution, maleic anhydride is added, the mixture is subjected to a reflux reaction for 40 min, then bi-carboxylphthalimide is added, the mixture reacts for 1 h and then is subjected to rotary evaporation and drying until a solvent is removed, and a cyanate prepolymer is obtained; the cyanate prepolymer is mixed with diphenylphosphine oxide, naphthol-phenolic resin and tetraglycidyl diamino-dimethylene benzene, the mixture is stirred for 1 h at the temperature of 110 DEG C, titanium dioxide whiskers and silyl enol ether are added, the mixture is continuously stirred for 50 min and naturally cooled, and a cyanate modifier is obtained; then the cyanate modifier is crushed and added to an extruder with polyphenylene sulfide and hollow aluminum oxide, extrusion is performed at the temperature of 155 DEG C, and halogen-free particles with high dielectric performance are obtained; the halogen-free particles with high dielectric performance are subjected to hot pressing, and the halogen-free electronic material with high dielectric performance is obtained. The prepared product has excellent dielectric performance and has a good flame-retardant effect and good thermal performance.
Owner:SUZHOU YIKETAI ELECTRONICS MATERIAL
Activity-controllable (gamma-methyl)-alpha-methylene-gamma-butyrolactone polymerizing method
The invention relates to an activity-controllable (gamma-methyl)-alpha-methylene-gamma-butyrolactone polymerizing method and belongs to the technical field of polymer synthesis. Silyl enol ether is applied as initiator and Lewis acid as catalyst to catalyzing activity-controllable polymerization and copolymerization of MMBL ((gamma-methyl)-alpha-methylene-gamma-butyrolactone) and MBL (alpha-methylene-gamma-butyrolactone). According to the activity-controllable (gamma-methyl)-alpha-methylene-gamma-butyrolactone polymerizing method, monomers to be polymerized are renewable resources and has a wide application prospect; the polymerization system of the monomers has the advantages of being convenient to operate, mild in reaction conditions, rapid, high in conversion rate and the like; the prepared polymer is controllable in molecular weight and narrow in molecular weight distribution and accordingly achieves activity-controllable polymerization.
