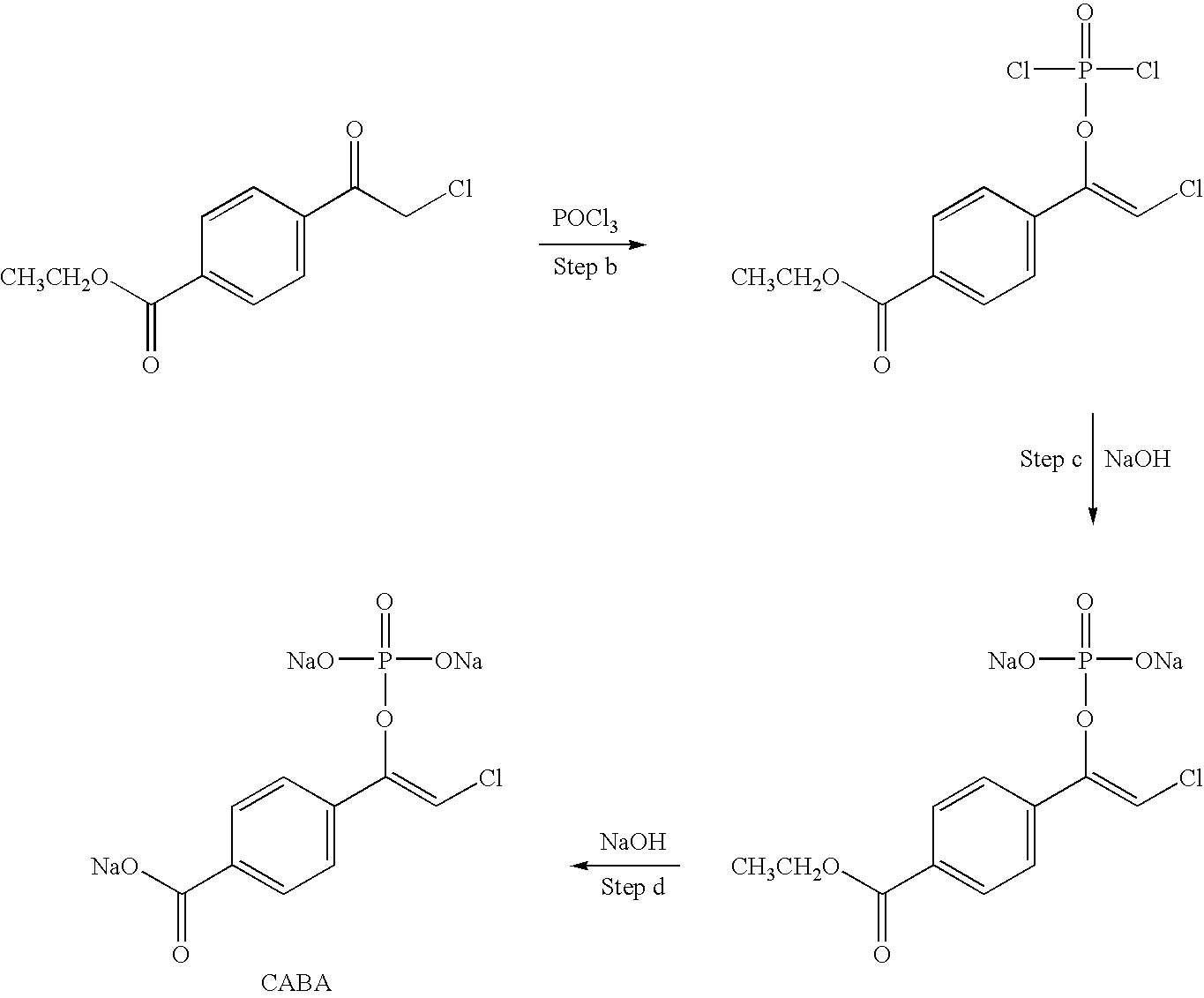
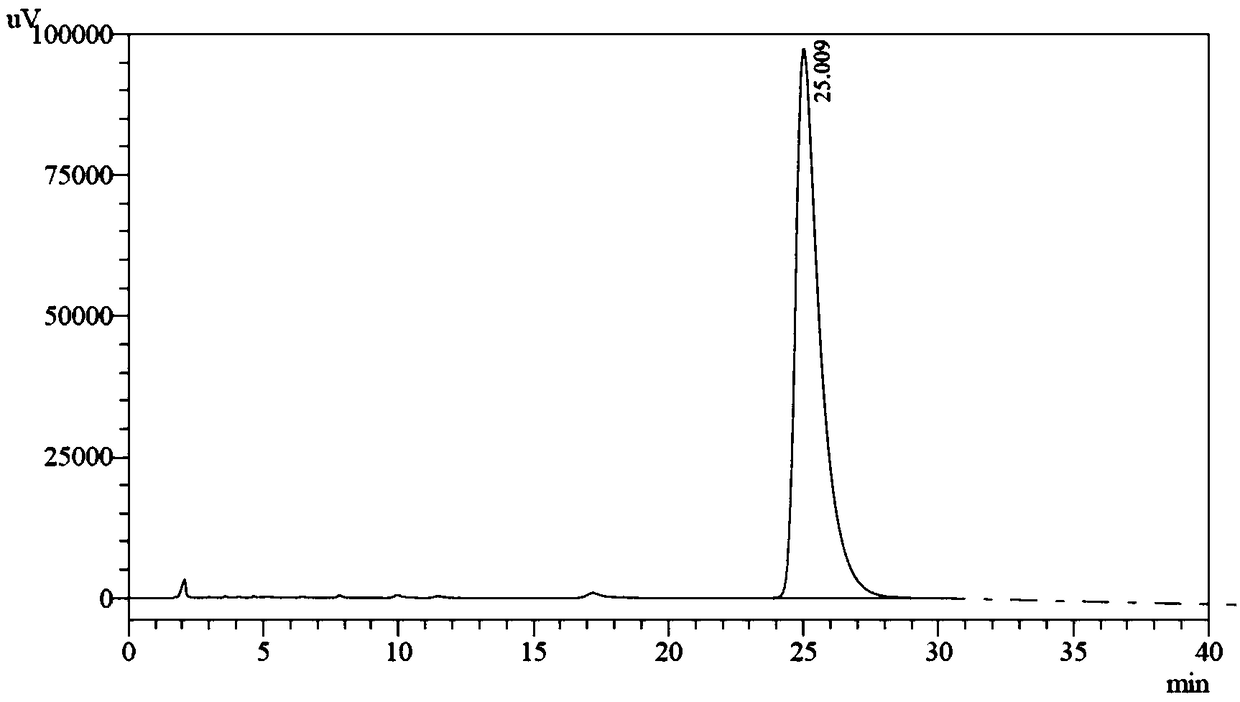
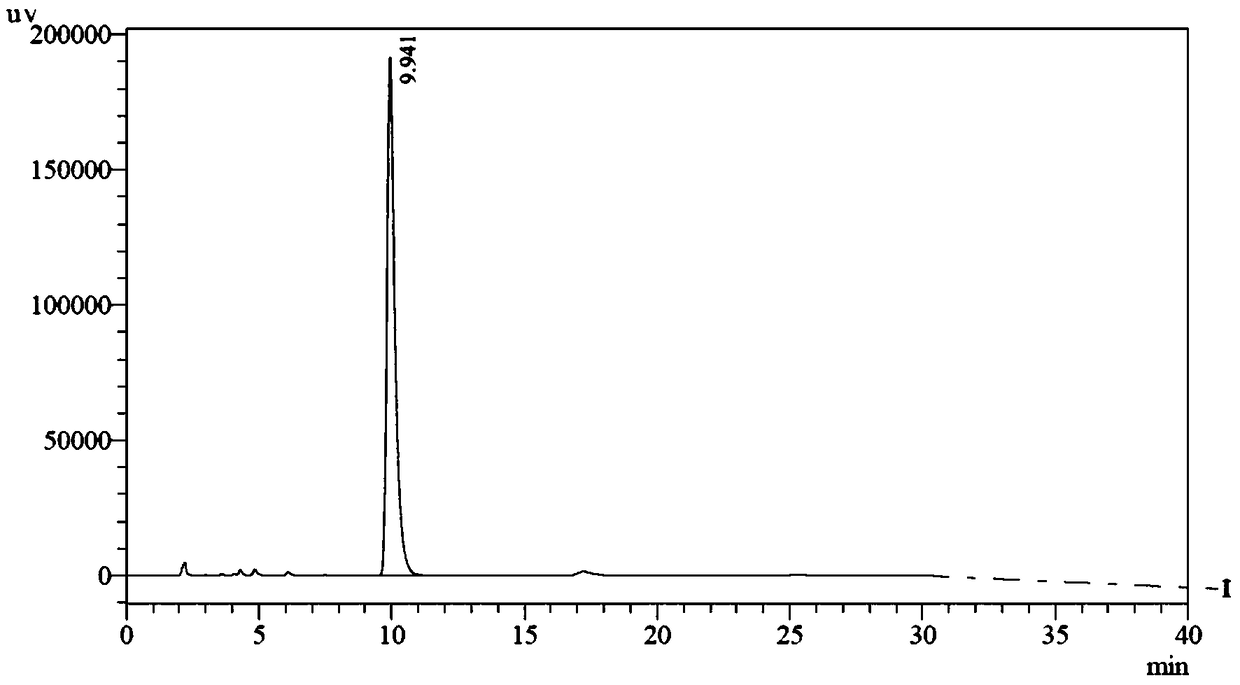
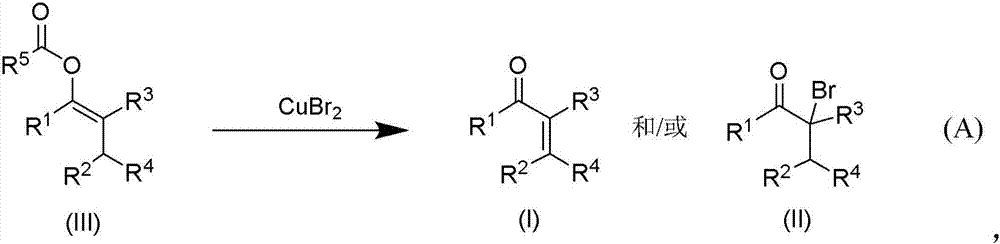
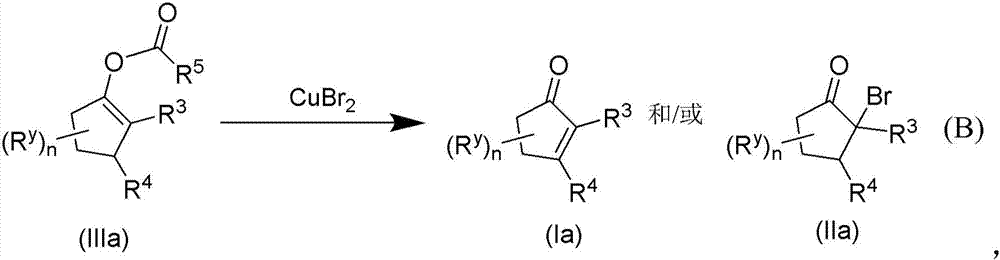
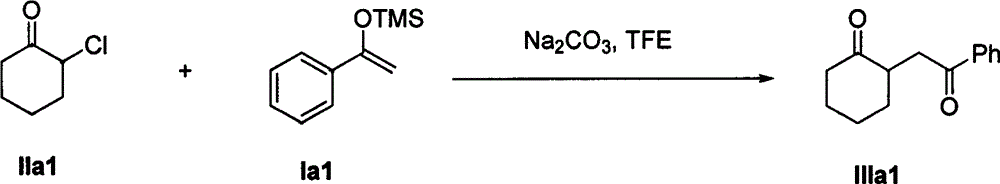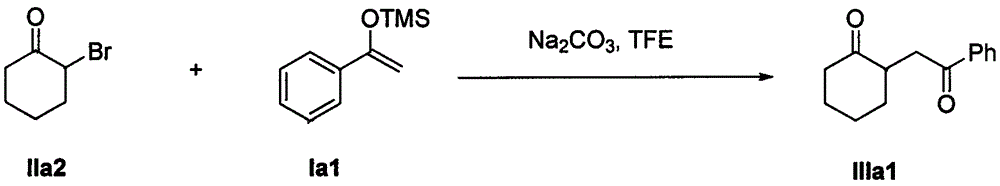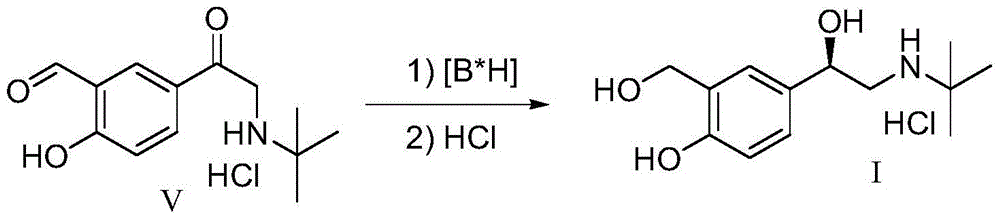Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Haloketone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
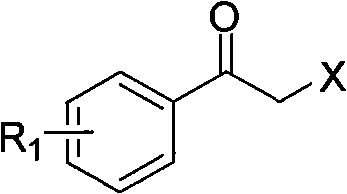
A haloketone in organic chemistry is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. The general structure is RR'C(X)C(=O)R where R is an alkyl or aryl residue and X any one of the halogens. The preferred conformation of a haloketone is that of a cisoid with the halogen and carbonyl sharing the same plane as the steric hindrance with the carbonyl alkyl group is generally larger.
