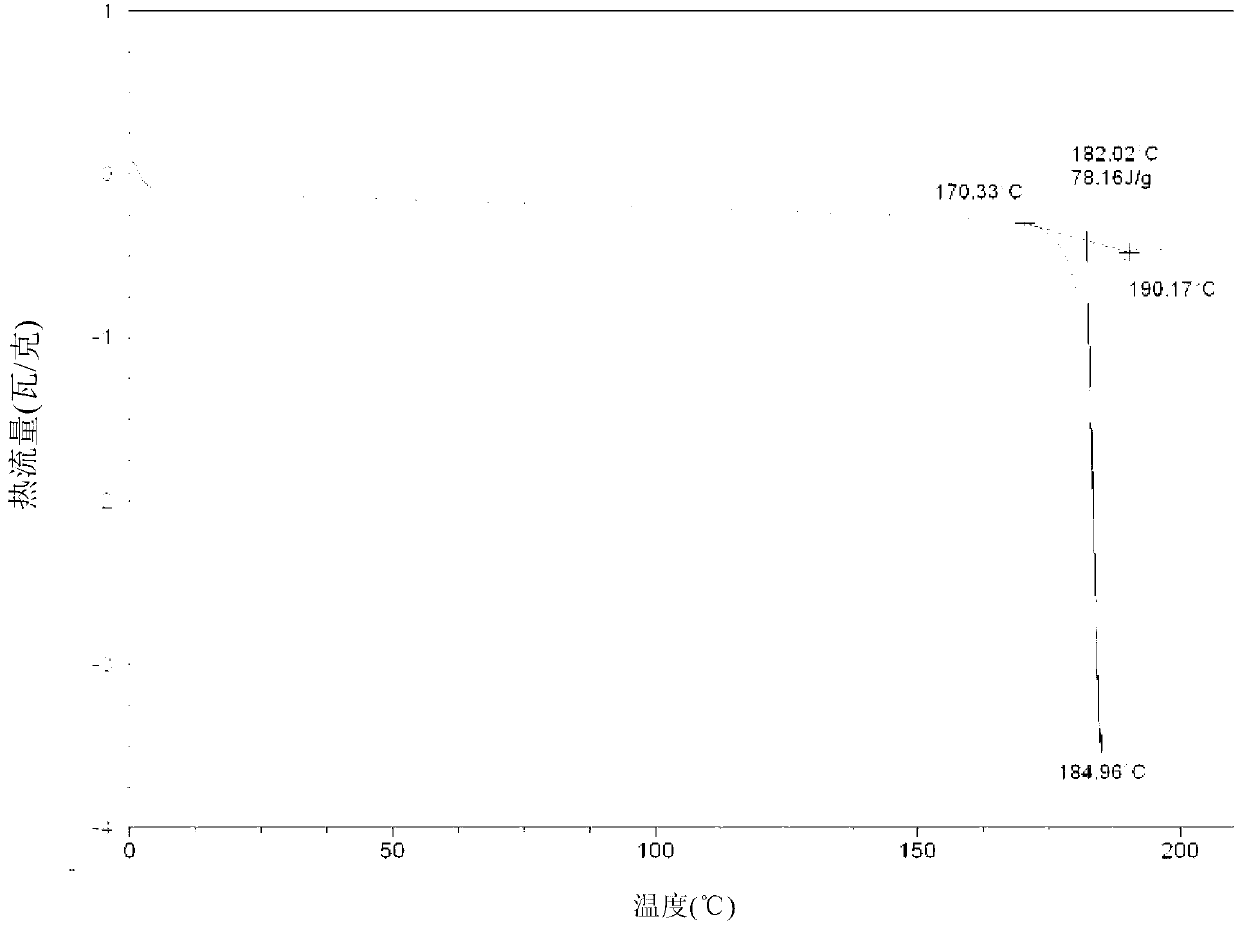
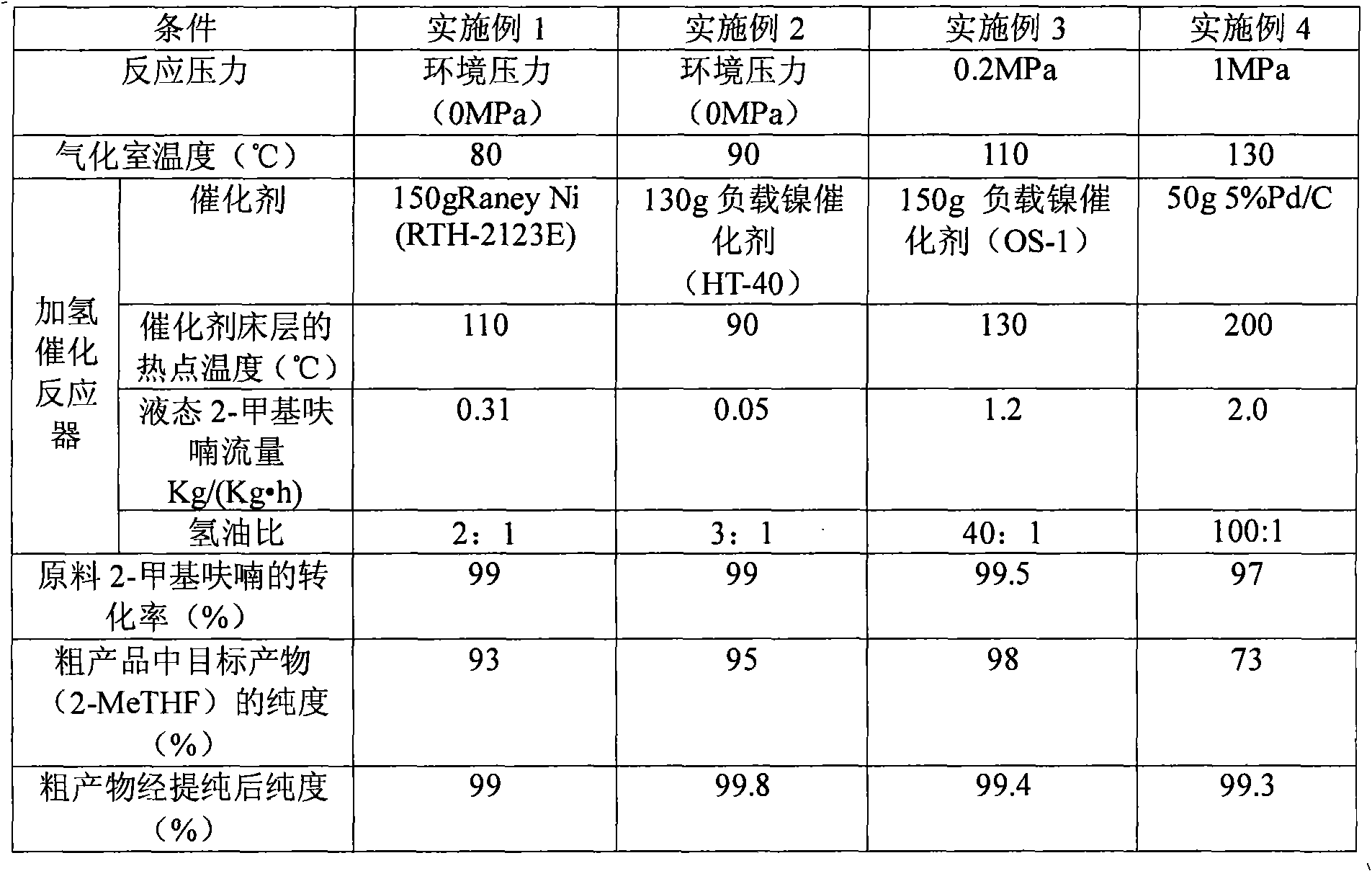
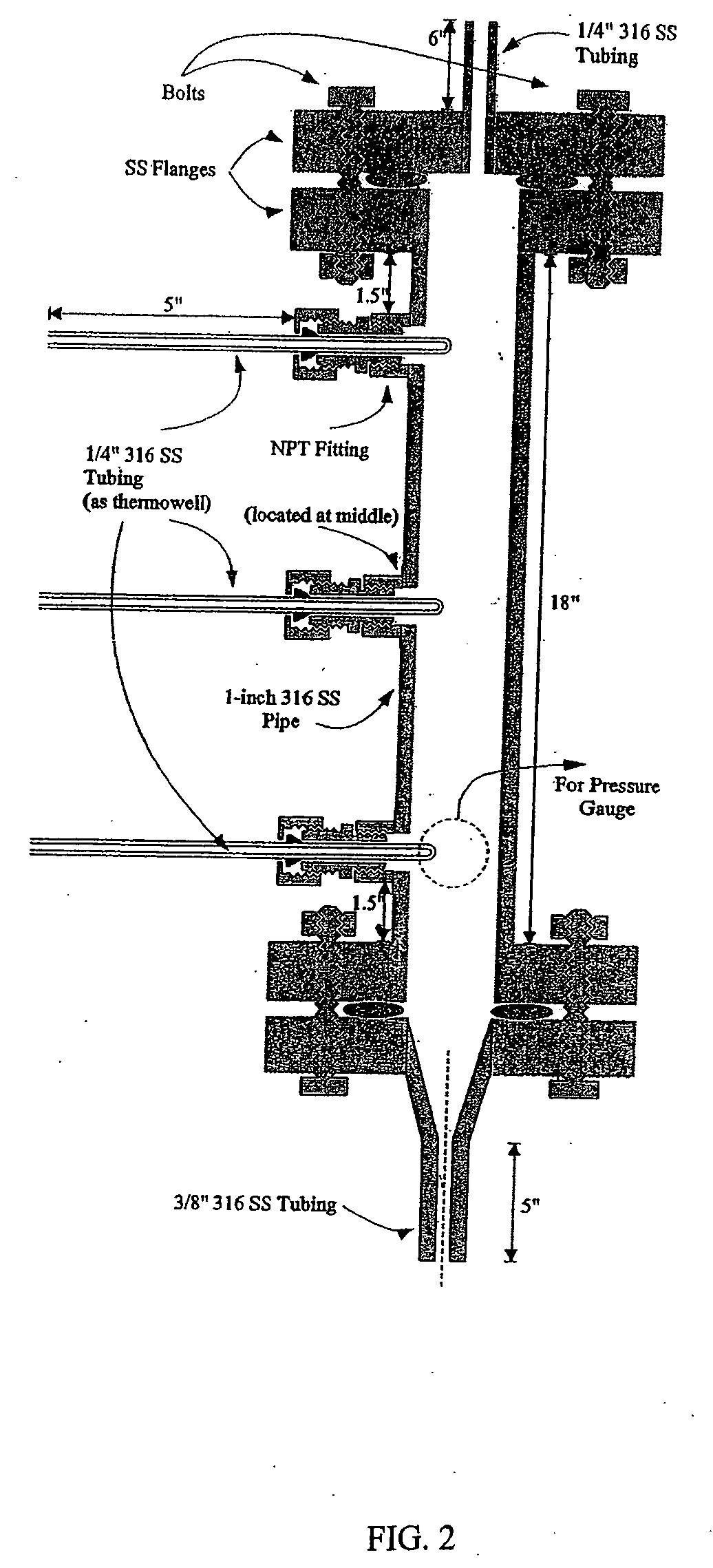
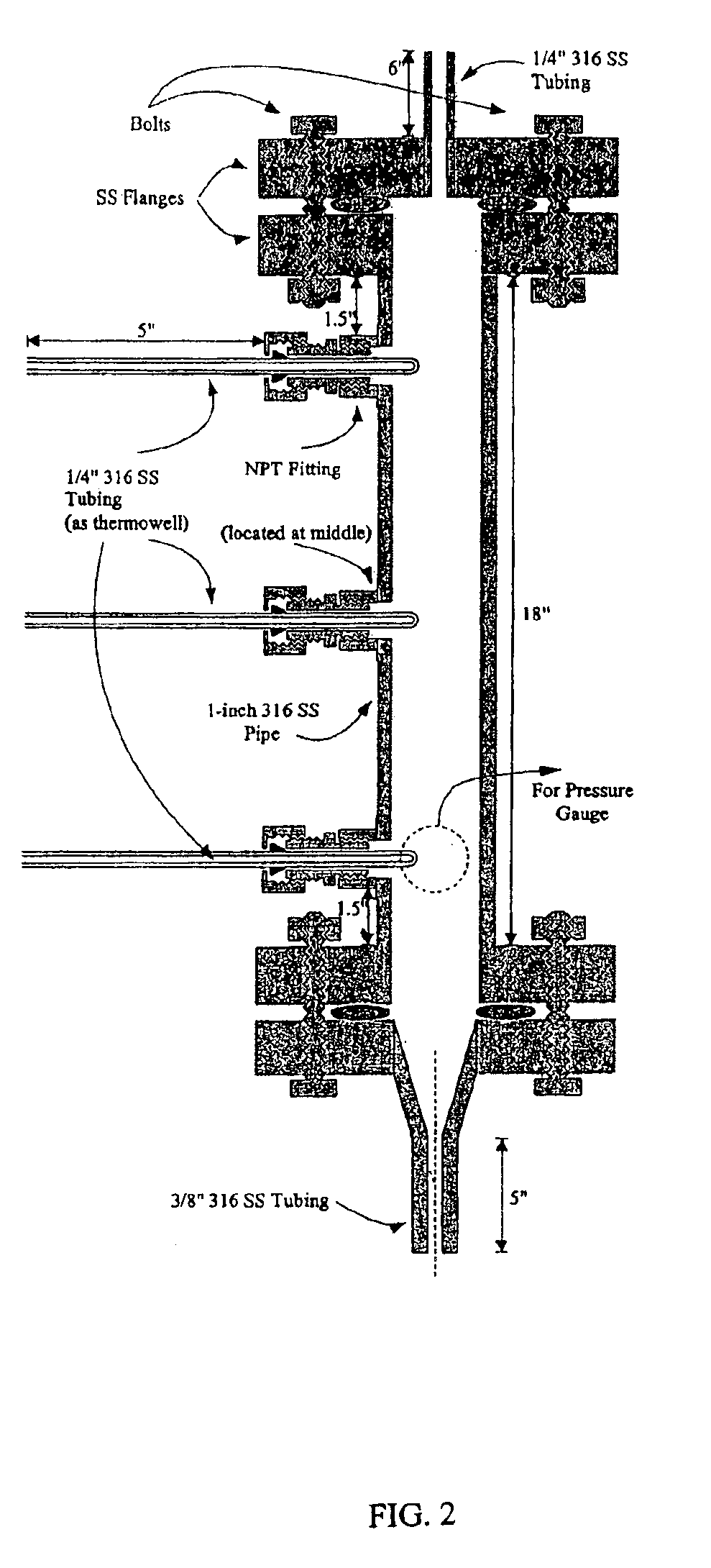
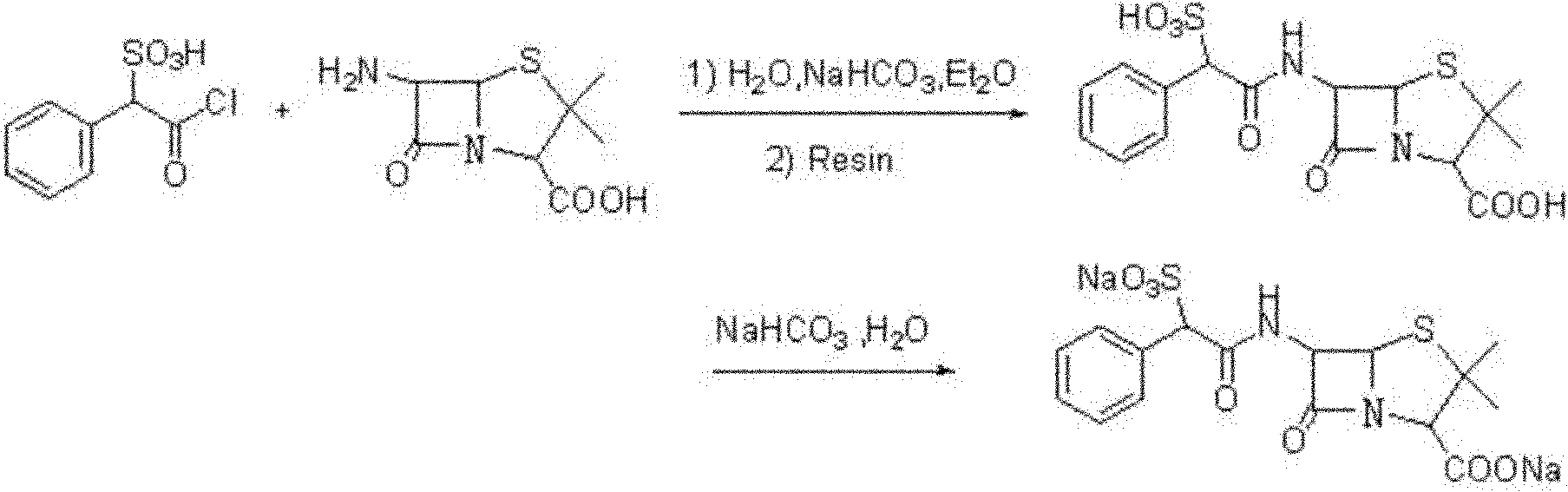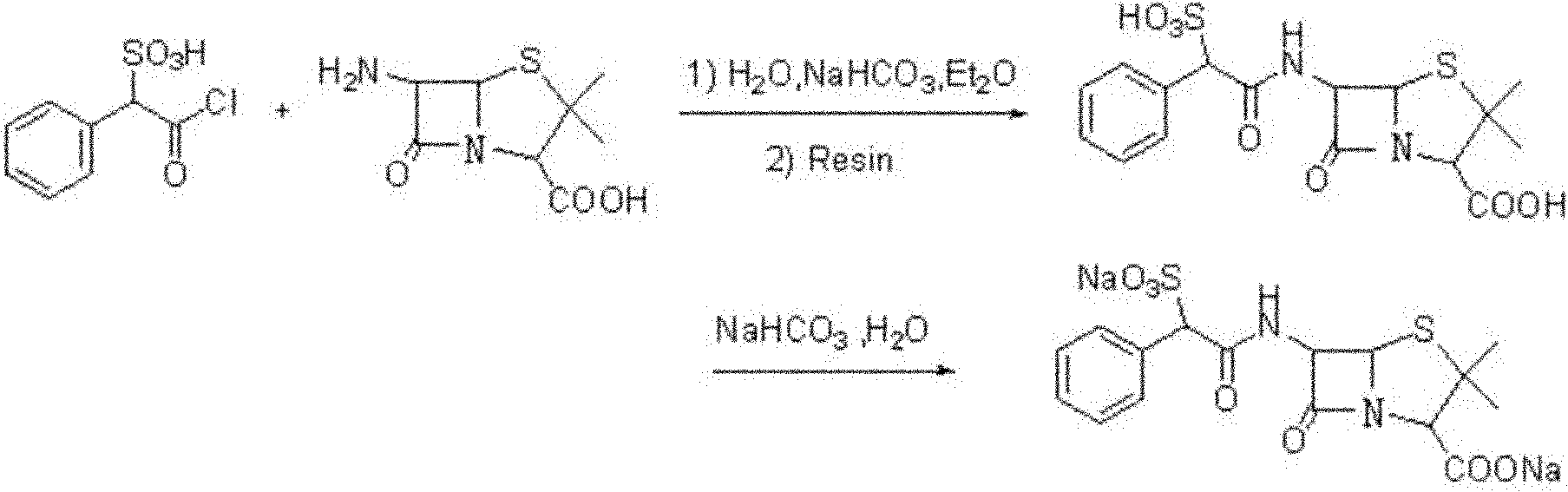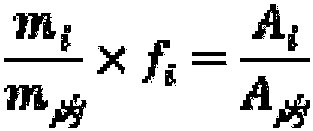Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
113 results about "2-Methyltetrahydrofuran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
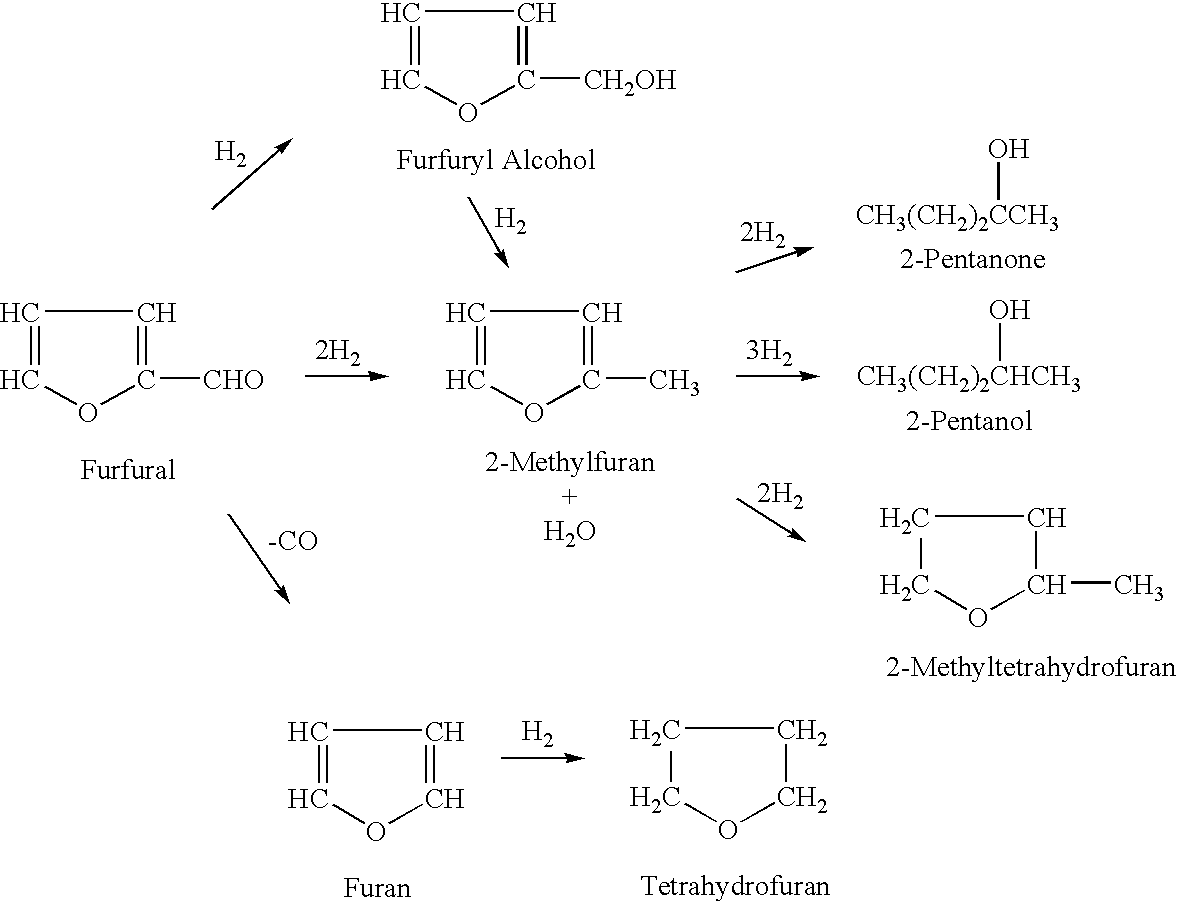
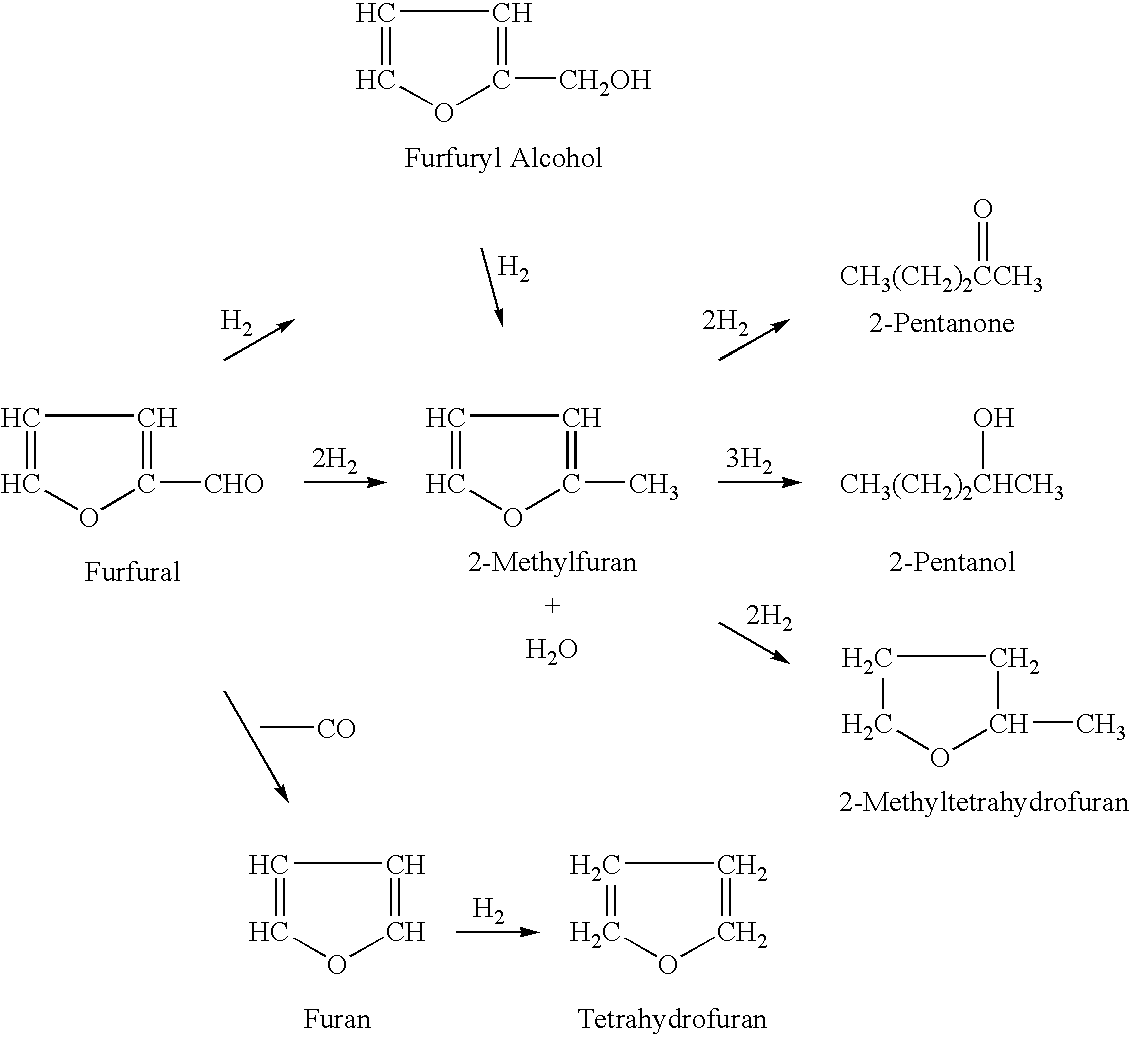
2-Methyltetrahydrofuran is an organic compound with the molecular formula CH₃C₄H₇O. It is a highly flammable mobile liquid. It is mainly used as a replacement for THF in specialized applications for its better performance, such as to obtain higher reaction temperatures, or easier separations (as, unlike THF, it is not miscible with water). It is derived from sugars via furfural and is occasionally touted as a biofuel.