Owner:JILIN UNIV
Method for synthesizing 1,4-diketone compound by using 2-halogenated cyclopentanone as raw material
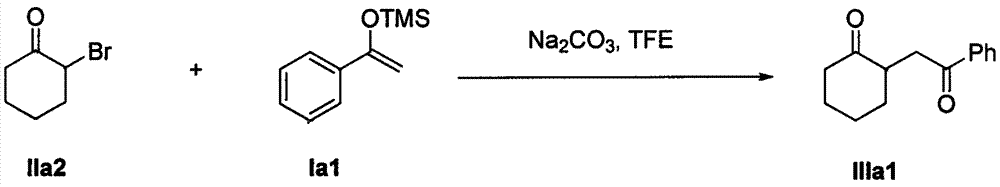
InactiveCN104945231AMild reaction conditionsFew reaction stepsCarbonyl compound preparation by condensationSynthesis methods2,2,2-Trifluoroethanol
The invention provides a method for synthesizing 1,4-diketone compound by using 2-halogenated cyclopentanone as a raw material. In the presence of alkali, 2-halogenated cyclopentanone reacts with silyl enol ether in perfluoroalkyl alcohol used as a solvent to obtain a 1,4-diketone compound, wherein perfluoroalkyl alcohol is trifluoroethanol or hexafluoroisopropanol, and the alkali is selected from sodium carbonate, potassium carbonate, sodium trifluoroethanol, triethylamine and pyrrolidine . The synthesis method provided by the invention has advantages as follows: raw materials are easily available; costs are low; reaction conditions are mild; operation is simple and easy to control; there exists less side reaction; post-treatment is simple; product yield is high; and the solvent can be recovered and recycled so as to greatly save production cost. The method has good environmental protection benefit and economic benefit and is suitable for industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Natural product (+)-Strictifolione synthetic method
InactiveCN105254604ASimple and fast operationHigh yieldOrganic chemistryChemical recyclingIndiumStrictifolione
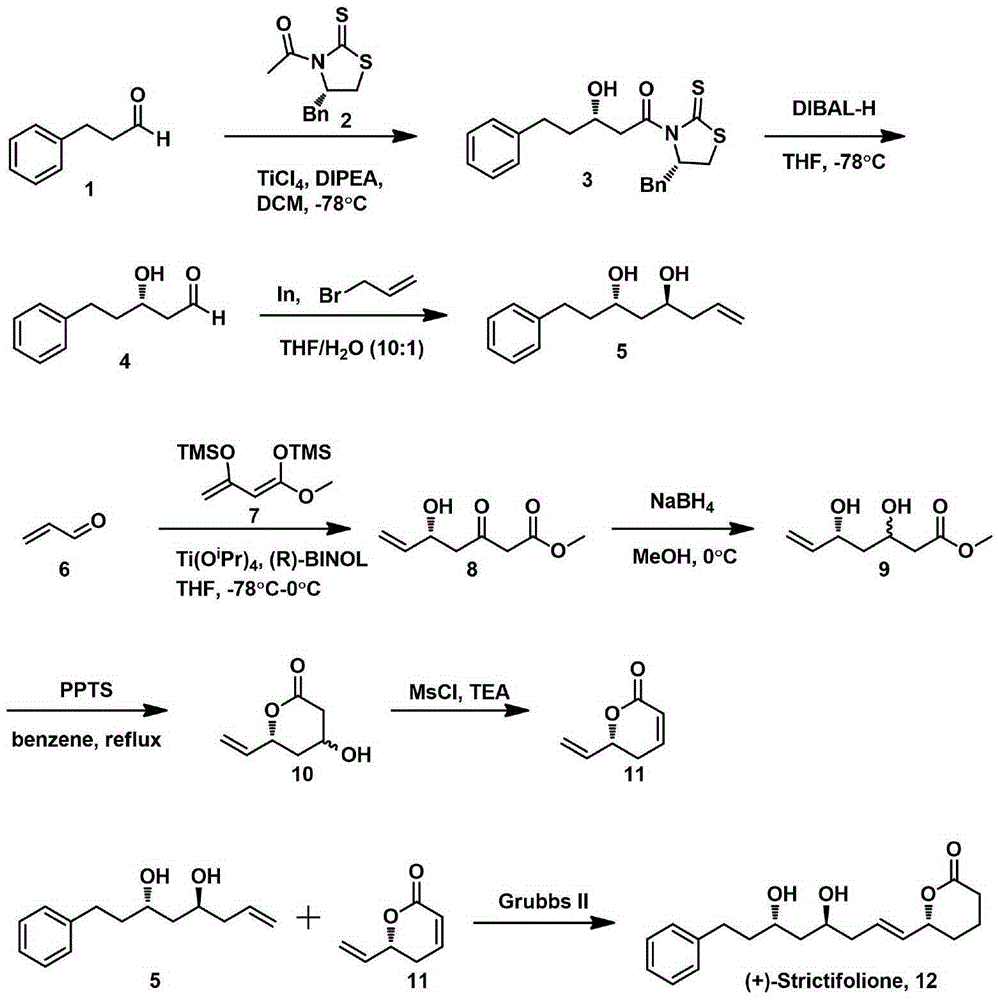
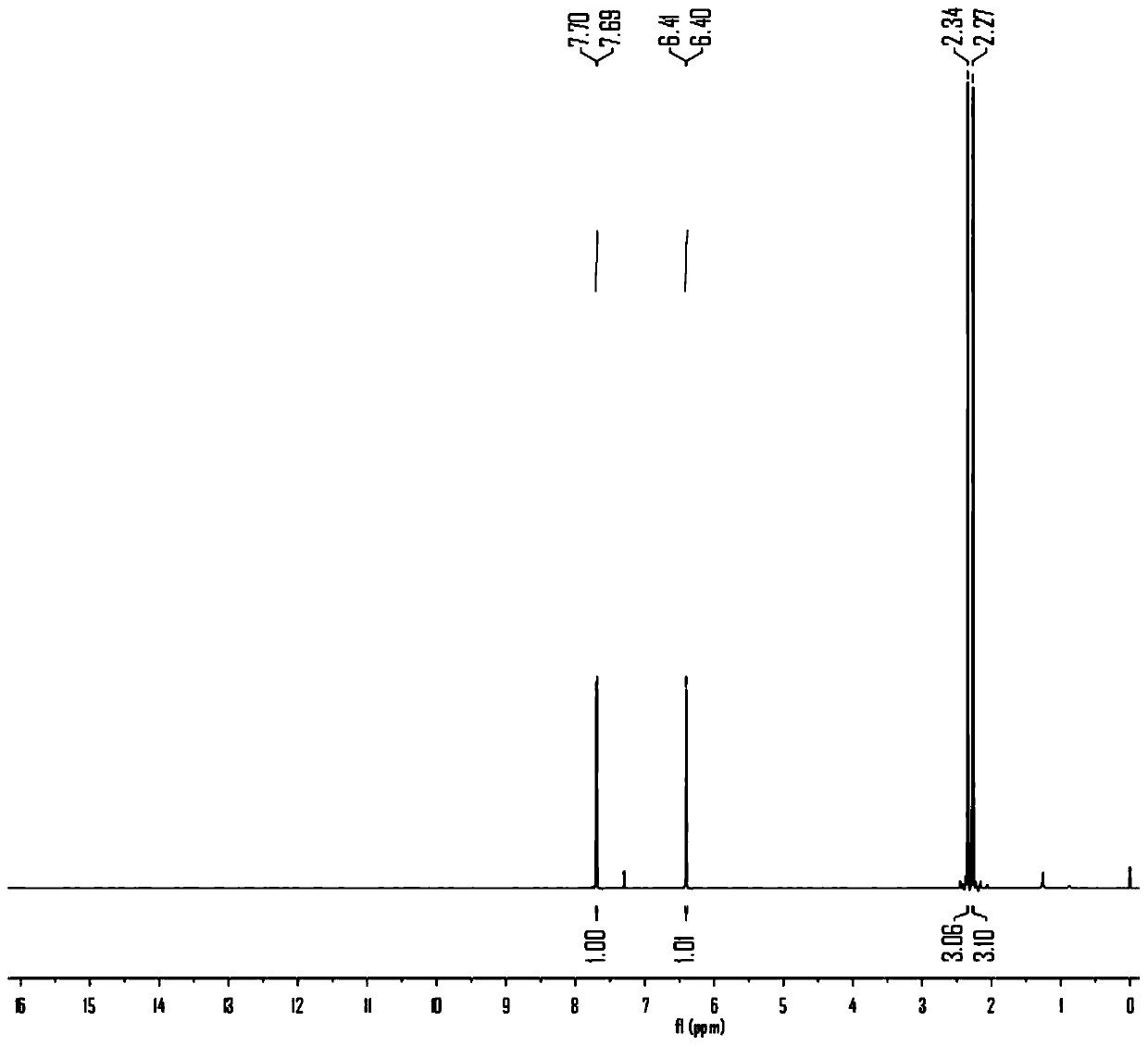
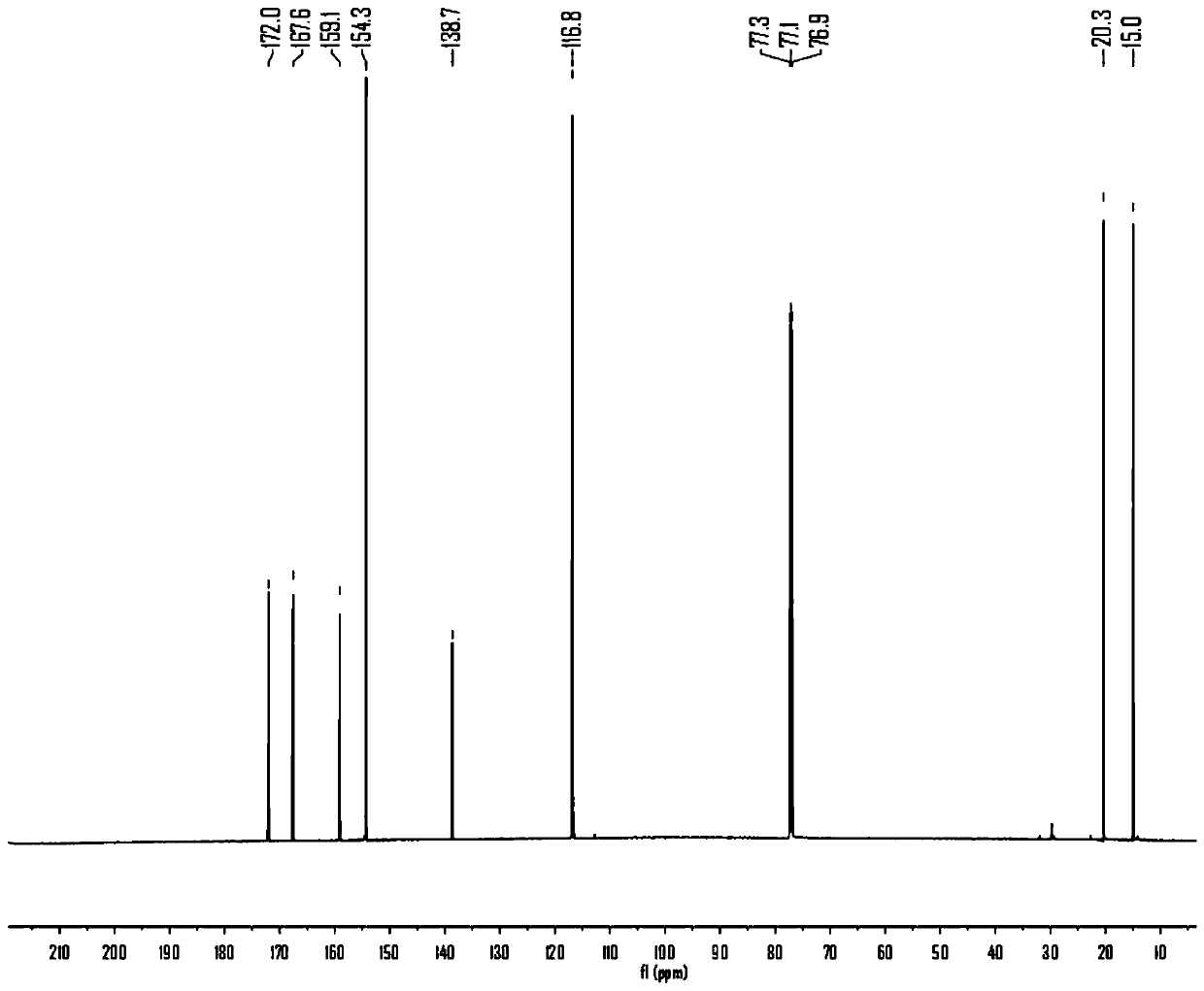
The invention discloses a natural product (+)-Strictifolione synthetic method. The method comprises the steps that 3-benzenepropanal and an Evans chiral auxiliary reagent are subjected to an aldol reaction for diisobutyl aluminum hydride reduction and then subjected to an addition reaction with metal indium activated 3-propylene bromine to