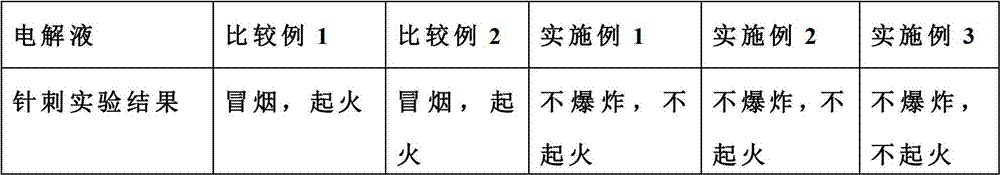
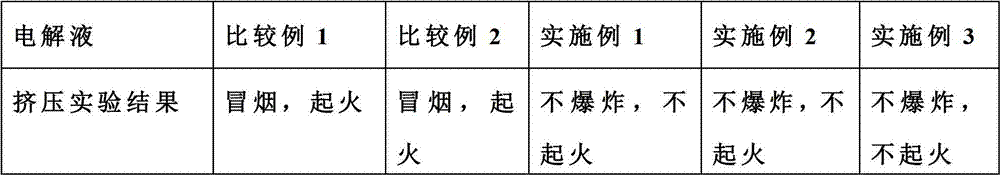
Flame retardant long-life electrolyte and lithium ion battery using same
InactiveCN102780040AImprove securityImprove cycle lifeSecondary cellsComposite filmPhysical chemistry
The invention discloses a multi-component electrolyte for lithium ion batteries. The multi-component electrolyte is good in ionic conductivity and is characterized by containing the following multiple components: (1) carbonic ester solvents, (2) flame retardant additives, (3) single or composite film-forming additives and (4) composite lithium salt. One of the film-forming additives (3) is selected from haloketones, the general formula of the haloketones is normally: XH2mCm-(C=O)-CnH2n+1, wherein both m and n are natural numbers, m is identical to or different from n, and X can be one of F, Cl, Br and I. The electrolyte can improve safety, magnification performance and cycle life of the lithium ion batteries.
Owner:LONG POWER SYST SUZHOU
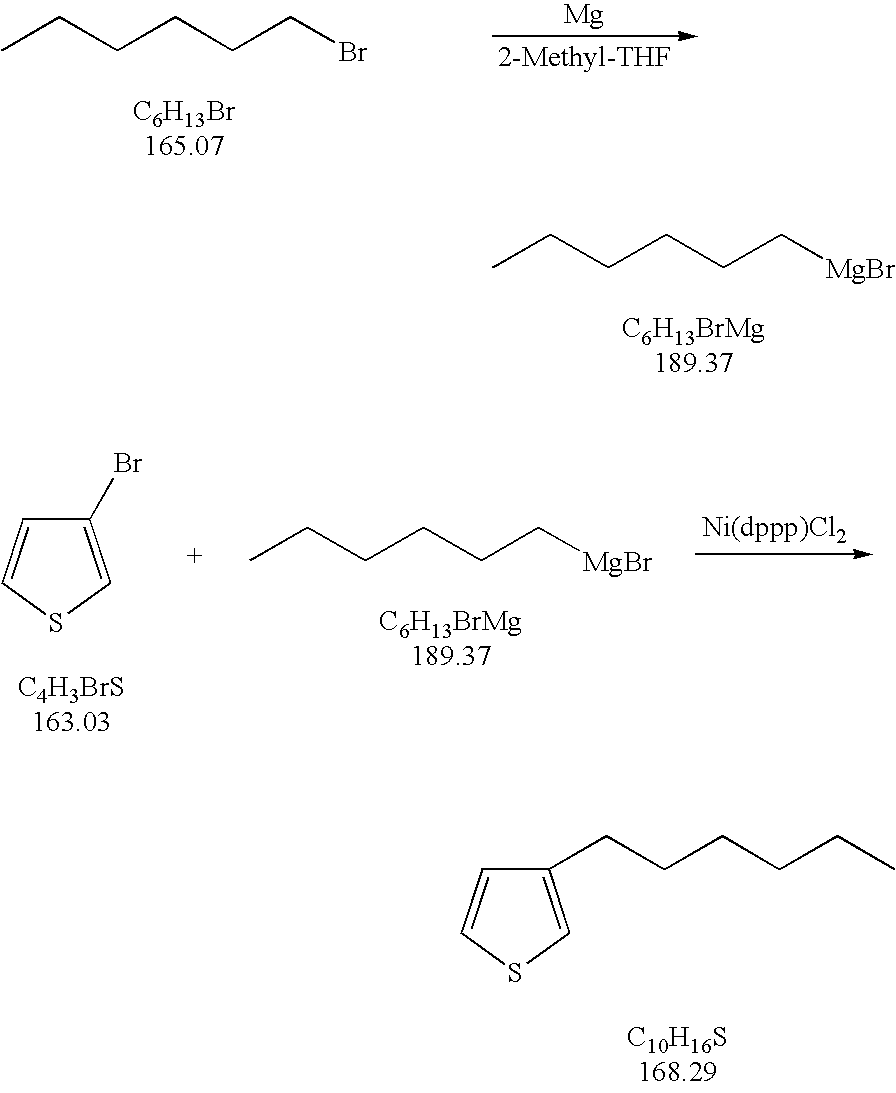
Process for the kumada coupling reaction
InactiveUS20060155134A1Silicon organic compoundsGroup 3/13 element organic compoundsHigh concentrationGrignard reagent
A method for the formation of 3-alkylthiophenes or 3-arylthiophenes from 3-halothiophenes. More particularly, improvements on the Kumada coupling reaction for the production of 3-alkylthiophenes or 3-arylthiophenes by reacting a 3-halothiophene with an alkylmagnesiumhalide or arylmagnesiumhalide Grignard reagent in the presence of a catalyst and a 2-methyl tetrahydrofuran solvent. The 2-methyl tetrahydrofuran solvent allows for higher concentrations of the Grignard reagent with minimal or no dithienyl side product generation, achieving higher product yields and at a lower cost than other known methods.
Owner:HONEYWELL INT INC
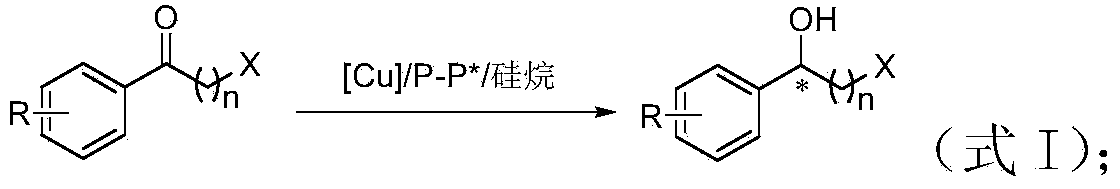
Method for preparing chirality halohydrin in copper-catalyzed asymmetry hydrosilation mode
InactiveCN103524307AHigh yieldHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholNitrogen
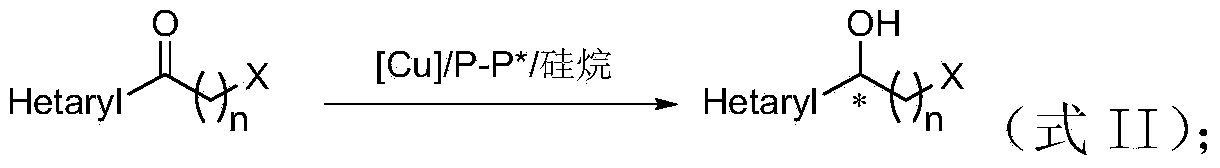
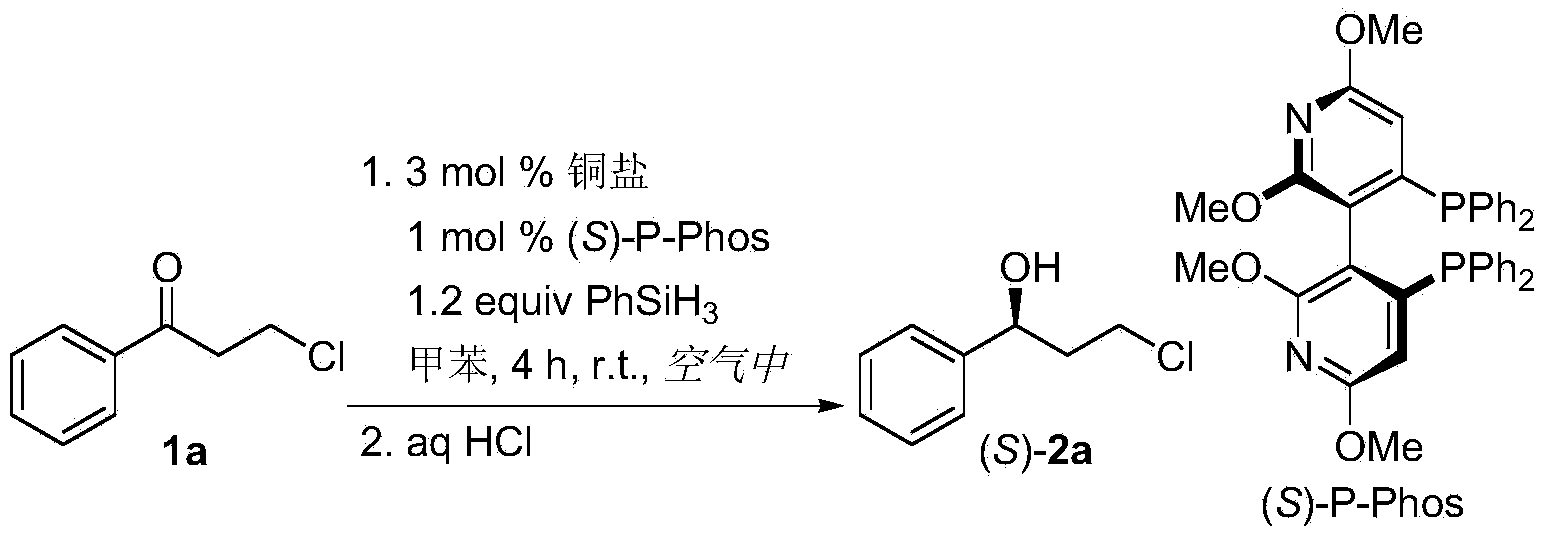
The invention discloses a method for preparing chirality halohydrin in a copper-catalyzed asymmetry hydrosilation mode. In the prior art, mostly a noble metal catalyst is needed, the research is generally limited in catalysis asymmetry reduction of alpha-haloketone, and documents about catalysis asymmetry reduction of beta-, gamma-, epsilon- or other haloketone substrates are less related. The method disclosed by the invention comprises preparing chirality gamma-, delta- or zeta-haloalkyl aryl alcohol from beta-, gamma-, epsilon-haloalkyl aryl ketone in a copper catalysis asymmetry hydrosilation reduction mode, or preparing chirality beta-, gamma- or delta-haloalkyl aryl alcohol from alpha-, beta- or gamma-haloalkyl aryl ketone in the copper catalysis asymmetry hydrosilation reduction mode. The method adopts a non-noble metal catalyst, the raw material is easy to obtain, nitrogen protection is not needed, the reaction condition is mild, the operation is simple, and the yield and the enantioselectivity of the reaction are high.