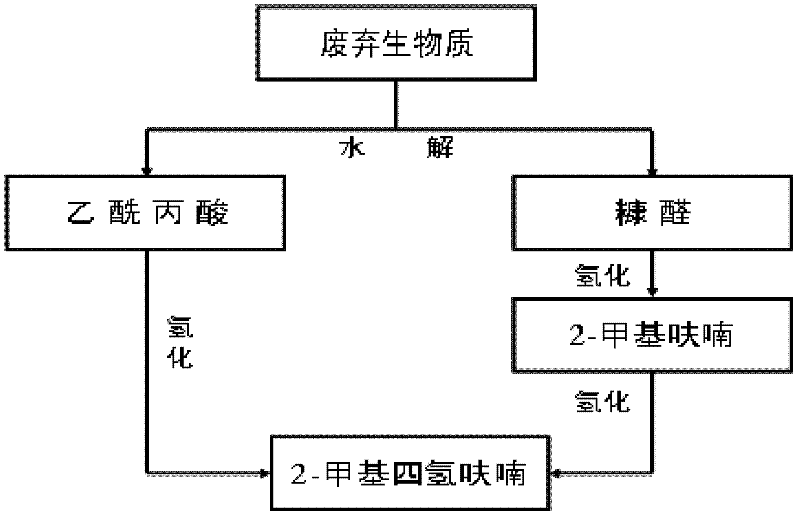
Method for preparing 2-methyltetrahydrofuran from waste biomass
ActiveCN102558106AReduce pollutionReduce manufacturing costOrganic chemistryLevulinic acidPropanoic acid
The invention discloses a method for preparing 2-methyltetrahydrofuran from waste biomass, which comprises the following steps: carrying out acid hydrolysis reaction on the waste biomass to obtain furfural and levulinic acid; sequentially carrying out first hydrogenation reaction and second hydrogenation reaction on the furfural to obtain the 2-methyltetrahydrofuran; and carrying out hydrogenation reaction on the levulinic acid to obtain the 2-methyltetrahydrofuran. The method provided by the invention more sufficiently utilizes the waste biomass, and adopts non-noble metal catalysts, thereby lowering the production cost.
Owner:北京雷恩新材料科技有限公司
Barbecue essence and production process thereof