obtain a compound shown in the formula 5; silyl enol ether reacts with acrolein under the action of titanium tetraisopropoxide and (R)-1,1-binaphthol, then carbonyl is reduced through sodium borohydride, p-toluenesulfonic acidpyridinium salt is used for cyclization, and acidification treatment is carried out after a methylsulfonyl group becomes a leaving group to obtain a compound shown in the formula 11; a Grubbs second-generation catalyst is used for carrying out a metal coupling reaction on the compounds shown in the formulas 5 and 11 to obtain (+)-Strictifolione. According to the bisection synthetic method, the design is simple and reasonable, operation is easy and convenient, the reaction condition is moderate, linear steps are simple, the product yield is high, and the production cost is greatly reduced.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Synthesis method of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one
ActiveCN110713476AHigh reactivityThe five-step reaction process is simpleOrganic chemistryMethyl palmoxiratePyran
The invention relates to a synthesis method of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, which creatively uses maltose as an initial raw material to synthesize the 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one. The synthesis method comprises: firstly, generating maltol acetate through acetylation; secondly, carrying out catalytic hydrogenation to obtain dihydromaltol acetate; adding asilylation reagent again to synthesize a dihydromaltol acetate silyl enol ether compound; increasing reaction activity of 5-position methylene and introducing hydroxy to the 5-position through peroxidation to obtain 5-hydroxy-dihydromaltol acetate; and performing a deacetylation reaction to obtain the 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one that is a target compound. According to the technical scheme, the five-step reaction process is simple, the yield is higher than 80%, the purity of the final product reaches 98% or above, large-scale production can be conducted, and the method has a wide application prospect.