Owner:HANGZHOU NORMAL UNIVERSITY
Method for synthesizing 1, 4-diketone compound without catalyst
InactiveCN104844401AMild reaction conditionsFew reaction stepsCarbonyl group formation/introductionCarbonyl compound preparation by condensationDiketoneAfter treatment
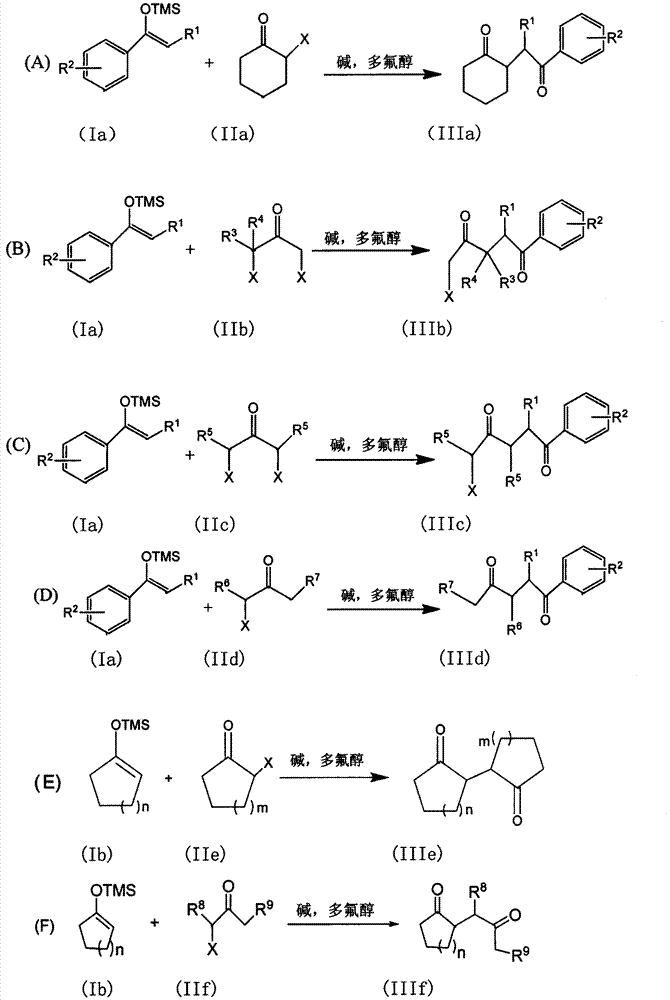
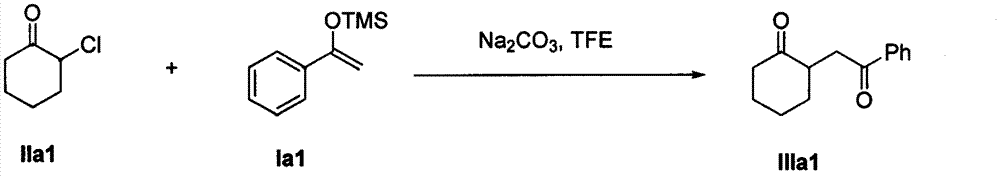
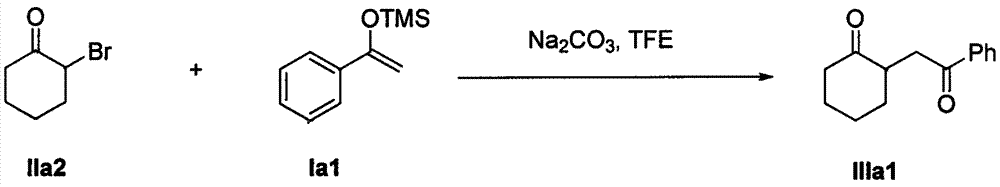
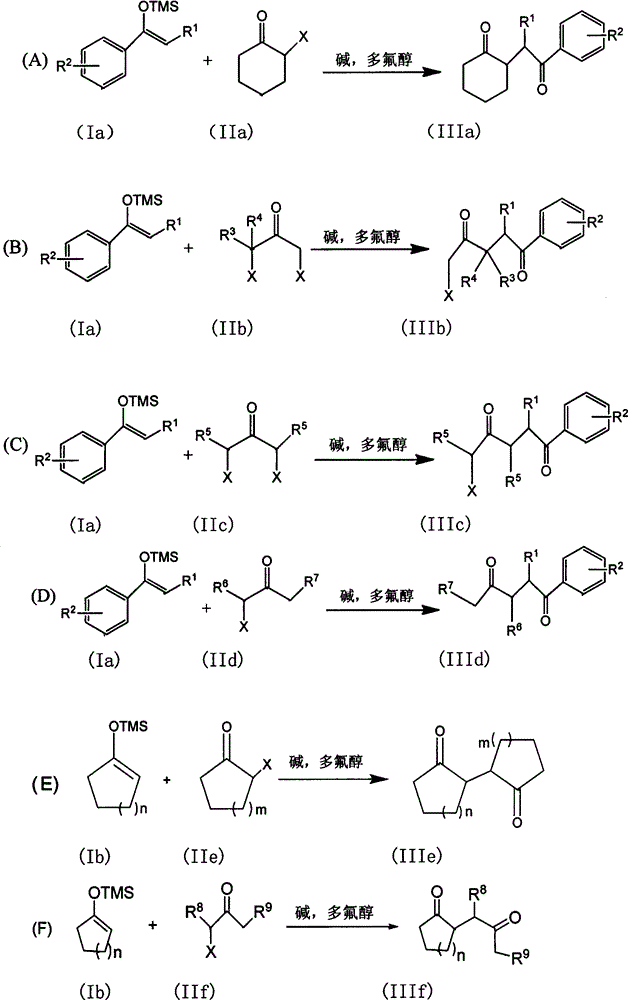
The invention discloses a method for synthesizing a 1, 4-diketone compound without a catalyst. In the existence of alkali, the corresponding 1, 4-diketone compound is obtained by reacting silyl enol ether and alpha-haloketone by taking perfluoroalkyl alcohol as a solvent. By adopting the method for synthesizing the 1, 4-diketone compound, the raw materials are easily obtained, the cost is low, the reaction conditions are mild, the operation is simple and easy to control, the side reactions are few, the after-treatment is simple, the product yield is relatively high, and the solvent can be recovered and recycled, so that the production cost is greatly saved; the method has good environment protection benefit and economical benefit and is suitable for industrial large-scale production.
Owner:CHONGQING MEDICAL UNIVERSITY
One-step catalytic process for the synthesis of isocyanates
InactiveUS20070293696A1Efficient and safe and cost-effectivePreparation by nitro-carbon monoxide reactionOrganic compound preparationKetoneHalocarbon
A one-pot process for the synthesis of isocyanates, polyisocyanates or mixtures thereof which includes the steps of: i. preparing a mixture comprising an amine, an alcohol, an oxygen-containing gas, carbon monoxide, a metal complex catalyst selected from the group consisting of macrocyclic complex catalysts and cobalt Shiff base catalysts; and a solvent selected from the group consisting of aliphatic or aromatic halocarbons, perhalogenated alcohols, halogenated ethers, halogenated ketones, perfluorinated hydrocarbons, polymers of chlorotrifluoroethylene having the formula —(CF2—CFCl)n wherein n is between 2 and 10, and mixtures thereof; ii. subjecting the resulting mixture to a first heating under pressure; iii. cooling and depressurizing the mixture resulting from the previous step; and iv. subjecting the mixture of the previous step to a second heating to separate out the isocyanate product from the mixture.