The invention discloses a barbecue essence, which is prepared from the following raw materials: ethyl maltol, 4-hydroxyl-2,5-dimethyl-3(2H)-furanone, 2,5-dimethyl-2,5-diyhydroxyl-1,4-dithiacyclo-hexane, methyl cyclopentenotone, 2-acetylpyrazine, 2-methyl tetrahydrofuran-3-mercaptan, bis(2-methyl-3-furyl) disulfide, propyl2-methyl-3-furyl disulfide, 4-methyl-5- hydroxyethyl-thiazole, 2-methylpyrazine, 2,3,5-trimethylpyrazine, 3-methylmercaptopropionaldehyde, difurfuryl disulfide, 4-methyl-4-furfurylthio-2-pentanone, 2,4,5-trimethylthiazole, 2,4,6-triisobutyl-1,3,5-dithiazine, beta-phenylethylmercaptan, 1,6-ethanthiol, 2-pentylthiophene, furfuryl mercaptan, 4,5-dimethyl-2-isobutyl-3-thiazoline, guaiacol, delta-dodecalactone, 2,4-decadienealdehyde, trans,trans-2,4- nonadienal, anisic aldehyde, butanoic acid, acetic acid, black pepper essential oil ginger essential oil, clove oil, cassia oil and an oil-soluble solvent. The invention also discloses a barbecue essence production process.