Owner:CHINA TOBACCO HENAN IND
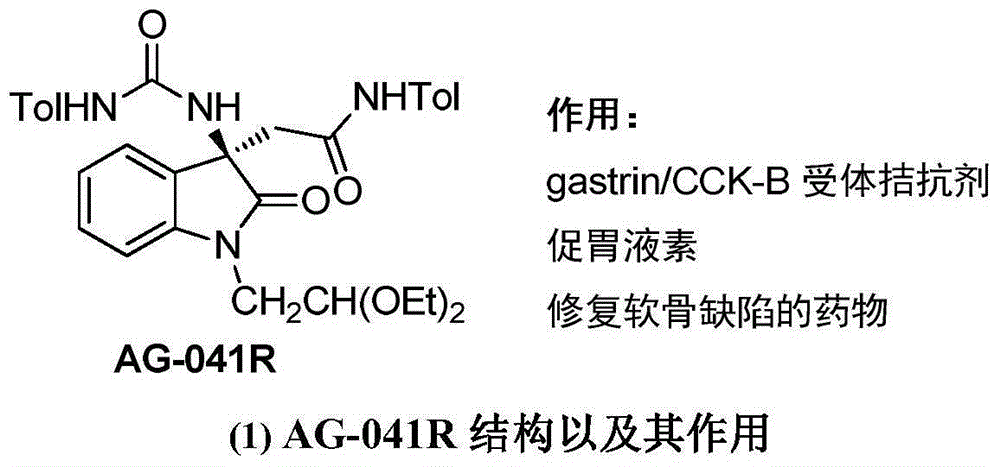
3-difluoroalkyl substituted amino oxindole derivative and synthesis method thereof
The present invention discloses an AG-041R difluoro analog, a 3-difluoroalkyl substituted 3-amino quaternary carbon oxindole derivative and a synthesis method thereof, wherein the compound has a structure represented by the following formula, has potential biological activity and efficacy, provides powerful technical support for development of new drugs, and has high practical values. The synthesis process of the compound comprises: adding a catalyst to a reaction container, sequentially adding isatin imine, a solvent and a nucleophilic reagent difluoro silyl enol ether, stirring at a temperature of -78 DEG C -100 DEG C until the TLC display reaction is completed, and separating through column chromatography to obtain the product. According to the present invention, the synthesis method has characteristics of simple and easily-available raw materials, mild reaction condition, simple operation and wide substrate application range, and the compounds CF2-AG-041R and 3-difluoroalkyl substituted amino oxindole have potential and important medicinal values.
Owner:EAST CHINA NORMAL UNIV
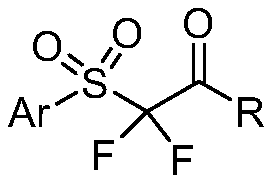
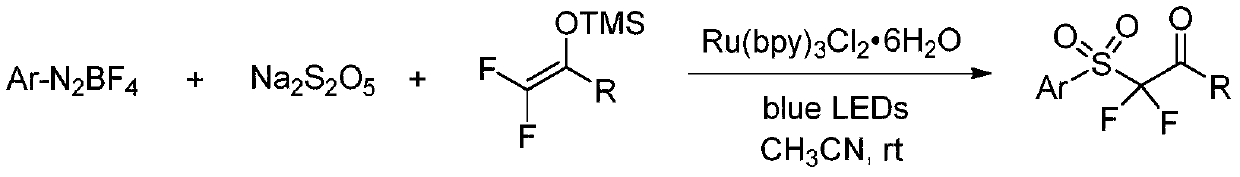
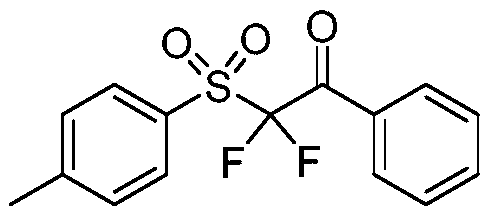
Synthesis method of alpha, alpha-difluoro-beta-carbonyl sulfone compound
The invention belongs to the technical field of organic chemistry, and particularly relates to a synthesis method of an alpha, alpha-difluoro-beta-carbonyl sulfone compound. The method comprises the following steps: with acetonitrile as a solvent, carrying out a reaction on aryl diazonium salt, sodium metabisulfite and 2,2-difluoroenol silyl ether with Ru(bpy)3Cl2.6H2O being a photosensitizer under blue light irradiation to obtain the 2, 2-difluoroenol silyl ether photosensitizer, thereby generating aryl sulfonyl free radicals from aryl diazonium salt and sodium pyrosulfite under the action of blue light and a photosensitizer, performing addition reaction on 2,2-difluoroenol silyl ether to obtain a free radical intermediate, and performing single-electron oxidation on the free radical intermediate and an excited photosensitizer to obtain the alpha, alpha-difluoro-beta-carbonyl sulfone compound. The synthesis method of the compound has the advantages that with the aryl diazonium salt,sodium metabisulfite and 2,2-difluoroenol silyl ether, which are easy to obtain, being raw materials, strict reaction conditions such as strong base and ultralow temperature are not needed, so that the method has the advantages of strong functional group compatibility, wide substrate application range and the like, a series of alpha, alpha-difluoro-beta-carbonyl sulfone compounds can be efficiently synthesized, and the method has good academic guidance significance and industrial application value.