Owner:REPSOL SA
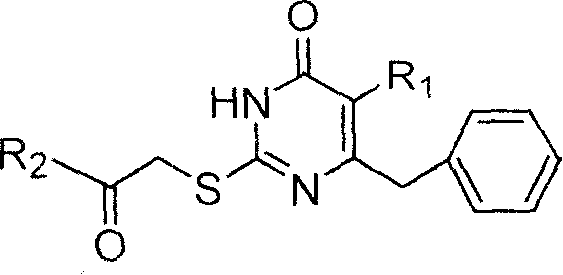
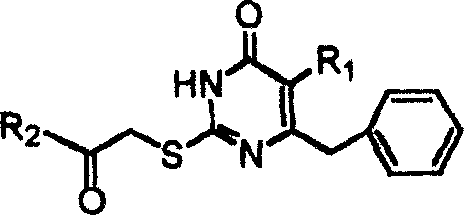
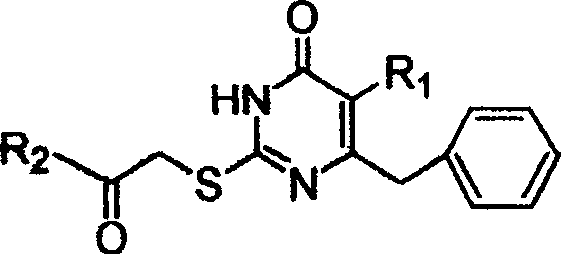
S-DABO compound, synthesizing method and usage
InactiveCN101037415AEasy to synthesizeLow cytotoxicityOrganic active ingredientsOrganic chemistryFuranKetone
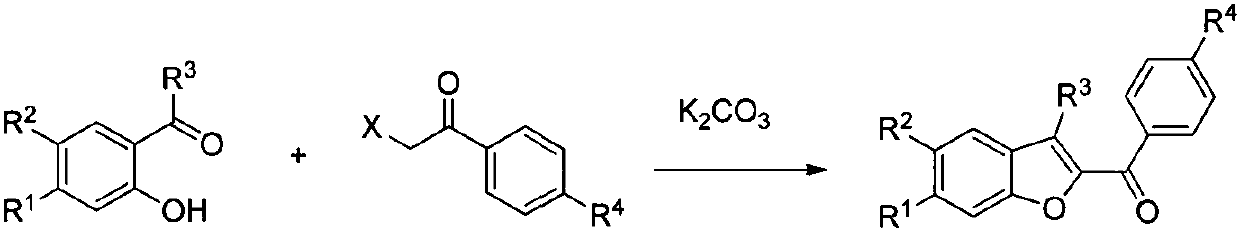
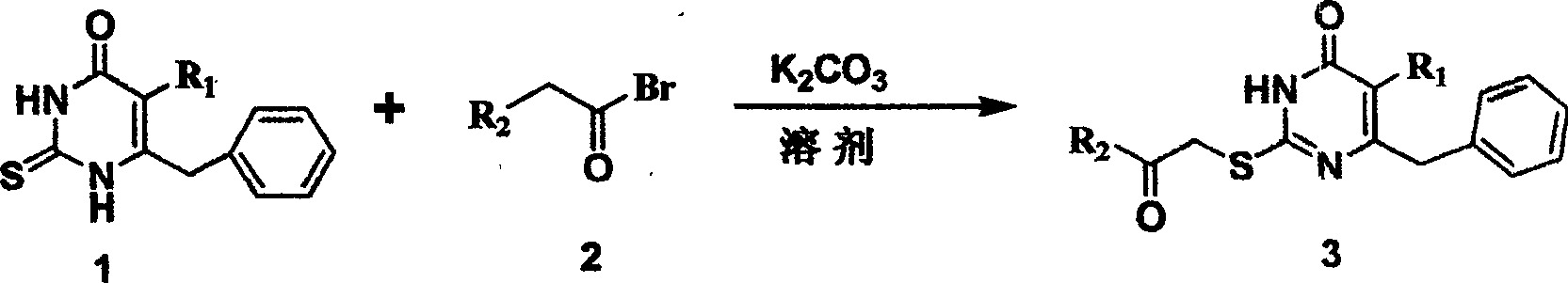
S-DABO composition and its synthesis method and function belong to medicine technical medicine. The invention relates to a 5-alkyl-6-phenyl-2-(substituted arylcarbonylmethylsulfur)uracil composition, having following general formula: wherein, R1 is C1-3 alkyl; R2=C1-6 alkyl, substituted furan ring, thiofuran ring, benzene ring (substituent on the benzene ring is H, OH, Cl3 alkoxy), having the 5-alkyl-6-phenylthiouracil as reagent, reacting with alpha-halogen ketone to get the inventive product which is catalyzed by K2CO3. The synthesis method is easy to operate. The product has an obvious anti-HIV virus activity, a low toxicity, a high selectivity index and can be an anti-HIV medicine candidate.
Owner:YUNNAN UNIV +1
Haloketone refrigerant compositions and uses thereof
Disclosed herein are haloketone refrigerant or heat transfer fluid compositions that are useful in refrigeration or air conditioning apparatus or as heat transfer fluids. The compositions of the present invention are also useful in centrifugal compressor systems that employ two-stage compressors or single slab / single pass heat exchangers.
Owner:EI DU PONT DE NEMOURS & CO
Protecting groups for biological labeling
InactiveUS6783947B1Sugar derivativesMicrobiological testing/measurementCombinatorial chemistryAqueous solution
Alpha-haloketones are useful alkylating agents for coupling to sulfhydryl-containing biomolecules. However, they react spontaneously with water, alkali and organic bases and therefore cannot be stored for extended periods of time in aqueous solutions, particularly in the presence of proteins at physiological pH. The present invention provides novel solutions to these problems, however, as novel compounds and compositions comprising protected haloketones are disclosed herein. Methods of preparing and using protected haloketones which are useful in a variety of applications-e.g., in assays and conjugation reactions-are also disclosed herein.
Owner:DADE BEHRING MARBURG GMBH
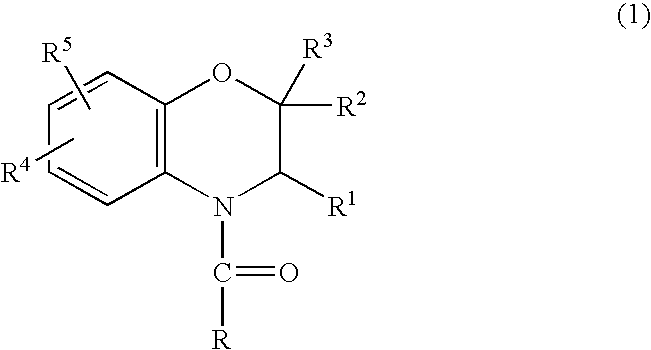
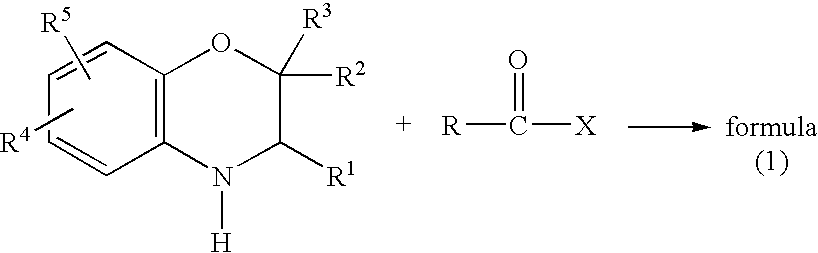
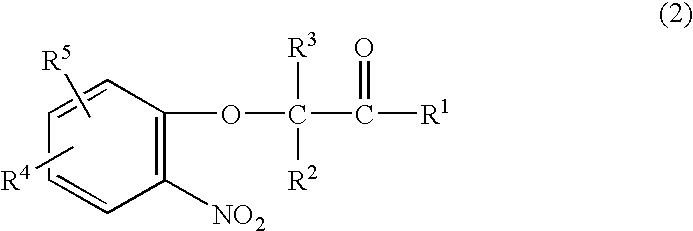
Method for making acylamides by synthesizing and acylating benzoxazines
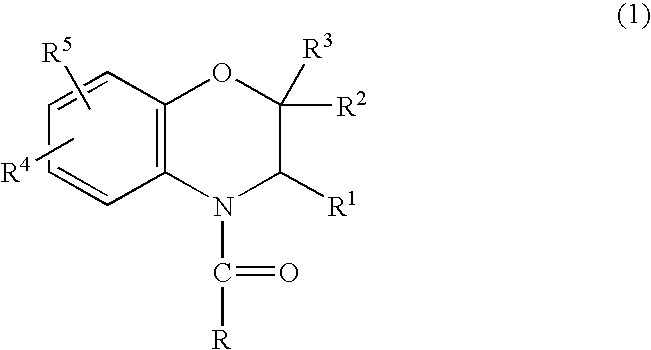
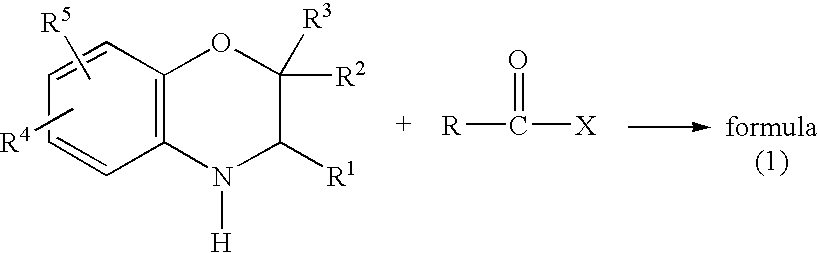
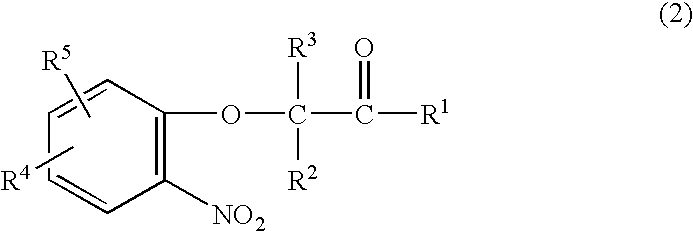
A method is provided for synthesizing acylamides, useful as protectant for cultivated plants. A benzoxazine, such as 3,4-dihydro-3-methyl-2H-1,4-benzoxazine, is made in a stepwise manner by initially forming an o-nitrophenoxyketone by reacting a haloketone and a nitrophenol. The o-nitrophenoxyketone is hydrogenated to form the corresponding benzoxazine, which is thereafter acylated with an acylhalide to produce the acylamide, such as 4-dichloroacetyl-3,4-dichloroacetyl-3,4-dihydro-3-methyl-2H-1,4-benzoxazine.
Owner:SYNGENTA CROP PROTECTION INC
Synthesis method of derivative of chiral Beta-amino-alcohol and part of final products thereof
InactiveCN101747211AMeet the needs of large-scale productionNo racemization was foundPhysical/chemical process catalystsOrganic compound preparationAlcoholStereochemistry
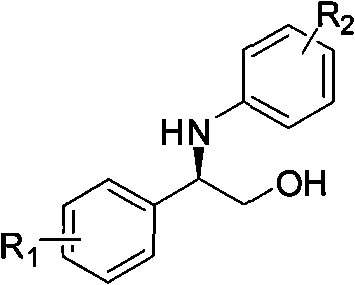
The invention relates to a synthesis method of a derivative of chiral Beta-amino-alcohol and part of intermediate products and final products thereof. The method selects a raw material which is commercialized on a market or a raw material of Alpha-halogeno ketone with easy preparation as an initial raw material, wherein X is Br or Cl, the final product with the chemical formula as the accompanying drawing is obtained by reaction or wherein R1=H, 2-Cl, 3-Cl, 4-Cl, 2, 4-Cl, 4-Br, 4-F, 4-CF3 and 4-NO2; R2=H, 2-OMe, 3-OMe, 4-OMe, 4-F, 4-Cl, 4-Br, 3-Br, 3-F, 3-Cl, 2-Br, 2-Cl, 2F, 4-Me, 3-Me and 2-Me, and the chiral center is S or R; the intermediate product with the chemical formula is shown in the accompanying drawing and the chiral center is in S configuration or R configuration; and the final product with the chemical formula is shown in the accompanying drawing and the chiral center is in S configuration or R configuration.
Owner:ASYMCHEM LIFE SCI TIANJIN
Process for producing optically active halohydrin compound
InactiveUS6888012B2Carbamic acid derivatives preparationOrganic compound preparationCompound aHalohydrin
A process of preparing an optically active halohydrin compound characterized by comprising asymmetric hydrogen transfer reduction of an α-haloketone compound in the presence of a group 9 transition metal compound having a substituted or unsubstituted cyclopentadienyl group and an optically active diamine compound. The asymmetric hydrogen transfer reduction is preferably conducted in the presence of a base.