Owner:厦门市顶味兴业香料发展有限公司
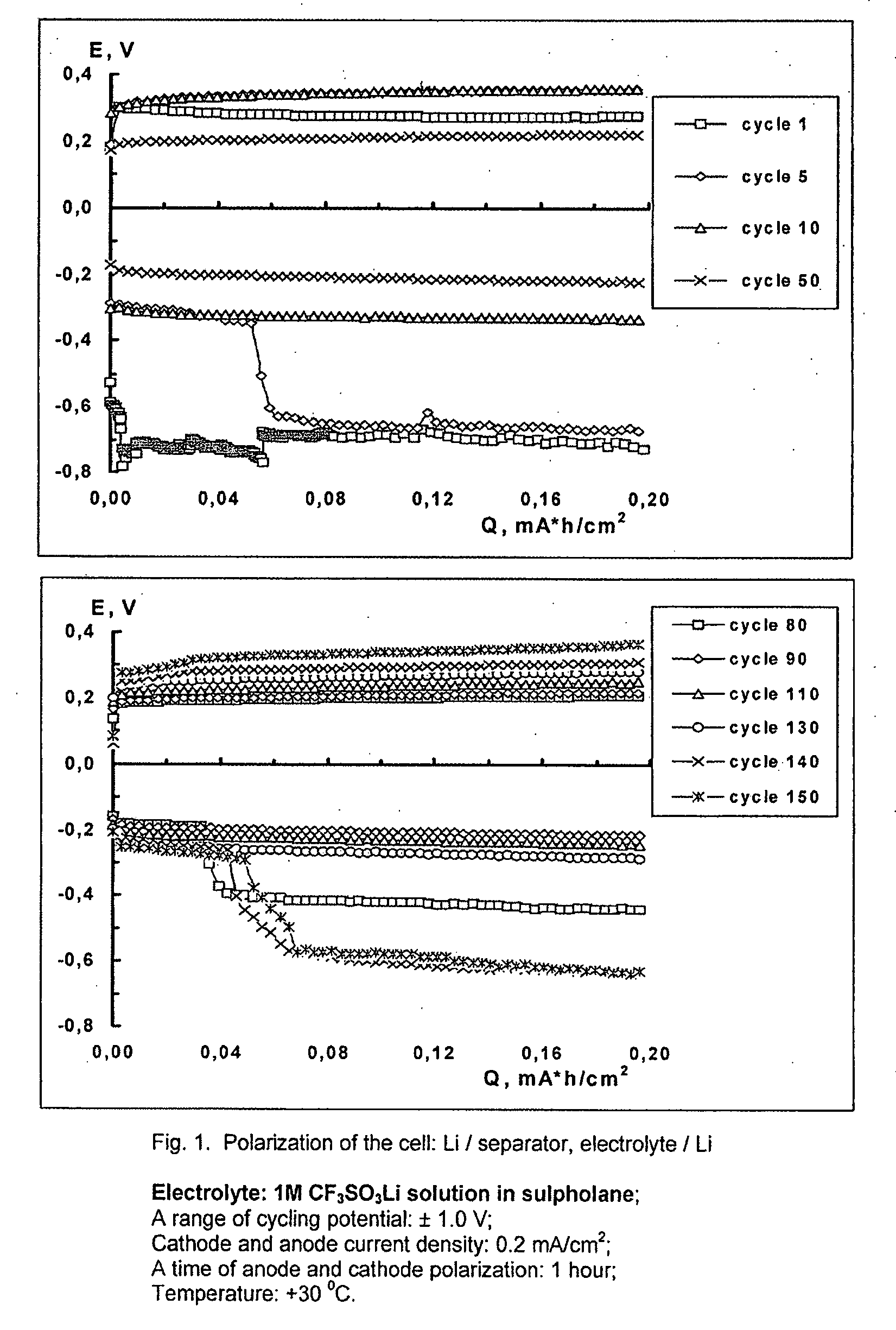
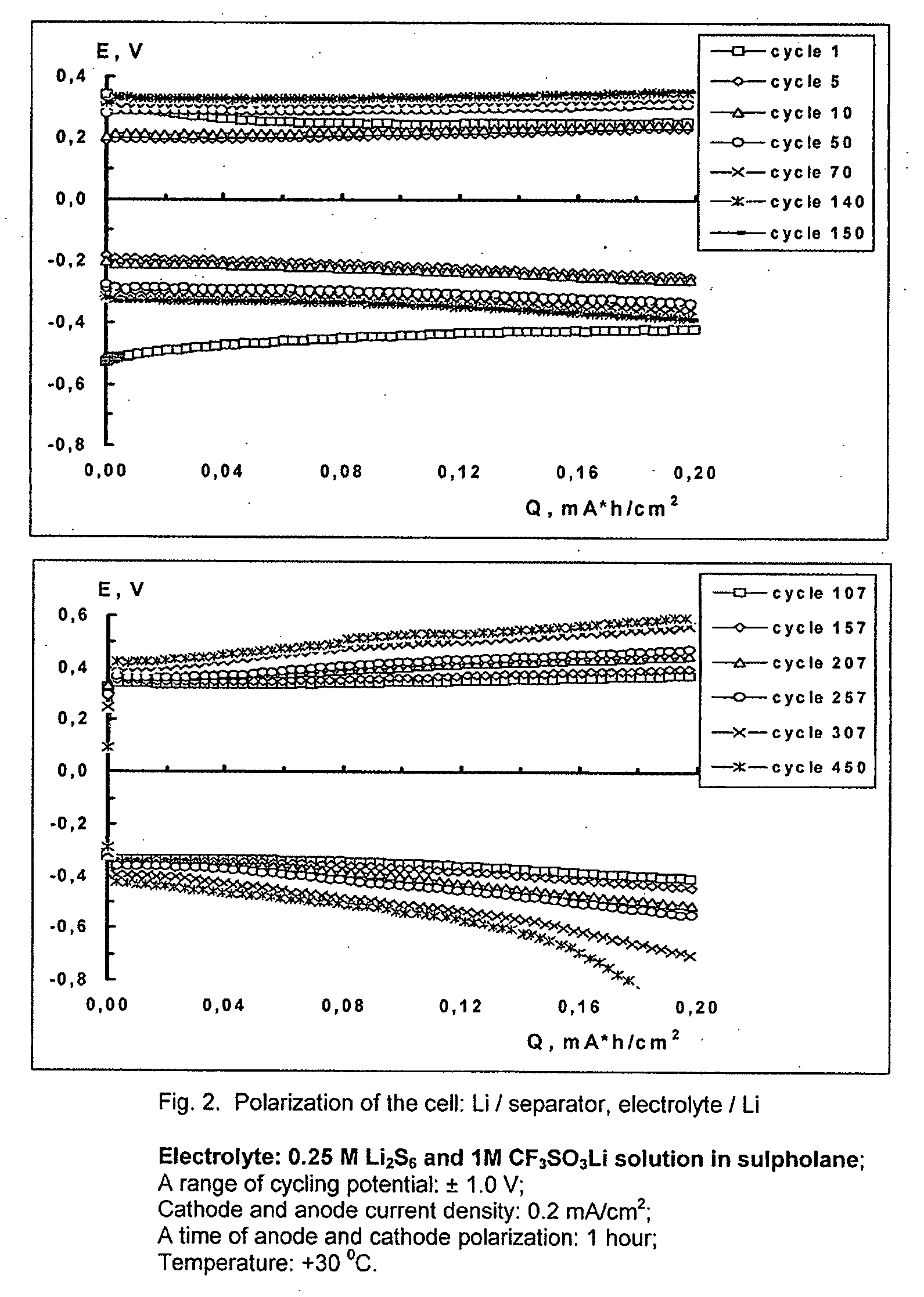
Cell or battery with a metal lithium electrode and electrolytes therefor
InactiveUS20080038645A1Improve cycle lifeOrganic electrolyte cellsLi-accumulatorsSulfolaneMetallic lithium
An electrolyte for rechargeable batteries with a negative electrode of lithium or lithium containing alloys comprising: one or several non-aqueous organic solvents, one or several lithium salts and one or several additives increasing the cycle life of the lithium electrode. The electrolyte solution may comprise one or several solvents selected from the group comprising: tetrahydrofurane, 2-methyltetrahydrofurane, dimethylcarbonate, diethylcarbonate, ethylmethylcarbonate, methylpropylcarbonate, methylpropylpropyonate, ethylpropylpropyonate, methylacetate, ethylacetate, propylacetate, dimetoxyethane, 1,3-dioxalane, diglyme (2-methoxyethil ether), tetraglyme, ethylenecarbonate, propylencarbonate, γ-butyrolactone, and sulfolane. The electrolyte solution may further comprise at least one salt or several salts selected from the group consisting of lithium hexafluorophosphate (LiPF6), lithium hexafluoroarsenate (LiAsF6), lithium perchlorate (LiClO4), lithium sulfonylimid trifluoromethane (LiN(CF3SO2)2)) and lithium trifluorosulfonate (CF3SO3Li) or other lithium salts or salts of another alkali metal or a mixture thereof. Also disclosed is an electrochemical cell or battery with an anode of metallic lithium or a lithium-containing alloy, and such an electrolyte.
Owner:OXIS ENERGY
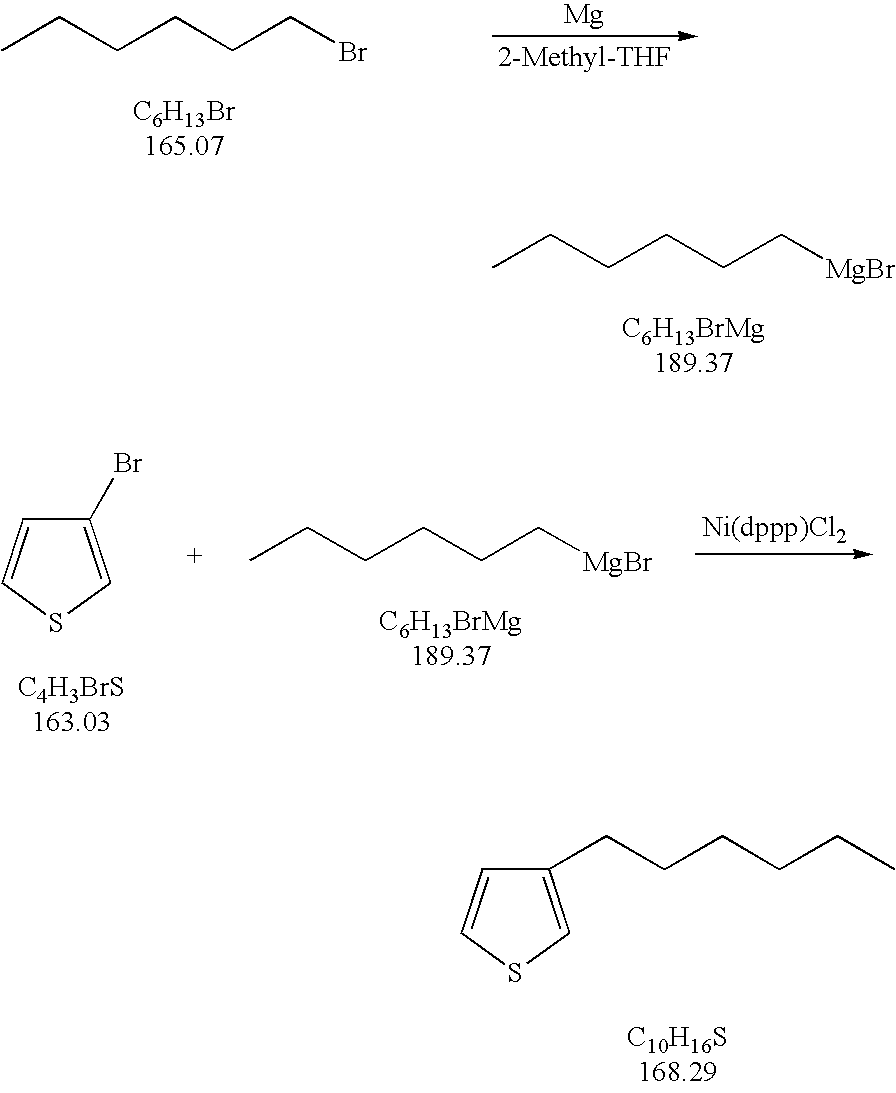
Process for the kumada coupling reaction
InactiveUS20060155134A1Silicon organic compoundsGroup 3/13 element organic compoundsHigh concentrationGrignard reagent
A method for the formation of 3-alkylthiophenes or 3-arylthiophenes from 3-halothiophenes. More particularly, improvements on the Kumada coupling reaction for the production of 3-alkylthiophenes or 3-arylthiophenes by reacting a 3-halothiophene with an alkylmagnesiumhalide or arylmagnesiumhalide Grignard reagent in the presence of a catalyst and a 2-methyl tetrahydrofuran solvent. The 2-methyl tetrahydrofuran solvent allows for higher concentrations of the Grignard reagent with minimal or no dithienyl side product generation, achieving higher product yields and at a lower cost than other known methods.