Owner:TAIZHOU UNIV
Method for difluoroalkylation after fatty amine deamination
ActiveCN111892489AImprove compatibilityImprove economyCarbamic acid derivatives preparationCarboxylic acid nitrile preparationAlkanePyridinium
Owner:ZUNYI MEDICAL UNIVERSITY
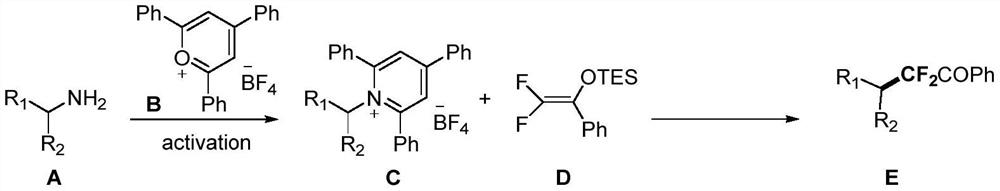
Method for preparing alpha-fluoro-beta-ethynyl ketone compound containing two chiral centers
ActiveCN106854125AStarting materials are cheap and readily availableEasy to synthesizeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisKetone
The invention provides a method for preparing an alpha-fluoro-beta-ethynyl ketone compound containing two chiral centers, belongs to the field of organic synthesis, and relates to a method for synthesizing an alpha-fluoro-beta-ethynyl ketone compound containing two chiral centers from fluorinated silyl enol ether and a propargyl compound by catalyzing an asymmetric propargyl substitution reaction, wherein the used chiral copper catalyst is generated from a copper salt and a chiral tridentate P,N,N-ligand in various polar and non-polar solvents in an in-situ manner. According to the present invention, with the method, the alpha-fluoro-beta-ethynyl ketone compounds containing two chiral centers and having various substituents can be conveniently synthzied, wherein the enantiomeric excess percentage is up to greater than 99%; and the method has characteristics of easily available raw materials, simple operation, mild reaction condition, high diastereoselectivity, high enantioselectivity, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
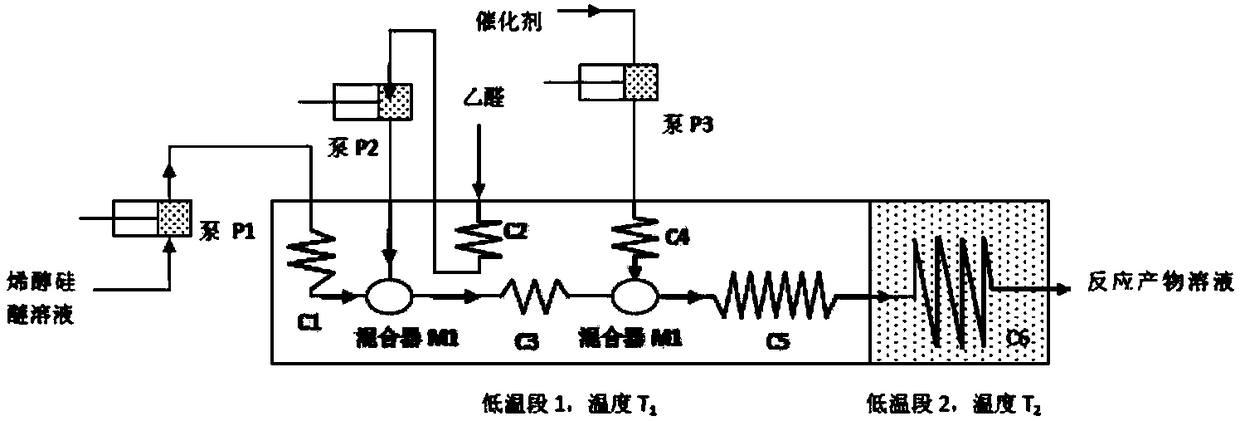
Method for producing 6-ethylidene chenodeoxycholic acid by continuous flow micro reactor
ActiveCN108299539AImprove cooling conditionsUniform and good reaction environmentSteroidsBulk chemical productionChenodeoxycholic acidContinuous flow
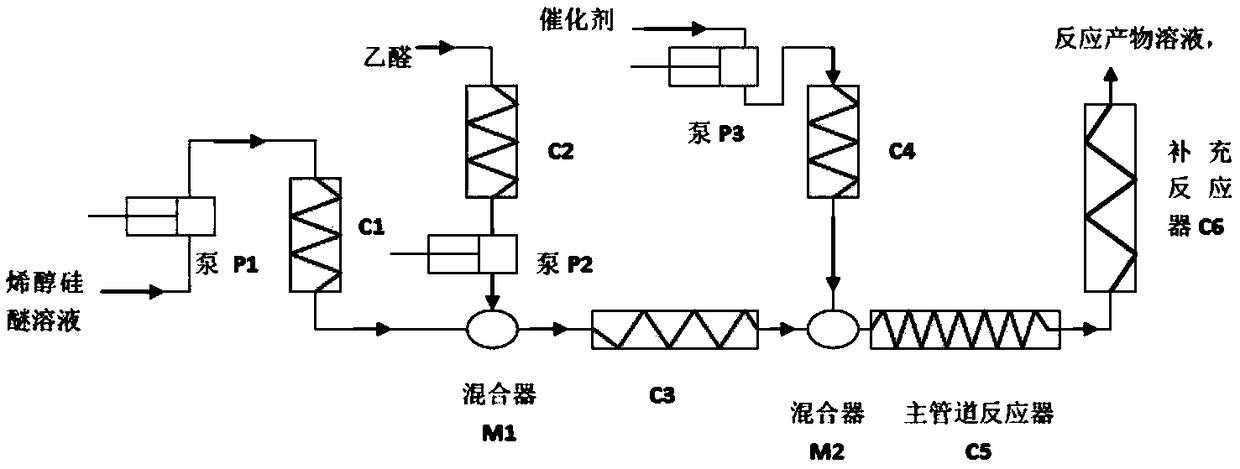
The invention discloses a method for producing 6-ethylidene chenodeoxycholic acid by a continuous flow micro reactor. According to the method, pumping and conveying a solution of silyl enol ether intoa pipeline reactor through a pump; after cooling, mixing the solution with the cooled acetaldehyde solution in a mixer; then, continuously performing cooling in a pipeline reactor; next, mixing the materials with the cooled catalyst solution in another mixer; then, completing the reaction in the pipeline reactor. The operation is simple; the operation and the control of the production are easy; byproducts in the production process are few; the yield is high; the synchronous amplification is easy.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
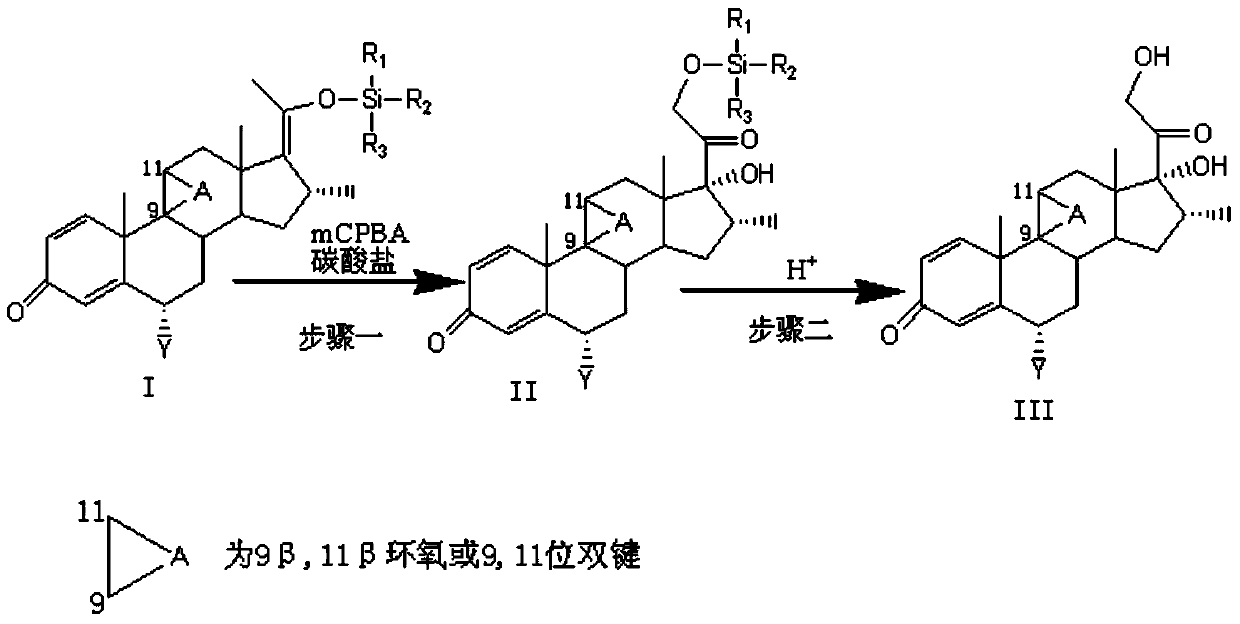
Preparation method of 17,21-dihydroxy steroid derivative
The invention relates to a method for preparing a 17,21-dihydroxy steroid compound by taking a steroid silyl enol ether compound I as an initiator.