Owner:AJINOMOTO CO INC
Bivalent copper complex containing diphosphine ortho-position carborane ligand, preparation method and application thereof
ActiveCN111635435AEasy to prepareHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsOrtho positionEthylic acid
The invention relates to a bivalent copper complex containing a diphosphine ortho-position carborane ligand, a preparation method and application thereof. The copper complex is prepared by the following method of: adding an n-BuLi solution dropwise into an ortho-position carborane o-C2B10H12 solution, and carrying out stirring reaction, then adding halogenated phosphine for continuous reaction, adding copper acetate Cu(OAc)2 into the reaction system for continuous reaction, after the reaction is finished, performing separating to obtain the diphosphine ortho-position carborane ligand, and applying the copper complex to catalytic synthesis of a beta-carbonyl phosphine oxide compound. Compared with the prior art, the synthesis process has excellent selectivity and high yield, the copper complex can catalyze alpha-haloketone and phosphite to react to synthesize the beta-carbonyl phosphine oxide compound under the room temperature condition, and the reaction is efficient, green and environmentally friendly.
Owner:SHANGHAI INST OF TECH
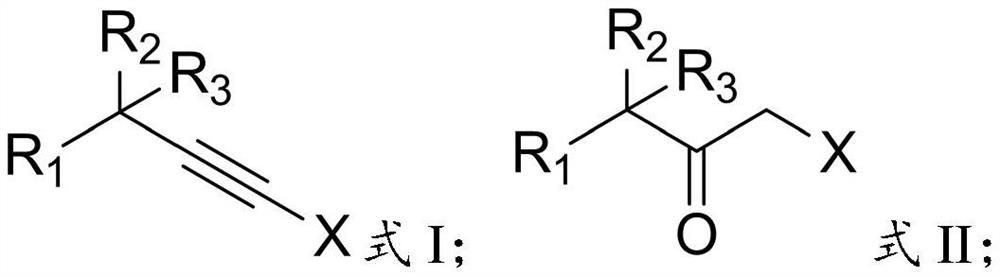
Preparation method of alpha-halogenated ketone compound
InactiveCN113387781AReduce usageHigh yieldOrganic compound preparationCarboxylic acid amides preparationOrganic solventPtru catalyst
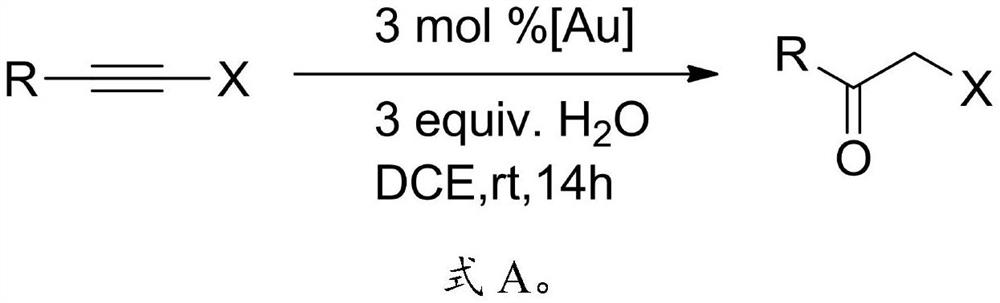
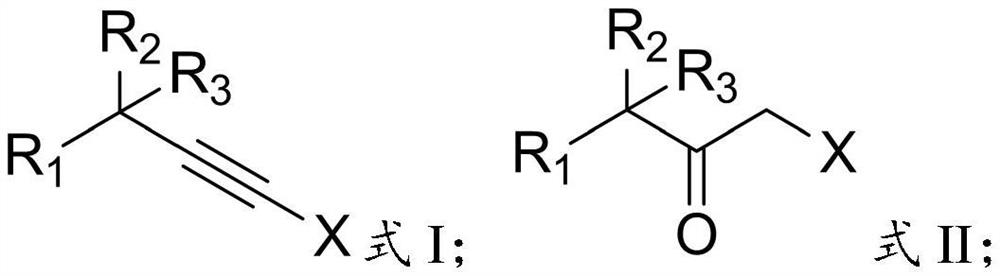
The invention relates to the technical field of pesticide chemistry, and provides a preparation method of an alpha-halogenated ketone compound. The preparation method comprises the steps: mixing terminal alkyne halide, an organic solvent, acid and water, and reacting to obtain the alpha-halogenated ketone compound. According to the preparation method provided by the invention, the terminal alkyne halide is directly reacted under the conditions of acid and water, and the use of a metal ion complex catalyst is avoided, so that the tedious operation of removing metal ions in the post-treatment process of the product is avoided, the whole process is simple and convenient to operate, green and environment-friendly, and the requirements of large-scale industrial production are met; and in addition, the method provided by the invention is high in raw material conversion rate, relatively high in product yield and purity, free of anhydrous and anaerobic operation and wide in substrate universality, and target molecules can be obtained from aliphatic, aromatic and other terminal alkyne halides at high yield. Furthermore, the preparation method provided by the invention is mild in reaction condition and easy to control.
Owner:DALIAN CHEMPHY CHEM
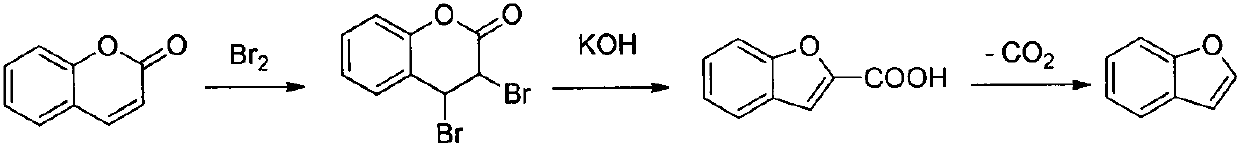
Method for synthesizing benzofuran derivative from phenol and alpha-haloketone
The invention provides a method for efficiently preparing a benzofuran derivative by using phenol and alpha-haloketone as raw materials through one-step reaction under the action of titanium tetrachloride. The method comprises the steps that under the protection of inert gas, phenol and perfluoroalkyl alcohol are added and then stirred and heated to reflux, titanium tetrachloride is added, finallya mixed solution of alpha-haloketone and perfluoroalkyl alcohol is dropped in, and after the reaction is completed, the benzofuran derivative is obtained by separation and purification. The synthesismethod can easily obtain raw materials, is low in cost, mild in reaction conditions, simple and easy in operation, few in side reactions, simple in post-treatment and high in product yield, greatly saves the production cost, has good economics benefits, and is suitable for industrialized production.
Owner:CHONGQING MEDICAL UNIVERSITY
METHODS OF PREPARING a,ß-UNSATURATED OR a-HALO KETONES AND ALDEHYDES
ActiveUS20170174607A1Low costReduce usageOrganic compound preparationPreparation by dehydrogenationKetoneCopper
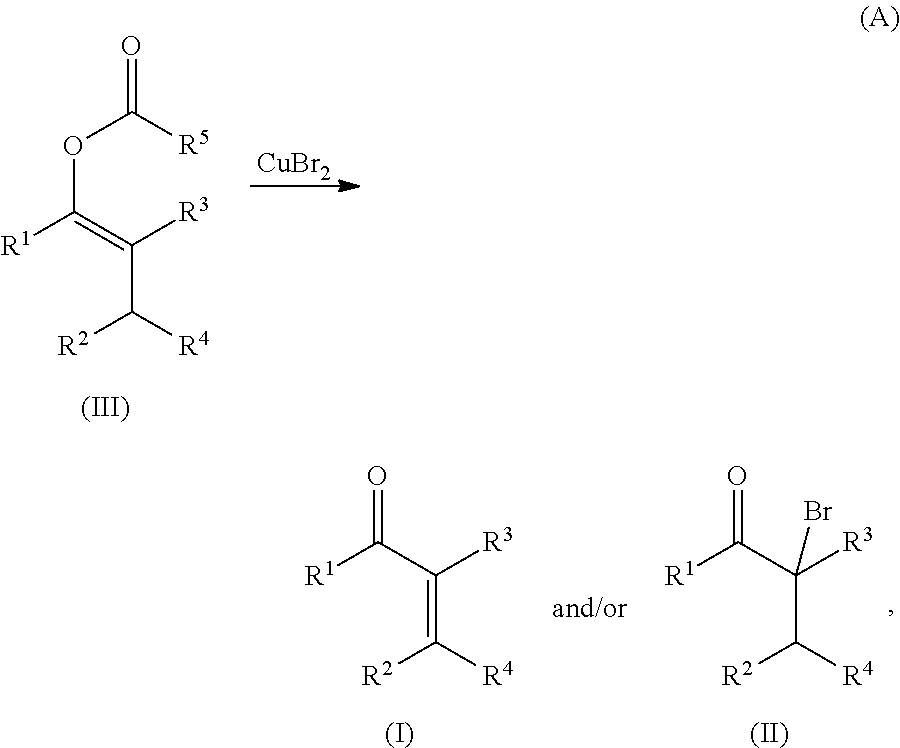
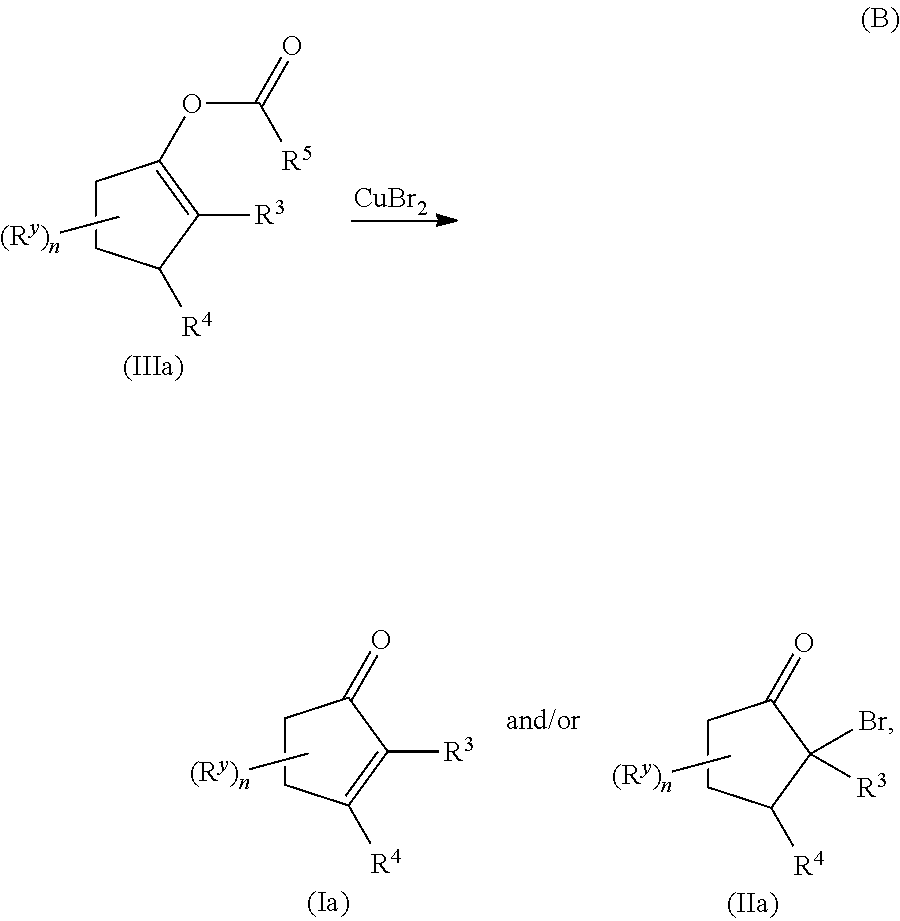
Copper(II) bromide mediated oxidation of acylated enol and use of the reaction in the synthesis of α,β-unsaturated or α-bromo ketones or aldehydes are disclosed. The method provides an efficient and practical process for manufacturing dehydrohedione (DHH) and many other versatile α,β-unsaturated or α-bromo ketones or aldehydes in large scales to avoid using precious metal compounds.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Method for making acylamides by synthesizing and acylating benzoxazines
InactiveUS6977299B2Significant reductionRecrystallization be eliminatedOrganic chemistry methodsNitrophenolMethyl group
A method is provided for synthesizing acylamides, useful as protectant for cultivated plants. A benzoxazine, such as 3,4-dihydro-3-methyl-2H-1,4-benzoxazine, is made in a stepwise manner by initially forming an o-nitrophenoxyketone by reacting a haloketone and a nitrophenol. The o-nitrophenoxyketone is hydrogenated to form the corresponding benzoxazine, which is thereafter acylated with an acylhalide to produce the acylamide, such as 4-dichloroacetyl-3,4-dichloroacetyl-3,4-dihydro-3-methyl-2H-1,4-benzoxazine.