Owner:HONEYWELL INT INC
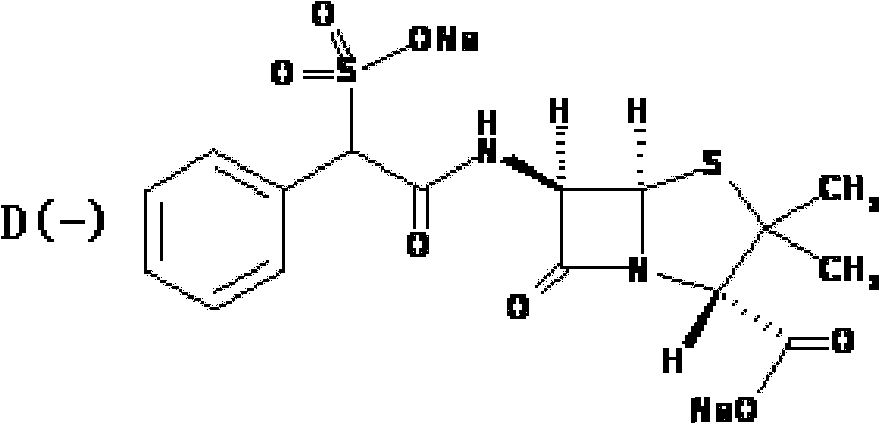
Preparation method of D(-)-sulbenicillin sodium
The invention provides a preparation method of D(-)-sulbenicillin sodium, which comprises the following steps: preparing D(-)-sulfophenylacetyl chloride from D(-)-sulfophenylacetic acid; preparing a D(-)-sulbenicillin sodium crude product from the D(-)-sulfophenylacetyl chloride and 6-APA; and purifying to obtain the final product D(-)-sulbenicillin sodium, wherein the 6-APA and the D(-)-sulfophenylacetyl chloride are added to a mixed solvent of water, ethanol and 2-methyltetrahydrofuran and react at the pH of 5.6-7.0 and the room temperature of 15-25 DEG C for 20-40 minutes. The preparation method has mild reaction condition, high yield and high purity of the obtained product.
Owner:HUNAN SANQING PHARMA +1
Method for preparing sulbenicillin disodium
The invention provides a method for preparing sulbenicillin disodium. The method comprises the following steps of: preparing alpha-sulfophenylacetyl chloride from alpha-sulfophenylacetic acid; reacting the alpha-sulfophenylacetyl chloride with 6-aminopenicillanic acid (APA) in the mixed solvents of water, ethanol and tetrahydro-2-methylfuran under the condition of the pH value of 5.6 to 7.0 and the temperature of 15 to 25 DEG C for 20 to 40 minutes to obtain crude sulbenicillin disodium; and obtaining the aqueous solution of the sulbenicillin disodium by post treatment and then cooling and drying the aqueous solution of the sulbenicillin disodium to obtain the final product, namely the sulbenicillin disodium. The method has the advantages of reducing energy consumption and saving production cost along with simple process, mild reaction condition and high yield.
Owner:HUNAN SANQING PHARMA +1
Preparation method of citalopram intermediate
InactiveCN105294496ACarboxylic acid nitrile preparationOrganic compound preparationGrignard reagentNitrogen
The invention provides a preparation method of a citalopram intermediate, and belongs to the technical field of pharmaceuticals. In the method, 2-methyltetrahydrofuran is taken as a reaction solvent. Under the protection of nitrogen, a 4-fluorophenylmagnesium bromide solution Grignard reagent, 5-cyanophthalein and a N,N-dimethylpropyl magnesium chloride Grignard reagent are taken as raw materials. A reaction is carried out to synthesize 4-(4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrilehydrobromide. The method is characterized by high yield and high purity, and is suitable for industrial production.
Owner:SUN YAT SEN UNIV +1
Compounding method for 3- ethyoxyl-4-ethoxycarbonyl phenylacetic acid
ActiveCN101891621AEasy to operateRealize industrial productionOrganic compound preparationCarboxylic acid esters preparationN dimethylformamidePhenylacetic acid
The invention discloses a compounding method for 3- ethyoxyl-4-ethoxycarbonyl phenylacetic acid, comprising the following steps: making 4-methyl salicylate and bromoethane which are used as starting raw materials to carry out double alkylation reaction in N,N-dimethylformamide to prepare 2-ethyoxyl-4-ethyl methylbenzoate; directly dissolving the unpurified 2-ethyoxyl-4-ethyl methylbenzoate into 2-methyl tetrahydrofuran, adding lithium diisopropylamide, and introducing carbon dioxide; acidizing the mixture by sulphuric acid to prepare the 3-ethyoxyl-4-ethoxycarbonyl phenylacetic acid. The product of the invention is white powders with a melting point of 78 to 80 degrees and the content of 99.4%. In the invention, the solvent of N, N-dimethylformamide and 2-methyl tetrahydrofuran can be recycled and repeatedly applied. The invention has the advantages of simple operation, environment friendliness, simple industrial operation, industrial production realization and low cost due to the adoption of the common industrial raw materials.
Owner:QIDONG HUDONG CHEM
Method used for preparing disperse blue 360
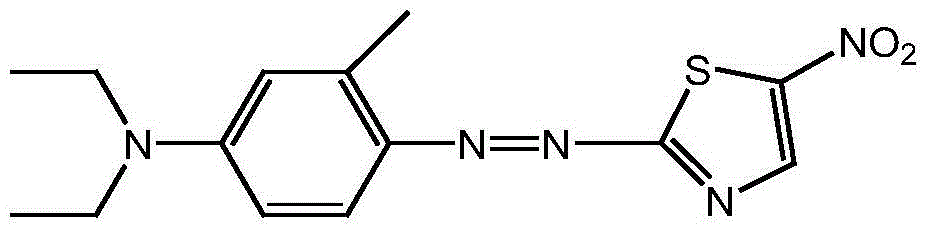
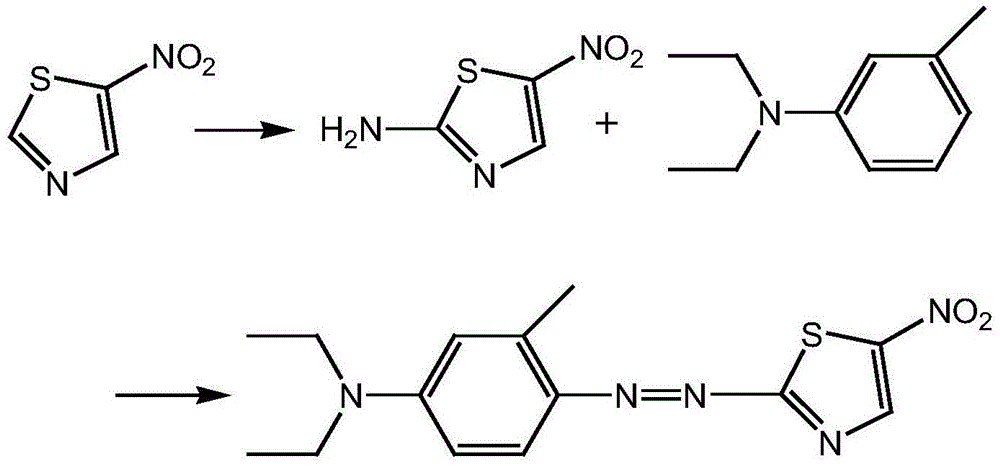
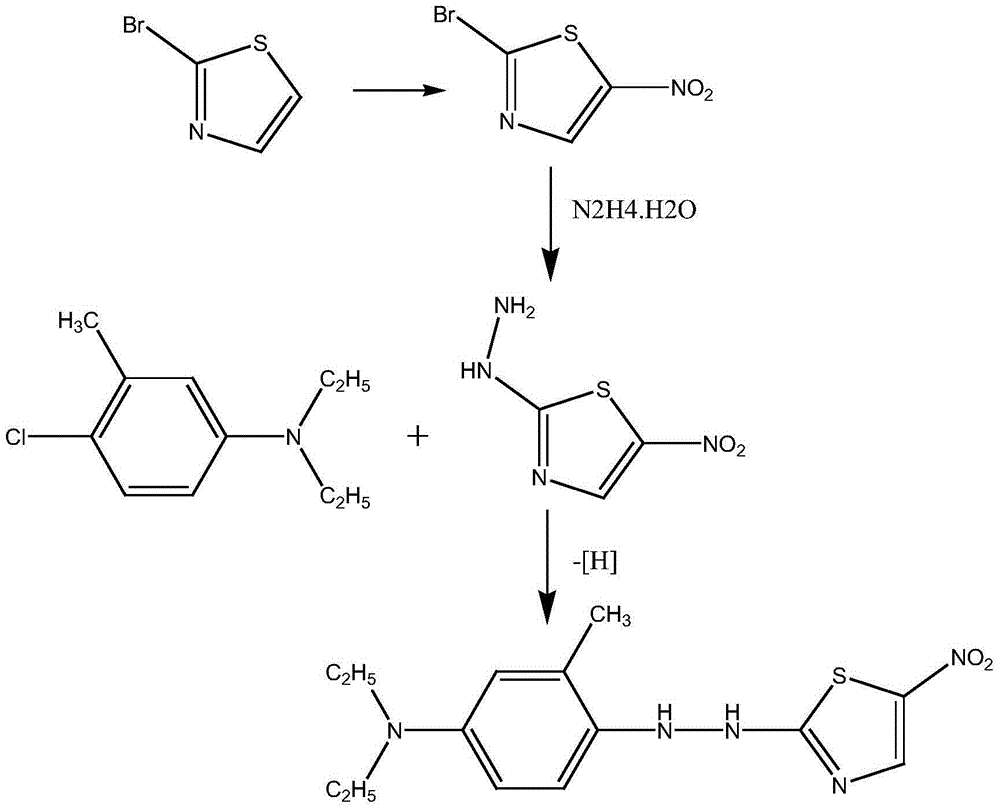
The present invention discloses a method for preparing disperse blue 360. 2-Bromothiazole is used as a raw material and is subjected to four unit reaction processes of nitration, hydrazination, amination and oxidation to obtain the target product. Its specific route is as follows: 2-bromothiazole is used as a raw material and subjected to a nitration reaction under the action of a mixture of concentrated nitric acid and concentrated sulphuric acid using to produce 2-bromo-5-nitrothiazole, the resulting 2-bromo-5-nitrothiazole is reacted using 2-methyltetrahydrofuran as a solvent under the action of hydrazine hydrate to produce 2-hydrazino-5-nitrothiazole, the resulting 2-hydrazino-5-nitrothiazole is reacted with 3-methyl-4-chloro-N,N-diethylaniline under the action of triethylamine, cuprous bromide and butanol to produce 2-[[4-(diethylamino)-2-methylphenyl]diamine]-5-nitrothiazole, the prepared 2-[[4-(diethylamino)-2-methylphenyl]diamine]-5-nitrothiazole is reacted under the action of an oxidant to produce the target product. In the present invention, sodium perborate and a composite catalyst are used for the hydrazine oxidation, which solves the problem of a low conversion rate of hydrazine oxidation using hydrogen peroxide as an oxidant, and provides a new preparation method for synthesizing the azo dye.