Owner:TIANJIN JINYAO GRP
Method for synthesizing alpha, alpha-difluoroketone compound
ActiveCN112500270AMild reaction conditionsImprove reaction efficiencySugar derivativesOrganic compound preparationOrganic solventAlcohol
The invention discloses a method for synthesizing an alpha, alpha-difluoroketone compound, which comprises the following steps: reacting an alcohol compound with a difluoroenol silyl ether compound inan organic solvent under the action of a catalyst (fluoro alcohol, Lewis acid and acid), and carrying out after-treatment after the reaction is finished to obtain the alpha, alpha-difluoroketone compound. Reaction conditions are mild, reaction efficiency is high, raw materials are cheap and easy to obtain, and important application significance is achieved.
Owner:TAIZHOU UNIV
Preparation method of chiral 1, 4-diphenyl-2-hydroxy-1, 4-dibutanone compound
PendingCN112979523ASolve tough problemsGroup 1/11 organic compounds without C-metal linkagesOrganic compound preparationFuranMeth-
The invention provides a preparation method of a chiral 1, 4-diphenyl-2-hydroxyl-1, 4-dibutanone compound. The method comprises the following steps: in the presence of a chiral metal compound, mixing silyl enol ether and phenylacetaldehyde monohydrate or substituted phenylacetaldehyde monohydrate in a solvent and performing action to obtain the chiral 1, 4-diphenyl-2-hydroxy-1, 4-dibutanone compound, wherein the reaction equation is as shown in the formula 1; R1 of silyl enol ether is of a phenyl or furan structure, a substituent R2 in substituted phenyl glyoxal monohydrate is selected from one or more of hydrogen atoms, halogen, methyl, methoxyl, nitryl and trifluoromethyl, and the substituent is at the ortho-position, meta-position or para-position of a benzene ring. The chiral metal compound can efficiently and highly enantioselectively catalyze the asymmetric Mukaiyama aldol reaction of the phenylacetaldehyde monohydrate compound, a new method for synthesizing the 1, 4-diphenyl-2-hydroxy-1, 4-dibutanone compound is provided, the problem that conditions are harsh in the original reaction is solved, and the chiral metal compound is more environmentally friendly.
Owner:UNIV OF SCI & TECH OF CHINA
Preparation method of alpha-alkenyl-alpha, alpha-difluoroaryl ketone compound and product
ActiveCN114507121ASimple post-processingOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystKetone
The invention discloses a preparation method of an alpha-alkenyl-alpha, alpha-difluoroaryl ketone compound and a product, and the preparation method comprises the following step: carrying out direct cross-coupling reaction on an aryl alkyne compound and difluoroenol silyl ether in a solvent under the action of a catalyst to obtain the alpha-alkenyl-alpha, alpha-difluoroaryl ketone compound. The difluoroenol silyl ether is used as a coupling substrate, so that the reaction has mild conditions and operation convenience, and the preparation method has the characteristics of simple post-treatment, cheap and small-amount catalyst, high economic benefit and the like.
Owner:NANJING UNIV OF TECH
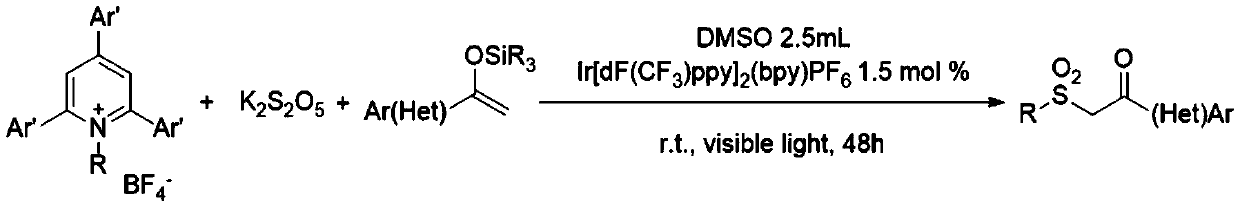
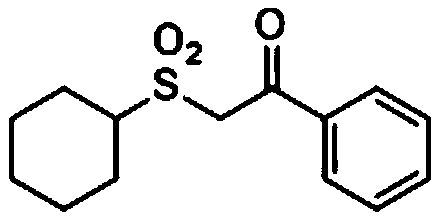
Preparation method of dialkyl sulfone compounds
ActiveCN110734389AEfficient constructionReduce usageOrganic chemistryOrganic compound preparationSulfonyl chloridePyridinium
The invention belongs to the technical field of organic chemistry, and particularly relates to a preparation method of dialkyl sulfone compounds. The preparation method comprises following steps: under catalysis of visible light, various simple or complex alkyl substituted N-alkyl pyridinium salts and a photocatalyst are adopted to generate alkyl free radicals, sulfonyl free radicals are obtainedthrough cascade reaction with sulfur dioxide, and silyl enol ether is attacked, so that a series of dialkyl sulfone compounds are efficiently constructed. According to the method, the dialkyl sulfonecompounds can be efficiently, simply and conveniently synthesized, sulfonyl-derived potassium metabisulfite required by the reaction is a cheap and easily available chemical raw material, the use of strongly acidic sulfonic acid and sulfonyl chloride in the traditional sulfonyl compound synthesis is avoided, and the method has the advantage of large-scale industrial preparation.