Owner:SYNGENTA CROP PROTECTION INC
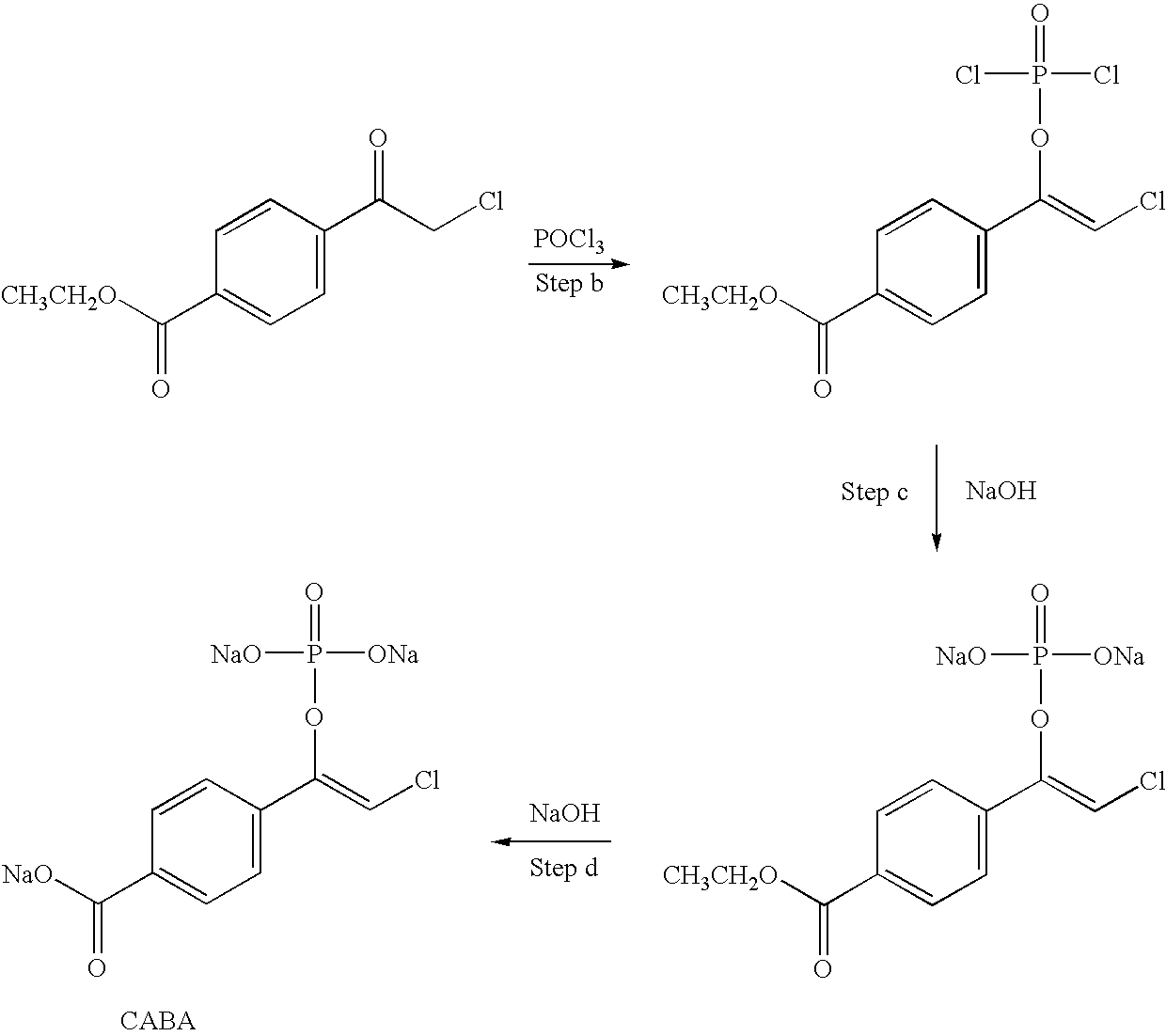
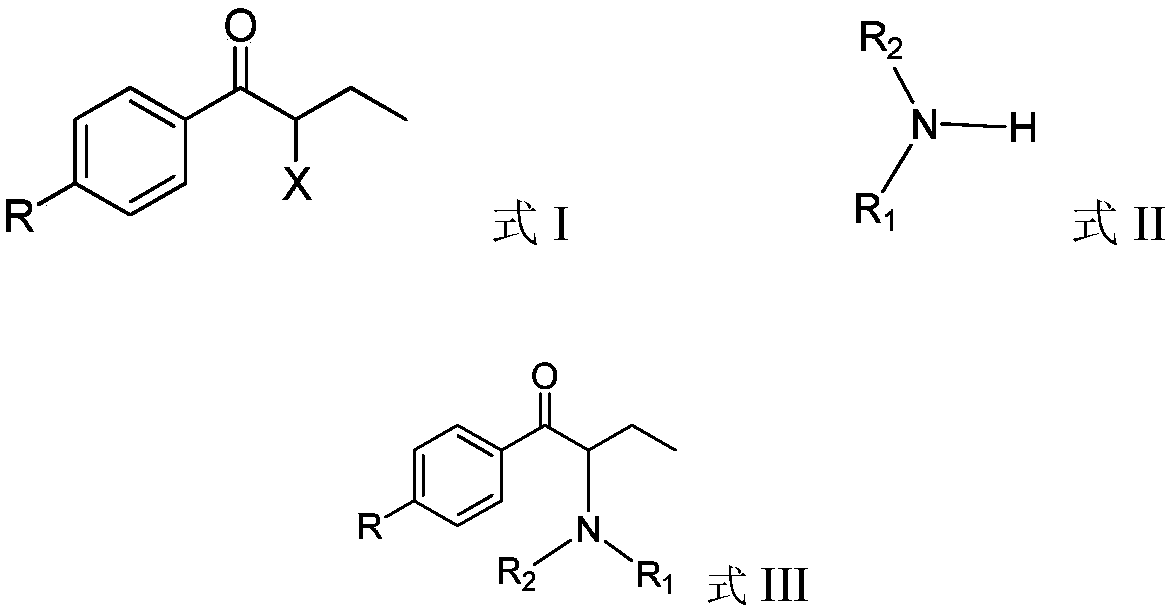
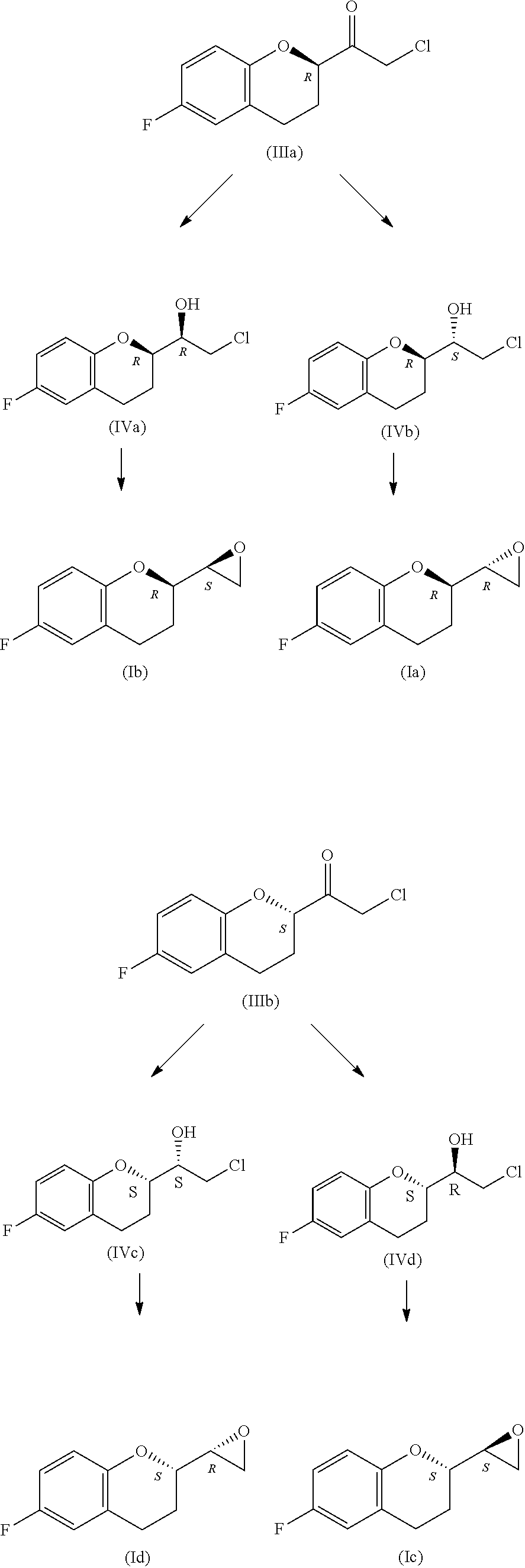
Preparation method of nebivolol intermediate, intermediate for preparing nebivolol intermediate, and preparation method of intermediate
ActiveCN108997297ARaw materials are easy to obtainSuitable for industrial applicationsOrganic chemistry methodsLithiumCarboxylic salt
The invention relates to a preparation method of alpha-haloketone of a nebivolol intermediate of a formula II, an intermediate compound I for preparing the alpha-haloketone, and a preparation method of the intermediate compound I. The preparation method disclosed by the invention comprises the following steps: taking chromane-2-carboxylic acid as a raw material, carrying out a reaction with dihalide or disulfonate, so as to obtain chromane-2-carboxylate I; further carrying out a reaction with dihalomethane under the effect of organo-lithium, so as to obtain the nebivolol intermediate alpha-haloketone II. According to the method disclosed by the invention, the defect that the intermediate for producing the alpha-haloketone is high in cost and low in purity is overcome. Moreover, the methoddisclosed by the invention has the advantages of being simple in operation, environmentally friendly, convenient for industrial production and the like.