Owner:JIANGSU DAOBO CHEM
Preparation method of catalyst for preparing 2-methyltetrahydrofuran from 2-methylfuran through gas phase hydrogenation
ActiveCN103977803AEasy to prepareGood repeatabilityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsGas phaseAmyl alcohol
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Preparation method of crystal form I of clopidogrel hydrogen sulfate
The invention provides a preparation method of a crystal form I of clopidogrel hydrogen sulfate. The preparation method comprises the following steps of (1) dropwise adding concentrated sulfuric acid or 2-methyltetrahydrofuran solution of the concentrated sulfuric acid into 2-methyltetrahydrofuran solution of clopidogrel free base to obtain mixed solution, wherein the temperature of the mixed solution is controlled to range from -10 DEG C to 5 DEG C when the dropwise adding is performed; and (2) heating the mixed solution to the temperature of 10-35 DEG C, performing crystal precipitation with stirring, separating precipitated crystals, and drying the precipitated crystals to obtain the crystal form I of the clopidogrel hydrogen sulfate. The preparation method is simple, easy to implement, good in reproducibility and suitable for industrial production. The prepared crystal form I of the clopidogrel hydrogen sulfate has the advantages of being high in crystal form purity and high-performance liquid chromatography (HPLC) purity, low in cost, good in stability, high in solvent recovery, environment-friendly and the like.
Owner:ZHEJIANG HISOAR PHARMA
Hydrogenation catalyst, preparation method of hydrogenation catalyst and application of hydrogenation catalyst to 2-methyltetrahydrofuran synthesis
ActiveCN102921415AHigh selectivityHigh catalytic activityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsFuranActive component
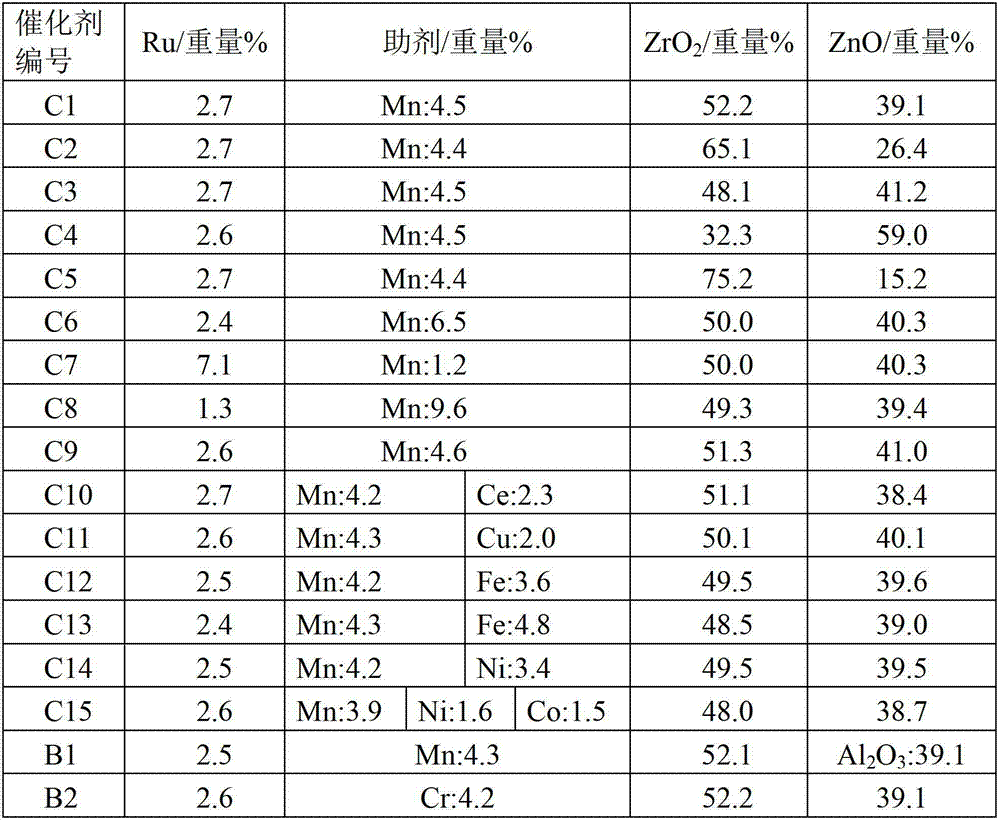
The invention discloses a hydrogenation catalyst which contains a carrier, an active component and an auxiliary. The active component and the auxiliary are loaded on the carrier, wherein the active component is ruthenium (Ru), the auxiliary is manganese (Mn) or a combination of Mn and at least one of metal components of an IB group, an IIB group, an IIIB group and a VIII group, and the carrier is a composite carrier of ZrO2 and ZnO. The invention further provides a preparation method of the hydrogenation catalyst and an application of the hydrogenation catalyst to 2-methyltetrahydrofuran synthesis. The hydrogenation catalyst has higher catalytic activity and 2-methyltetrahydrofuran selectivity and longer service life in the reaction that raw materials containing 2-methyl furan synthesize the 2-methyltetrahydrofuran through hydrogenation, and the raw materials containing the 2-methyl furan are not required to be rectified so that process is simplified, and energy consumption is reduced.
Owner:JIANGSU QINGQUAN CHEM CO LTD
Continuous production method of 2-methyl tetrahydrofuran
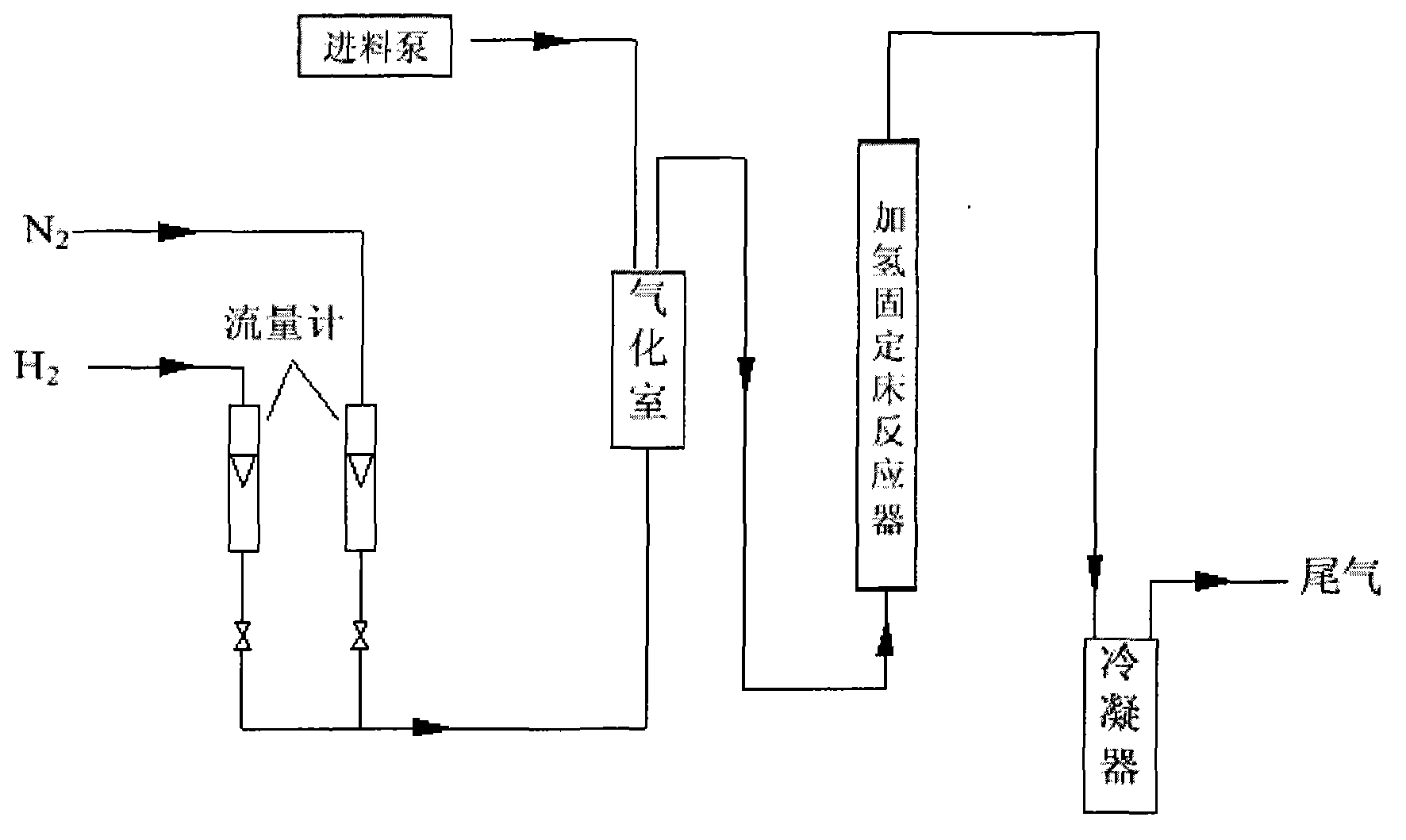
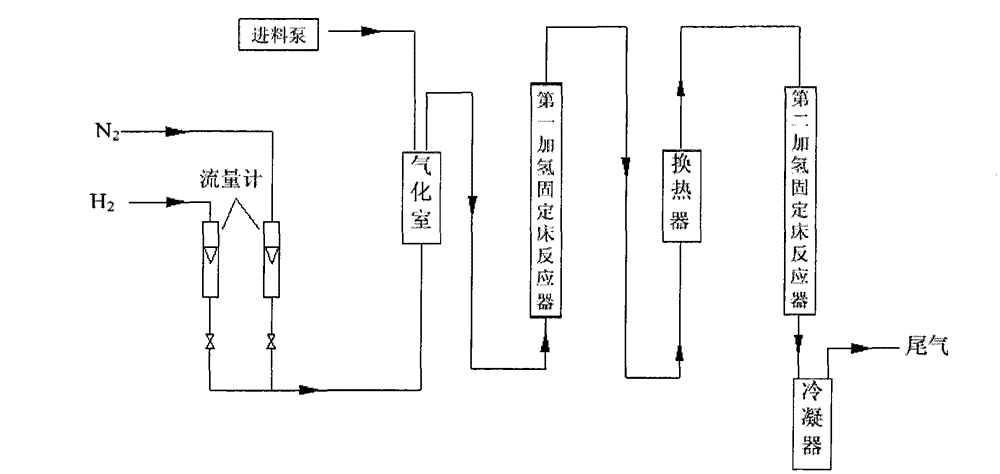
The invention discloses a continuous production method of 2-mehtyl tetrahydrofuran. The continuous production method comprises the following steps of: pumping 2-methyl furan to a gasifying chamber for gasifying; mixing the gasified 2-methyl furan with hydrogen gas to obtain a gas mixture; inputting the gas mixture to a hydrogenation fixed-bed reactor for carrying out a catalytic hydrogenation reaction; and inputting the gas output by the hydrogenation fixed-bed reactor to a condensing device for condensing to obtain 2-methyl tetrahydrofuran, wherein the pressure of the gasifying chamber and the hydrogenation fixed-bed reactor is 0-1.0MPa, and catalyst for aromatic saturation hydrogenation is filled in the hydrogenation fixed-bed reactor. The continuous production method of the 2-methyl tetrahydrofuran can be used for changing the high-pressure high-investment and high-risk process for conventionally producing 2-MeTHF and can be used for reducing the use of toxic and noble metal catalyst. Besides, the continuous production method has the advantages of being low in investment, small in risk, large in material throughput within unit time, and high in yield and purity, and is suitable for industrial production.