Owner:TAIZHOU UNIV
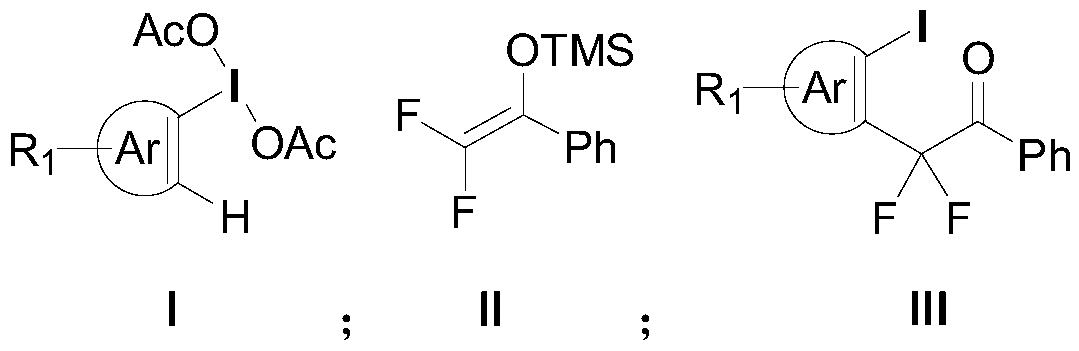
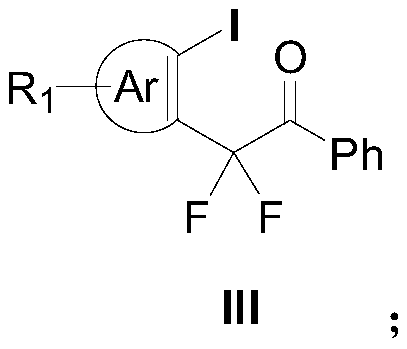
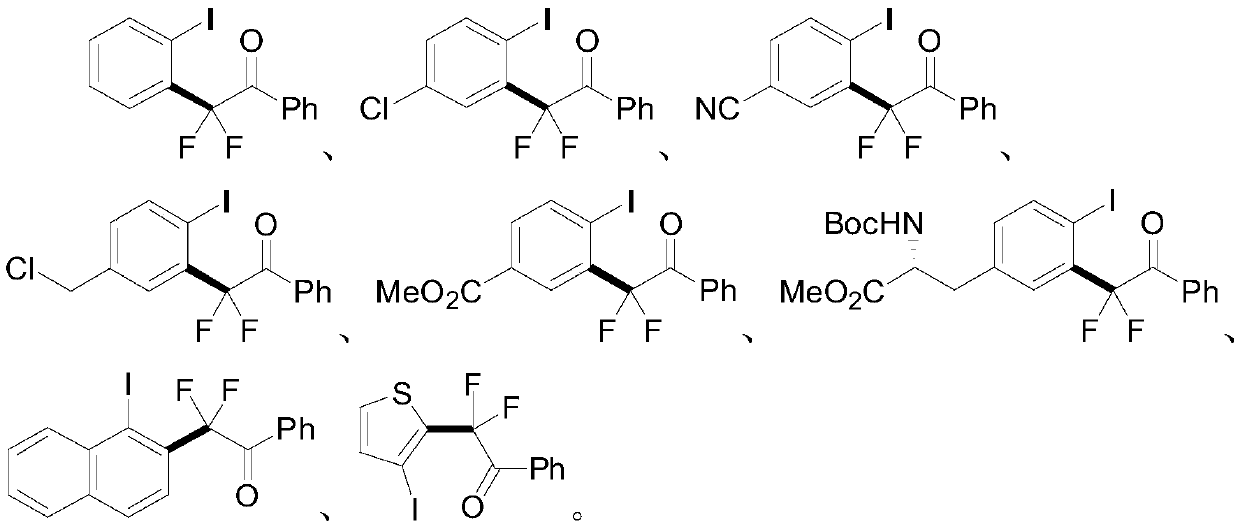
Aryl iodine compounds containing difluoromethylene at the ortho-position and preparing method thereof
ActiveCN109776295AEasy to separateMild reaction conditionsCarbamic acid derivatives preparationCarboxylic acid nitrile preparationArylTrimethylsilyl trifluoromethanesulfonate
A method for preparing aryl iodine compounds containing difluoromethylene at the ortho-position is disclosed. The method is characterized in that aryliodine diacetate shown as a structural formula (I)and difluoroacetophenone silyl enol ether shown as a structural formula (II) are subjected to a rearrangement reaction under the existence of trimethylsilyl trifluoromethanesulfonate to obtain the aryl iodine compound containing difluoromethylene, shown as the structural formula (III), wherein R1 is selected from hydrogen, halogen, alkyl, alkoxy, alkoxycarbonyl, halogenated alkyl, halogenated alkoxy, alkoxycarbonyl substituted alkyl, amino substituted alkyl, alkyl substituted with alkoxycarbonyl and amino, cyano or nitro, and Ar is selected from a benzene ring, a naphthalene ring and a thiophene ring. The method has following advantages: mild reaction conditions, good selectivity, a high yield, easy product separation, simple operation, and the like.