Owner:ZHEJIANG HISOAR PHARMA +1
Methods of preparing alpha,beta-unsaturated or alpha-halo ketones and aldehydes
InactiveCN106986768AEasy to understandOrganic compound preparationPreparation by dehydrogenationCupric bromideCombinatorial chemistry
Copper (II) bromide mediated oxidation of acylated enol and use of the reaction in the synthesis of alpha,beta-unsaturated or alpha-bromo ketones or aldehydes are disclosed. A method provides an efficient and practical process for manufacturing dehydrohedione (DHH) and many other versatile alpha,beta-unsaturated or alpha-bromo ketones or aldehydes in large scales to avoid using precious metal compounds.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Process for producing alpha-halo ketones, alpha-halohydrine and epoxides
InactiveCN1091088CEfficient productionCarbamic acid derivatives preparationOrganic compound preparationClosed loopKetone
Process for the efficient production of alpha-haloketones, alpha-halohydrins and epoxides on an industrial scale. These methods include a decarboxylation of the reaction product between a carboxylic acid derivative of general formula (1) and a metal enolate prepared from an α-haloacetic acid of general formula (2) or an acceptable salt thereof to produce formula ( 3) A method for α-haloketones, a method for reducing α-haloketones (3) to produce α-halohydrins, and a method for treating α-halohydrins (11) with alkali to close the ring to produce epoxy compound method. The methods described above are particularly suitable for the production of the corresponding optically active α-haloketones, α-halohydrins and epoxides from α-amino acid derivatives. R1COA (1)
Owner:KANEKA CORP
Method for preparing poly-substituted furan through Feist-Benary reaction under alkali-free and solvent-free condition
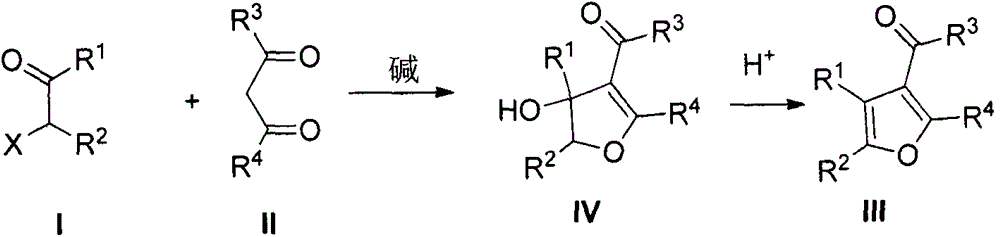
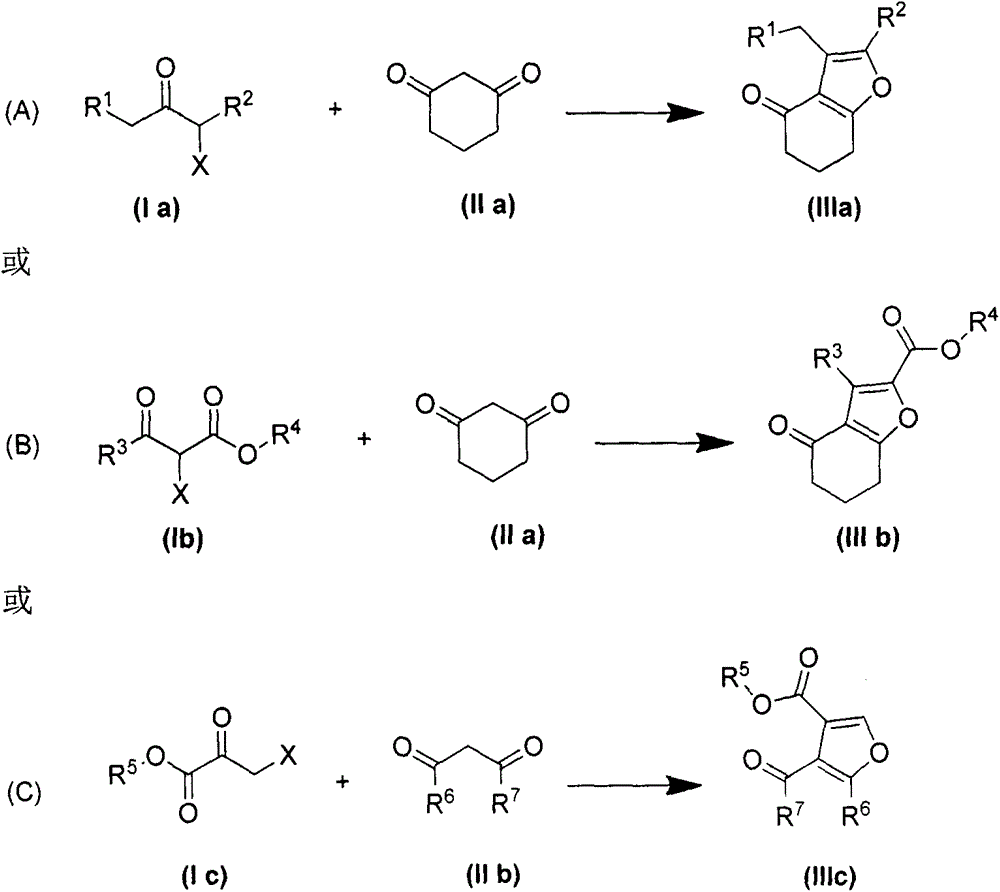
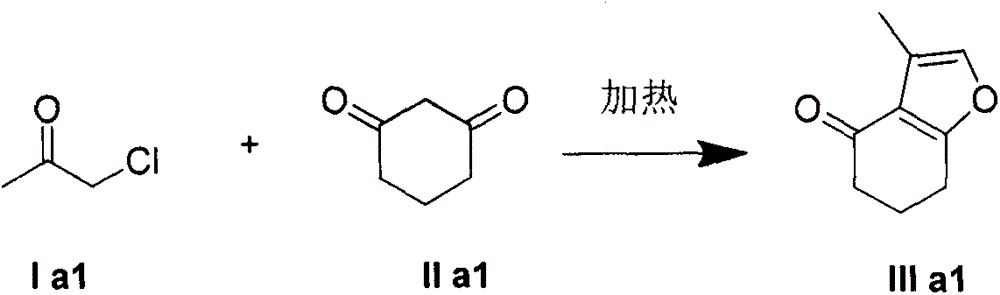
The present invention provides a poly-substituted furan preparation method, wherein alpha-halogenated ketone reacts with a beta-dicarbonyl compound under an alkali-free and solvent-free condition to obtain the poly-substituted furan compound. According to the present invention, the preparation method has characteristics of easily available raw materials, low cost, mild reaction conditions, simple and easily-controllable operation, less side reaction, simple post-treatment, high product yield, no requirement of any catalysts and solvents, substantially saved production cost, good environmental protection benefits and good economic benefits, and is suitable for the industrial large-scale production.
Owner:CHONGQING MEDICAL UNIVERSITY
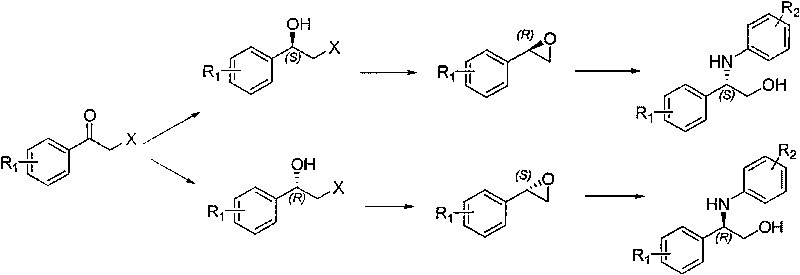
Optically active halohydrin derivative and process for producing optically active epoxy alcohol derivative from the same
InactiveUS20060155136A1Operational securityEasy to handle industriallySilicon organic compoundsOrganic compound preparationTriazole antifungalsEpoxy
The present invention provides an industrially safe, easily operable process for producing an optically active epoxy alcohol derivative useful as an intermediate for pharmaceuticals from inexpensively available materials, and also provides a novel halohydrin derivative serving as an important intermediate for the epoxy alcohol derivative. Furthermore, the present invention provides a process for producing an intermediate for a triazole antifungal agent by allowing a halohydrin to react with a triazole sulfonamide, the process including a small number of steps. A process for producing an optically active epoxy alcohol derivative includes allowing an optically active α-substituted propionate derivative to react with a haloacetic acid derivative in the presence of a base to prepare an optically active haloketone derivative, allowing the resulting haloketone derivative to react with an aryl metal compound to stereoselectively prepare a halohydrin derivative, eliminating a substituent for the hydroxy group of the halohydrin derivative, and performing epoxidation with a base. Furthermore, a process for producing an intermediate for a triazole antifungal agent includes allowing a halohydrin derivative to react with a triazole sulfonamide, the process including a small number of steps.
Owner:KANEKA CORP
S-DABO compound, synthesizing method and usage
InactiveCN100519540CEasy to synthesizeLow cytotoxicityOrganic active ingredientsOrganic chemistryFuranKetone
The S-DABO compound, its synthesis method and application belong to the technical field of medicine. The invention relates to a 5-alkyl-6-phenyl-2-(substituted arylcarbonylmethylthio)uracil compound, which has the following general formula: wherein: R1 is a C1-3 alkyl group; R2=C1-6 alkyl, substituted furan ring, thiophene ring, benzene ring (substituents on the aromatic ring are H, OH, C1-3 alkoxy). The product of the present invention is obtained by reacting 5-alkyl-6-phenylthiouracil with a-halogenated ketone under K2CO3 catalysis. The synthesis method is simple and easy, and the product has significant anti-HIV virus activity, low toxicity and high selectivity index, and can be used as a candidate anti-HIV drug.
Owner:YUNNAN UNIV +1
Protecting groups for biological labeling
InactiveUS20040198703A1BiocideGroup 5/15 element organic compoundsCombinatorial chemistryProtecting group
Alpha-haloketones are useful alkylating agents for coupling to sulfhydryl-containing biomolecules. However, they react spontaneously with water, alkali and organic bases and therefore cannot be stored for extended periods of time in aqueous solutions, particularly in the presence of proteins at physiological pH. The present invention provides novel solutions to these problems, however, as novel compounds and compositions comprising protected haloketones are disclosed herein. Methods of preparing and using protected haloketones which are useful in a variety of applications-e.g., in assays and conjugation reactions-are also disclosed herein.
Owner:KECZER STEYE DE +4
Substituted pyridine-2-ketone compounds and preparation method thereof
ActiveCN103044423BWide variety of sourcesLow priceOrganic chemistryAntineoplastic agentsSide chainKetone
The present invention relates to a pyridin-2-one compound and a preparation method thereof, in particular to a substituted pyridin-2-one compound and a preparation method thereof. The method mainly solves the technical problems that the existing pyridin-2-one compound derivatives are few and the synthesis methods are few. The technical scheme of the present invention is: a substituted pyridin-2-one compound, the general structural formula is as follows:, Formula 1; wherein R1 is hydrogen, a straight-chain alkyl group with 1 to 6 carbon atoms, and a side chain containing a substituent One of alkyl, substituted aryl, substituted benzyl or a-haloketone. R2 is one of hydrogen, a straight-chain alkyl group with 1 to 6 carbon atoms, an alkyl group containing a substituent side chain, a substituted aryl group or a substituted benzyl group.
Owner:SHANGHAI STA PHARMA R&D CO LTD
Catalyst-free method for synthesizing 1,4-diketone compounds
InactiveCN104844401BMild reaction conditionsFew reaction stepsCarbonyl group formation/introductionCarbonyl compound preparation by condensationDiketoneAlcohol
The invention discloses a method for synthesizing a 1, 4-diketone compound without a catalyst. In the existence of alkali, the corresponding 1, 4-diketone compound is obtained by reacting silyl enol ether and alpha-haloketone by taking perfluoroalkyl alcohol as a solvent. By adopting the method for synthesizing the 1, 4-diketone compound, the raw materials are easily obtained, the cost is low, the reaction conditions are mild, the operation is simple and easy to control, the side reactions are few, the after-treatment is simple, the product yield is relatively high, and the solvent can be recovered and recycled, so that the production cost is greatly saved; the method has good environment protection benefit and economical benefit and is suitable for industrial large-scale production.