Owner:ASYMCHEM LAB TIANJIN +4
Chicken oily essence and preparation method thereof
InactiveCN104256499ARealistic fragranceNatural aromaFood preparation2-methyl-3-furanthiol4-methyl-5-thiazoleethanol
The invention relates to chicken oily essence and a preparation method thereof. The chicken oily essence is prepared from 2-mercapto-3-butanol, 2-methyl-3-furathiol, 3-methylthiopropanol, 3-(methylthio)propionaldehyde, 2,3,5-trimethylpyrazine, 2-methyl-3-furanethiol, 2-amylthiophene, trans-2-trans-4-decadienal, trans-2-trans-4-nonadienal, trans-2-decenal, 4-methyl-5-thiazoleethanol, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, ethyl maltol, 2-acetylpyrazine, Chinese prickly ash essential oil, cold-pressed ginger oil, geranium oil and soybean salad oil. The chicken oily essence has a natural chicken local flavor, a lifelike, natural and lasting fragrance and good heat stability. The chicken oily essence is irreplaceable in the fields of flavorings, chickens' extract and instant noodles.
Owner:TIANJIN CHUNFA BIO TECH GRP
Green synthesis of 2-methylte-trahydrofuran
ActiveCN101492433BReduce manufacturing costSuitable temperatureOrganic chemistryChemical recyclingReaction temperature2-Methylfuran
Owner:ZHEJIANG UNIV OF TECH +1
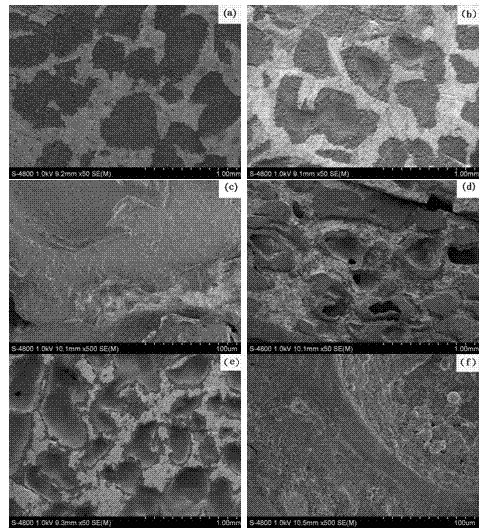
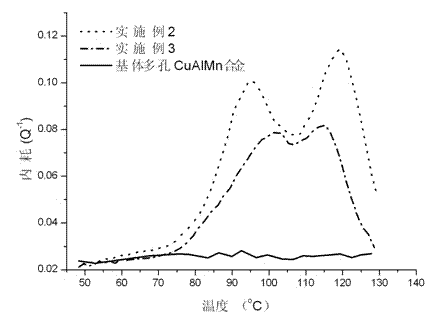
Preparation method for porous copper-based shape memory alloy-based damping composite material
The invention discloses a preparation method for a porous copper-based shape memory alloy-based damping composite material and relates to preparation of damping materials. The method comprises the following steps of: performing thermal circulation and ultrasonic cleaning on a quenching-state porous CuAlMn shape memory alloy sample to remove stains adhered to the outer surface of the sample and the inner surfaces of pores; immersing the sample in a polystyrene-2-methyltetrahydrofuran solution, and performing ultrasonic oscillation until the polystyrene-2-methyltetrahydrofuran solution is fully permeated into the pores of the porous CuAlMn shape memory alloy sample, which are communicated in a three-dimensional way; and finally drying until the 2-methyltetrahydrofuran solvent is volatilized completely to prepare a porous copper-based shape memory alloy-based damping composite material finished product, wherein polystyrene layers are deposited in the pores of the porous CuAlMn shape memory alloy. The finished product is high and controllable in damping property, and overcomes the defect that the stress concentration or microcracks are easily generated on edges of pore walls under the external load in the conventional CuAlMn shape memory alloy.
Owner:HEBEI UNIV OF TECH
Method for preparing biogasoline by catalytic hydrogenolysis on straws
InactiveCN109161395AAvoid lostMaximize utilizationLiquid hydrocarbon mixture productionHydrocarbon oils treatment productsFuranIodide
The invention provides a method for preparing biogasoline by catalytic hydrogenolysis on straws. The method comprises the following steps: successively adding the straws, iodide, a metal catalyst, water, an organic solvent and inorganic acid in a reaction container according to a certain rate of charge, after feeding hydrogen until pressure in the reaction container reaches a certain range, heating the reaction container to a certain temperature and then reacting for a certain time, then cooling to a room temperature and collecting an organic phase. The prepared product mainly comprises 2-methyltetrahydrofuran, 2-methyl furan, 2,5-dimethyl tetrahydrofuran, 2,5-dimethylfuran, 2-hexanone, 3-methylcyclopentanone, 2,5-hexanedione and 5-methylfurfural. By the method, the straws are catalytically hydrogenated by metal under the conditions of low temperature and low pressure, and cellulose and hemicellulose portions in the straws are directly converted into furan, furfural and a cyclic ketones compound at high yield.
Owner:NANCHANG UNIV
Green synthesis of 2-methylte-trahydrofuran
ActiveCN101492433AReduce manufacturing costSuitable temperatureOrganic chemistryChemical recyclingReaction temperaturePressure response
The invention discloses a green synthetic method of 2-methyltetrahydrofuran as is shown in formula (I): in a pressure reaction kettle, the liquid phase hydrogenation is carried out on the 2-methyltetrahydrofuran shown in formula (II) and composite catalysts at the reaction temperature of 50-100 DEG C and the reaction pressure of 1.0-4.0MPa until hydrogen is absorbed completely, and after the reaction is finished, reaction liquid is separated to obtain the 2-methyltetrahydrofuran as is shown in the formula (I); the composite catalysts are noble metal and non-noble metal simultaneously loaded on an activated carbon support. The noble metal is ruthenium, and the non-noble metal is one or more than one of zinc, copper or iron. As a green chemical synthetic method with perfect extension and application prospect, the green synthetic method of the 2-methyltetrahydrofuran provided by the invention has the advantages of little noble metal usage in the catalysts, low catalyst preparing cost, low reaction pressure, proper temperature, convenient operation, high yield, pollution-free property, easy recovery of the catalysts, etc.
Owner:ZHEJIANG UNIV OF TECH +1
One-step catalytic conversion of biomass-derived carbohydrates to liquid fuels
The invention relates to a method for manufacture of hydrocarbon fuels and oxygenated hydrocarbon fuels such as alkyl substituted tetrahydrofurans such as 2,5-dimethyltetrahydrofuran, 2-methyltetrahydrofuran, 5-methylfurfural and mixtures thereof. The method generally entails forming a mixture of reactants that includes carbonaceous material, water, a metal catalyst and an acid reacting that mixture in the presence of hydrogen. The reaction is performed at a temperature and for a time sufficient to produce a furan type hydrocarbon fuel. The process may be adapted to provide continuous manufacture of hydrocarbon fuels such as a furan type fuel.
Owner:PENN STATE RES FOUND
Continuous production method of 2-MeTHF (2-methyltetrahydrofuran)
The invention discloses a continuous production method of 2-MeTHF (2-methyltetrahydrofuran), which comprises the following steps of: inputting gasified furfural and hydrogen into a first reaction area and conducting primary catalytic hydrogenation reaction; inputting gas output by the first reaction area into a second reaction area and conducting secondary catalytic hydrogenation reaction; and condensing gas output by the second reaction area to obtain the 2-MeTHF, wherein the first reaction area is filled with catalyst for reducing aldehyde groups and the second reaction area is filled with catalyst for aromatic saturated hydrogenation. By using low-toxicity, low-cost and easy-to-obtain catalyst to produce high-purity 2-MeTHF through gas-phase continuous reaction under low pressure or low ambient temperature, the traditional technology which has the disadvantages of high pressure, great investment and great risk is changed, and the use of high-toxicity precious metals can be reduced. The production technology is simple, the investment is small, the risk is small, the furfural treatment capacity per unit time is large, the yield is high, the purity of the obtained crude product is high and the impurities are easy to separate.
Owner:ASYMCHEM LAB TIANJIN +4
Processes for the preparation of 2-methylfuran and 2-methyltetrahydrofuran
Processes are disclosed for the preparation of 2-methylfuran and 2-methyltetrahydrofuran. The continuous vapor-phase processes are commercially viable and efficient because they permit the preparation of 2-methylfuran and 2-methyltetrahydrofuran using commercially-available catalysts, namely, a reduced copper-based catalyst consisting essentially of cupric oxide, chromium (III) oxide, manganese oxide and barium chromate and a reduced nickel-based catalyst consisting essentially of nickel, nickel (II) oxide, aluminum oxide and silica. An apparatus comprising two inline hydrogenators is used for preparing the 2-methylfuran or 2-methyltetrahydrofuran.