Owner:ZHEJIANG NORMAL UNIVERSITY
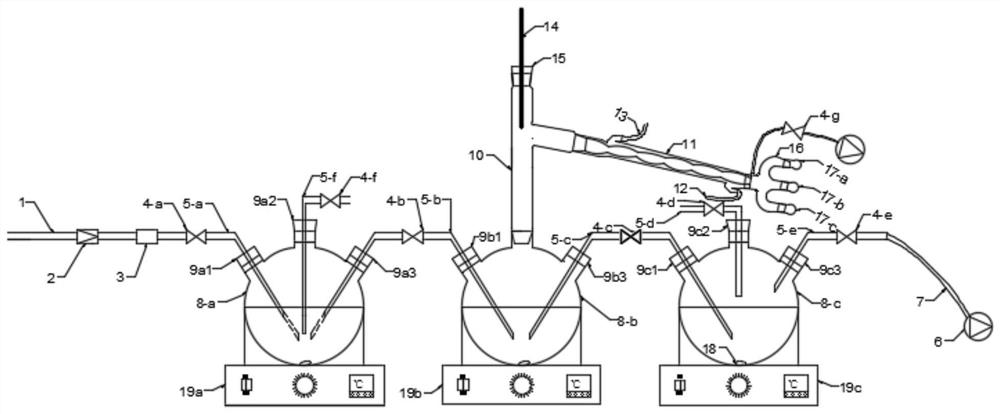
Integrated device for preparing, separating and purifying silyl enol ether
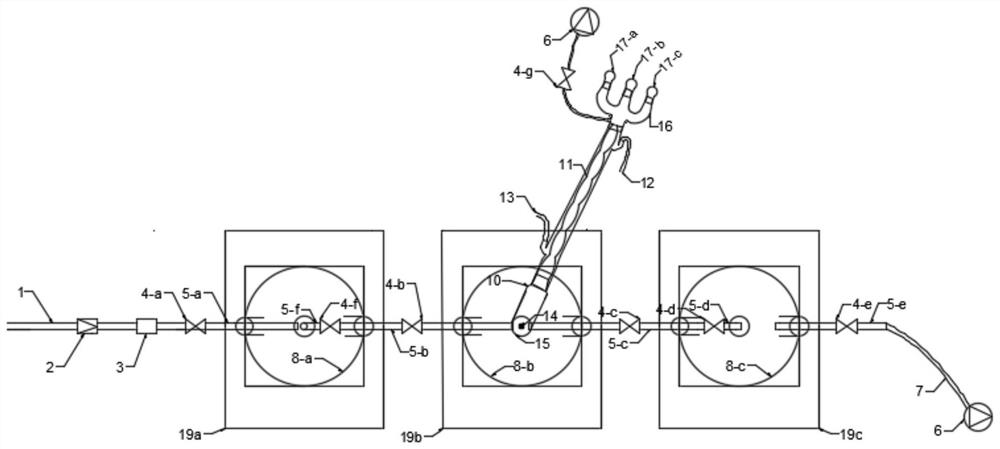
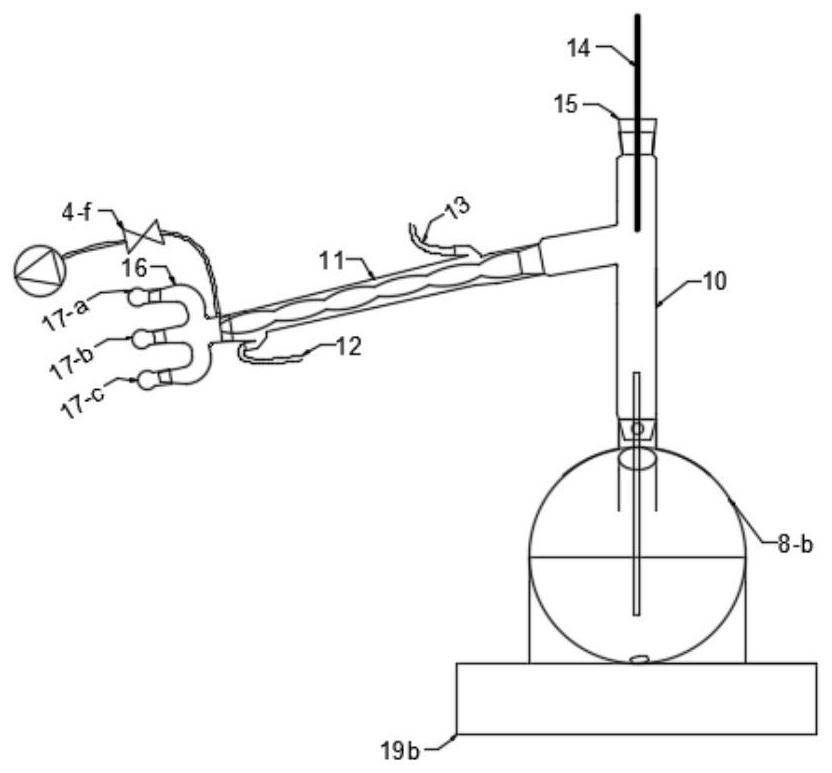
PendingCN112791687ARealize solid-liquid separationSimple and fast operationGroup 4/14 element organic compoundsDispersed particle separationTrimethylsilyl chlorideDistillation
The invention discloses an integrated device for preparing, separating and purifying silyl enol ether. The integrated device comprises a reaction unit, a purification unit and an absorption unit which are connected in sequence, wherein the reaction unit comprises a reactor, and the left upper end face, the top end face and the right upper end face of the reactor are provided with a reactor left opening, a reactor center opening and a reactor right opening; the purification unit comprises a distiller; a distiller left opening, a distiller center opening and a distiller right opening are respectively formed in the left upper end surface, the top end surface and the right upper end surface of the distiller; the absorption unit comprises an absorber, and the upper left end face, the top end face and the upper right end face of the absorber are provided with an absorber left opening, an absorber center opening and an absorber right opening respectively. Through the device, preparation of silyl enol ether, treatment of trimethylchlorosilane, distillation and purification can be realized, a high-purity silyl enol ether product is obtained, the operation is simple and convenient, residual acidic substances in a reaction system can be completely treated, and the device is green and environment-friendly.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com