Owner:CHONGQING MEDICAL UNIVERSITY
In-situ recycling technology of alkylamine
ActiveCN109678735AReduce dosageAtom economy is highOrganic chemistryOrganic compound preparationHalogenHaloketone
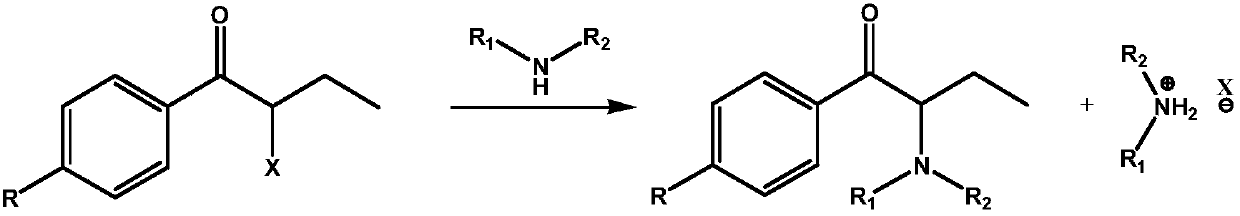
The invention relates to an in-situ recycling technology of amine during alpha-aminoketone production, in particular to a technology by which a certain amount of alkali compounds or other low-cost acid binding agents are added to bind with substituted halogen anions while amine compounds react with alpha-haloketone. By adopting the in-situ recycling technology, the using amount of amine compoundssuch as dimethylamine and the like can be greatly reduced and the atom economy is improved.
Owner:SHANDONG JIURI CHEM TECH CO LTD
Optically active halohydrin derivative and process for producing optically active epoxy alcohol derivative from the same
InactiveUS7582781B2Easy to operateAvailable materialSilicon organic compoundsOrganic compound preparationEpoxyAryl
The present invention provides an industrially safe, easily operable process for producing an optically active epoxy alcohol derivative useful as an intermediate for pharmaceuticals from inexpensively available materials, and also provides a novel halohydrin derivative serving as an important intermediate for the epoxy alcohol derivative. Furthermore, the present invention provides a process for producing an intermediate for a triazole antifungal agent by allowing a halohydrin to react with a triazole sulfonamide, the process including a small number of steps. A process for producing an optically active epoxy alcohol derivative includes allowing an optically active α-substituted propionate derivative to react with a haloacetic acid derivative in the presence of a base to prepare an optically active haloketone derivative, allowing the resulting haloketone derivative to react with an aryl metal compound to stereoselectively prepare a halohydrin derivative, eliminating a substituent for the hydroxy group of the halohydrin derivative, and performing epoxidation with a base. Furthermore, a process for producing an intermediate for a triazole antifungal agent includes allowing a halohydrin derivative to react with a triazole sulfonamide, the process including a small number of steps.
Owner:KANEKA CORP
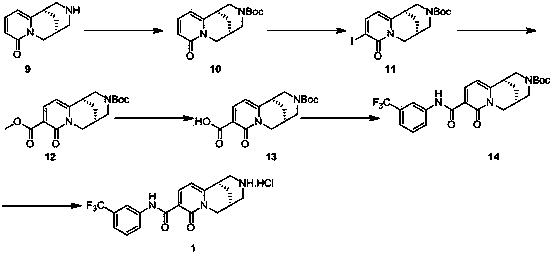
Process for the synthesis of intermediates of Nebivolol
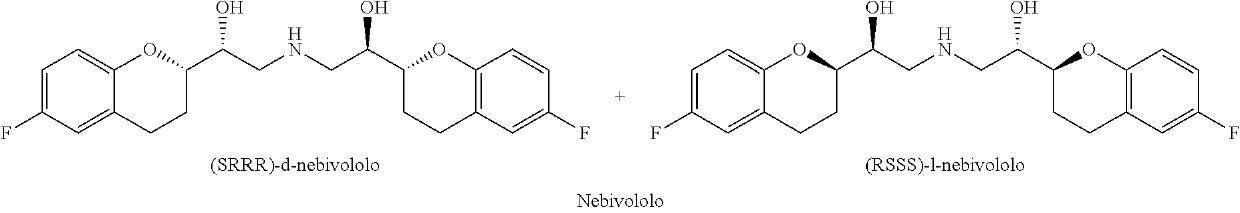
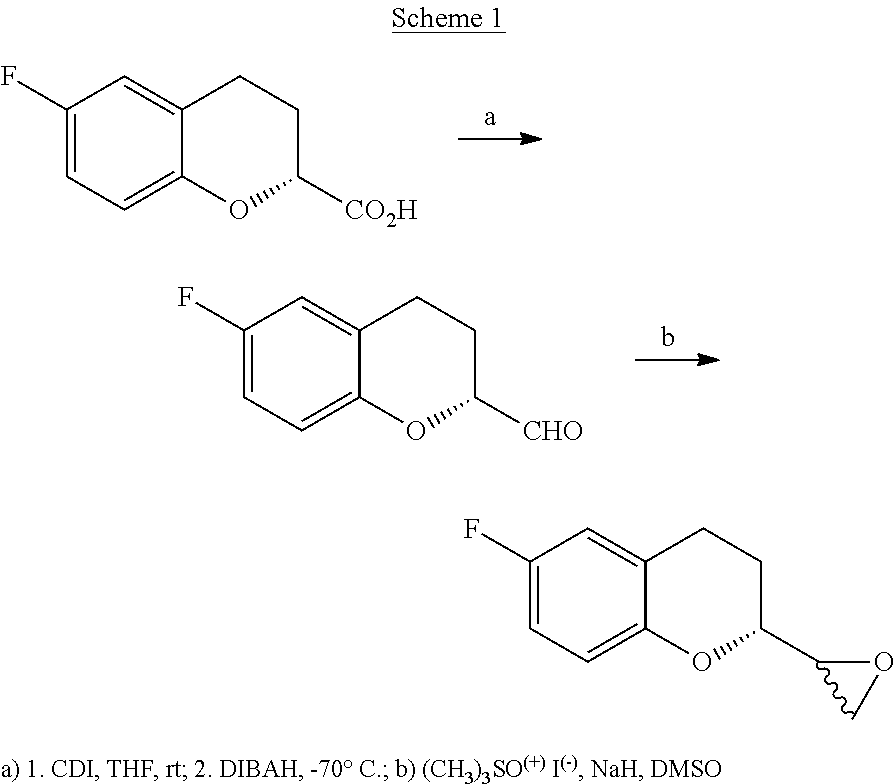
ActiveUS10392361B2High chemical purityEfficient synthesisOrganic chemistryNebivololCombinatorial chemistry
Owner:MENARINI INT OPERATIN S LUXEMBORG SA
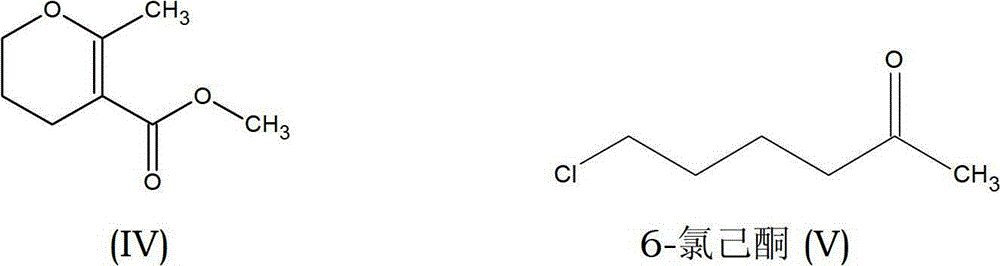
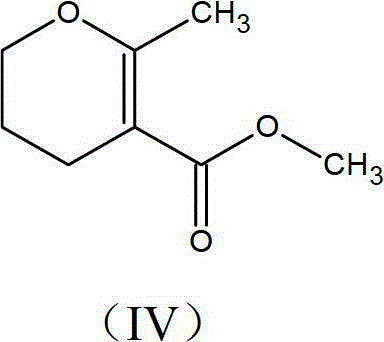
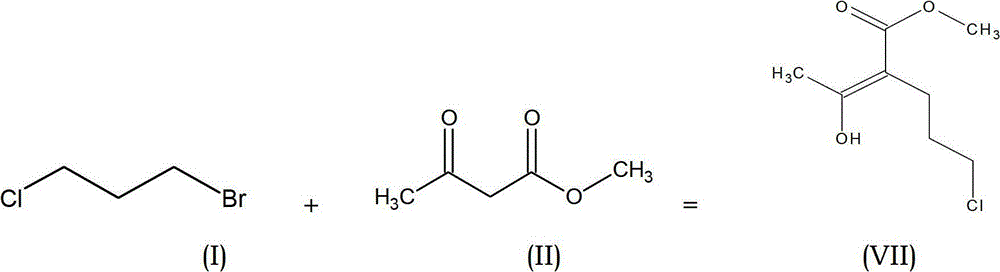
Preparation process of methyl-methyl-3, 4-dihydro-2H-pyran-5-carboxylate
The present invention provides an improved process for the preparation of methyl-methyl-3,4-dihydro-2H-pyran-5-carboxylate of formula (IV), comprising the steps of: (IV) (i) alkylating 1-Bromo-3-chloropropane of formula (I) with Methylacetoacetate of formula (II) to prepare haloketone of formula (VII) in presence of an alcoholic solvent; (I) (II) (VII) (ii) O-alkylating the compound of formula (VII) with sodium methoxide to obtain the desired molecule methyl-methyl-3,4-dihydro-2H-pyran-5-carboxylate of formula (IV) in its crude form; (VII) (IV) (iii) Purifying the content of step (ii) above by fractional distillation to obtain the purified molecule of formula (IV).
Owner:SPC LIFESCI PVT LTD
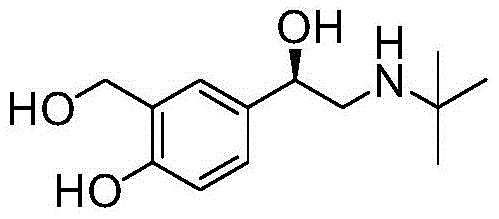
(r)-Asymmetric preparation method of albuterol hydrochloride
InactiveCN104829468BHigh yieldHigh optical purityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSalicylaldehydePtru catalyst
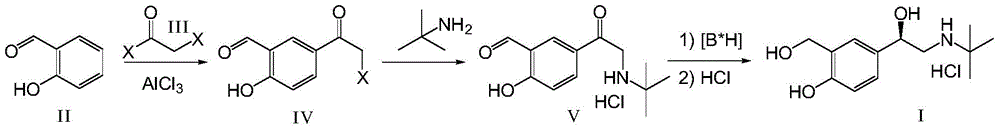
The invention relates to a novel method for asymmetric syntheses of (R)-salbutamol hydrochloride by using a high-efficiency chiral catalyst. The method comprises the following steps: 1) subjecting salicylaldehyde and halogenoacetyl halide to Friedel-Crafts acylation so as to obtain halogenated ketone; 2) subjecting the obtained halogenated ketone to aminolysis with tert-butylamine and then carrying out hydrolysis and deprotection so as to obtain salicylaldamino ketone; and 3) reducing the salicylaldamino ketone into a crude product of (R)-salbutamol in the presence of a chiral borane catalyst derived from chiral aminoalcohol and carrying out purification and salt formation of hydrochloric acid so as to obtain the high purity (R)-salbutamol hydrochloride.
Owner:CHENGDU XIANJI BIOCHEM SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com