Owner:PURE ENERGY
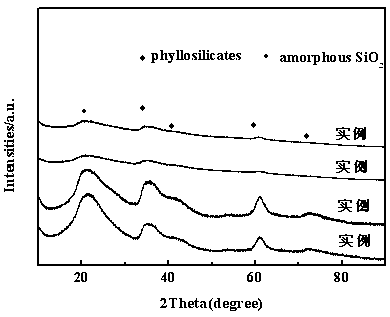
Method for preparing 2-methyltetrahydrofuran catalyst through gas phase hydrogenation of 2-methylfuran
InactiveCN109569611AEasy to prepareEasy to operateOrganic chemistryHeterogenous catalyst chemical elementsMetal nitrateNickel salt
The invention discloses a method for preparing a 2-methyltetrahydrofuran catalyst through gas phase hydrogenation of 2-methylfuran, and relates to a method for preparing the catalyst. The method includes dissolving soluble nickel salt or nickel salt and other metals in water to prepare a metal salt solution with a certain concentration; adding ammonia water into the solution for sealing and stirring; adding silica sol for sealing and stirring again for a certain time after the solution is evenly stirred; evaporating ammonia gas at a certain temperature to regulate the pH value of the obtainedsuspension to reach a certain value; and washing, drying and roasting the precipitate to finally prepare the NiO / SiO2 catalyst, wherein the mass percentage of NiO is 15%-45%, the mass percentage of SiO2 is 55%-85%, and the mass percentage of the metal auxiliary agent is 0.2%-8%; and the soluble metal salts are all metal nitrates. The preparation method of the catalyst is simple and easy to operateand has good repeatability; and the prepared catalyst can form a special layered silicate structure to maintain good activity, selectivity and stability.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
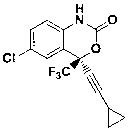
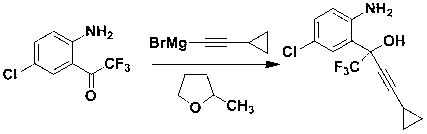
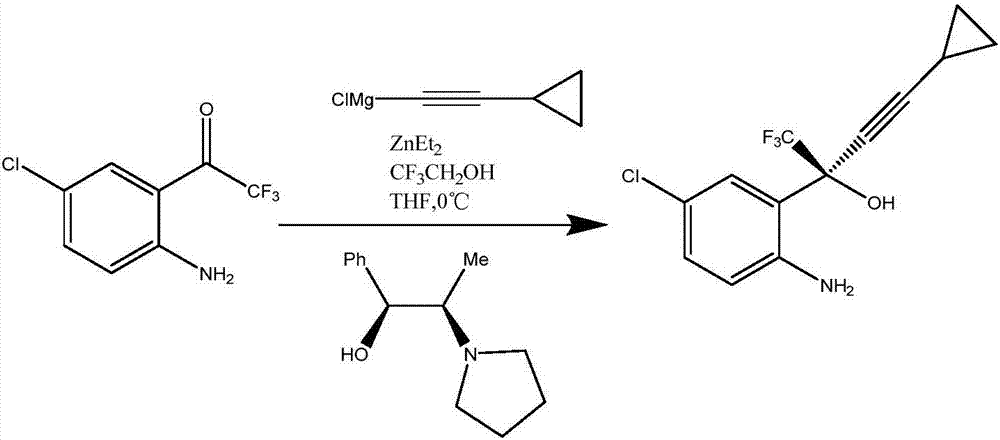
Preparation method of efavirenz intermediate
InactiveCN103254087AImprove stabilityReduce dosageOrganic compound preparationAmino-hyroxy compound preparationP-chloroanilineAnti virus
The invention discloses a preparation method of an efavirenz intermediate, relating to synthesis of an anti-virus medicine, namely an efavirenz key intermediate by adopting a green solvent, namely 2-methyltetrahydrofuran as a Grignard reaction solvent, and belonging to the technical field of organic synthesis. The preparation method comprises the following steps of: taking the 2-methyltetrahydrofuran as a solvent, enabling metal magnesium to react with ethyl bromide to obtain ethyl magnesium bromide, then dripping cyclopropylacetylene to generate cyclopropyne ethyl magnesium bromide, and finally performing addition reaction with 2-trifluoroacetyl p-chloroaniline to obtain 2-(2-amino-5-chlorophenyl)-4-cyclopropyl-1, 1, 1-trifluoro-3-butyn-2-ol. According to the method, the 2-(2-amino-5-chlorophenyl)-4-cyclopropyl-1, 1, 1-trifluoro-3-butyn-2-ol can be prepared with high selectivity and high yield, the product purity is more than 99.8%, and the yield can achieve 95.2-97.1%. Compared with traditional technologies, the preparation method disclosed by the invention has the following advantages: as the green solvent, namely the 2-methyltetrahydrofuran is adopted in Grignard reaction, the yield is high, the selectivity is good, the product is easy to separate, the reaction conditions are easy to control, the using quantity of the solvent is small, and the solvent is easy to recover, so that the preparation method is in line with a green chemical idea and is suitable for industrial production.
Owner:ZHENGZHOU UNIV +1
Processes for the preparation of 2-methylfuran and 2-methyltetrahydrofuran
Processes are disclosed for the preparation of 2-methylfuran and 2-methyltetrahydrofuran. The continuous vapor-phase processes are commercially viable and efficient because they permit the preparation of 2-methylfuran and 2-methyltetrahydrofuran using commercially-available catalysts, namely, a reduced copper-based catalyst consisting essentially of cupric oxide, chromium (III) oxide, manganese oxide and barium chromate and a reduced nickel-based catalyst consisting essentially of nickel, nickel (II) oxide, aluminum oxide and silica. An apparatus comprising two inline hydrogenators is used for preparing the 2-methylfuran or 2-methyltetrahydrofuran.
Owner:PURE ENERGY
Method for preparing phenyl dimethylchlorosilane
ActiveCN102993226AHigh yieldLess side effectsGroup 4/14 element organic compoundsChlorobenzeneGrignard reagent
The invention discloses a method for preparing phenyl dimethylchlorosilane. The method for preparing phenyl dimethylchlorosilane comprises the following steps of: carrying out a Grignard reaction on chlorobenzene and magnesium so as to generate a Grignard reagent, and carrying out condensation reaction on the obtained Grignard reagent and dimethyl dichlorosilane under a novel catalyst so as to generate the phenyl dimethylchlorosilane; and carrying out the Grignard reaction and the condensation reaction respectively in a solvent system formed by methyl tertiary butyl ether and / or 2-methyltetrahydrofuran. Through adopting the solvent formed by methyl tertiary butyl ether and / or 2-methyltetrahydrofuran for reaction, when the Grignard reaction and the condensation reaction are carried out, the side reaction is less, and the reaction is much slow and is easy to control; the productivity of phenyl dimethylchlorosilane is improved; and the waste liquid amount during the production process is reduced.
Owner:ANHUI BIOCHEM BIO PHARMA
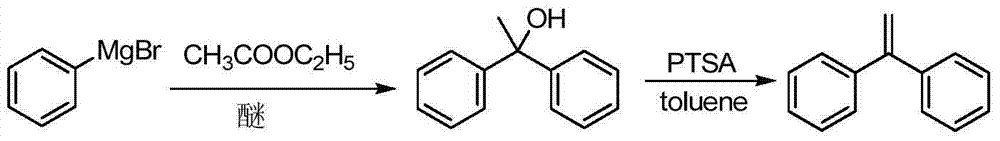
Preparation method of 1,1-diphenylethylene
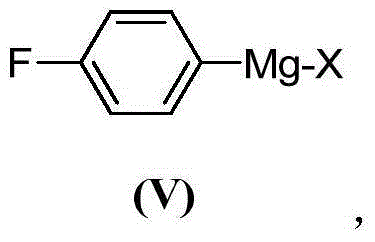
ActiveCN103755516AModerate boiling pointStrong Lewis alkalineHydrocarbonsBulk chemical productionPhenylmagnesium bromideGrignard reagent
The invention discloses a preparation method of 1,1-diphenylethylene shown in a formula (V). The method comprises the steps of firstly carrying out reaction on bromobenzene and magnesium chips in anhydrous 2-methyltetrahydrofuran to obtain a phenylmagnesium bromide Grignard reagent, and then dripping acetophenone into the phenylmagnesium bromide Grignard reagent to react, so as to generate 1,1-diphenylethanol; finally, dewatering 1,1-diphenylethanol in the presence of a sulfoacid functional ionic liquid catalyst, so as to obtain 1,1-diphenylethylene shown in the formula (V). The preparation method disclosed by the invention is short in reaction step, mild in condition, simple and convenient to operate, high in product yield, low in production cost, friendly to environment, and applicable to industrial production.
Owner:ZHEJIANG UNIV OF TECH +1
Efavirenz intermediate synthesizing method
InactiveCN106946718AEasy to recycleLow costOrganic compound preparationOrganic chemistry methodsDistillationAniline
The invention relates to an efavirenz intermediate synthesizing method. The efavirenz intermediate synthesizing method comprises the following steps of: adding cyclopropyl acetylene magnesium bromide to a coordination compound prepared from neopentyl alcohol, a zinc salt or copper salt and (1R, 2S)-1-phenyl-2-(1-pyrrolidyl)-1-propyl alcohol by using 2-methyltetrahydrofuran as a solvent, reacting, adding 4-chlorine-2-(trifluoroacetyl) aniline, and carrying out heat-preservation stirring till ending; and transferring to a saturated citric acid solution for quenching, carrying out reduced pressure distillation after separating to obtain an organic phase, adding an polar organic solvent and L-amino acid after obtaining racemate, heating and mixing, carrying out cooling crystallization after resolution, and carrying out recrystallization after obtaining crystals to obtain white powder. Compared with the prior art, the efavirenz intermediate synthesizing method has the advantages that the 2-methyltetrahydrofuran which is a green solvent is used in reaction; the product with relatively high optical purity is obtained by using very few organic ligands; the yield is relatively high; the selectivity is good; the separation of products and the control of reaction conditions are easy; and the method conforms to green and environmental protection concept and is suitable for industrialized production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Probenazole low-toxicity bactericide and preparation method thereof
The invention relates to a probenazole low-toxicity bactericide and a preparation method thereof, and belongs to the technical field of pesticide. The preparation method comprises the following steps: dissolving bromine in ethanol to obtain solution A; adding pyruvic acid into concentrated sulfuric acid, adding the solution A and obtaining a mixture I; adding P2S5 and formamide into 2-methyltetrahydrofuran to obtain solution II; adding the mixture I into the solution II, filtering, collecting precipitate, drying the precipitate, and obtaining thiazole-4-carboxylate; adding o-phenylenediamine and thiazole-4-carboxylate into polyphosphoric acid, reacting, mixing with water, filtering, drying and obtaining coarse probenazole; and adding coarse probenazole in absolute ethanol, adding active carbon, filtering, cooling filtrate to room temperature, filtering, drying, precipitating and obtaining probenazole. In the invention, bromine is dissolved in absolute ethanol and reacted, the operability of bromine is improved, the volatilization of a hydrogen bromide gas is slowed, the subsequent absorption process is continuous and controllable, and the unsafe factors in the production are reduced.
Owner:SHANDONG MEI LUO FU AGRI POLYTRON TECH
Soy sauce essence and preparation method thereof
Owner:TIANJIN CHUNFA BIO TECH GRP
Method for preparing 2-methyltetrahydrofuran catalyst through liquid phase hydrogenation of 2-methyl furan
InactiveCN110180547AEasy to prepareEasy to operateCatalyst carriersOrganic chemistryFuranNickel salt
The invention provides a method for preparing a 2-methyltetrahydrofuran catalyst through liquid phase hydrogenation of 2-methyl furan, and relates to a catalyst preparation method, which comprises: weighing a silica sol; taking glacial acetic acid, and adding into the silica sol in a dropwise manner; taking zirconium oxychloride (ZrOCI2.8H2O), and dissolving in deionized water; taking ammonia water, and adding into the ZrOCI2.8H2O solution in a dropwise manner; mixing the two precipitates, stirring, and aging for 12 h at a temperature of 80 DEG C; filtering the precipitate, washing, and calcining to obtain a ZrO2-SiO2 carrier; and preparing a catalyst, wherein the ZrO2-SiO2 solid powder is accurately weighed as the carrier, a soluble nickel salt and the soluble salts of other metals are taken and dissolved in water as an impregnation liquid, the carrier is immersed in the excess impregnation liquid, slow stirring and complete standing are sequentially performed, the excess solution isdrained after the adsorption equilibrium is achieved, the obtained material is dried, and the dried material is calcined to completely activate so as to prepare the finished NiO / ZrO2-SiO2 catalyst, wherein the mass percentage of NiO is 30%, and the mass percentage of the metal aid is 0.2-8%. According to the present invention, the used active component is non-precious metal Ni, and the carrier isZrO2-SiO2, such that the activity, the selectivity and the stability of the catalyst are substantially improved.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com