Preparation method of alpha-halogenated ketone compound
A halogenated ketone and compound technology, applied in the field of preparation of α-halogenated ketone compounds, can solve the problems of complex catalyst system structure, limited industrial use, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
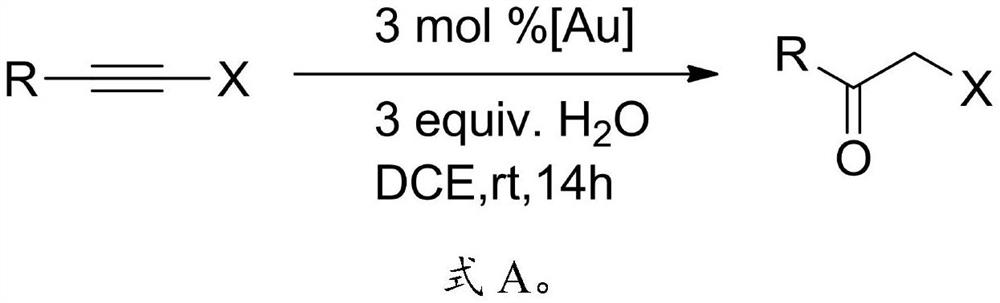
[0025] The present invention provides a kind of preparation method of α-halogenated ketone compound, comprises the following steps:
[0026] The terminal alkyne halide, organic solvent, acid and water are mixed and reacted to obtain α-halogenated ketone compounds.
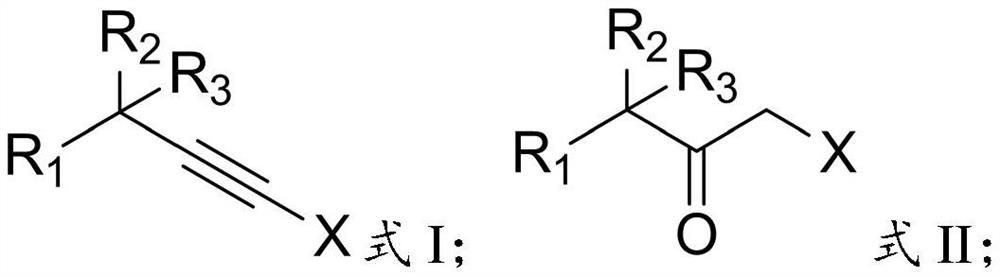
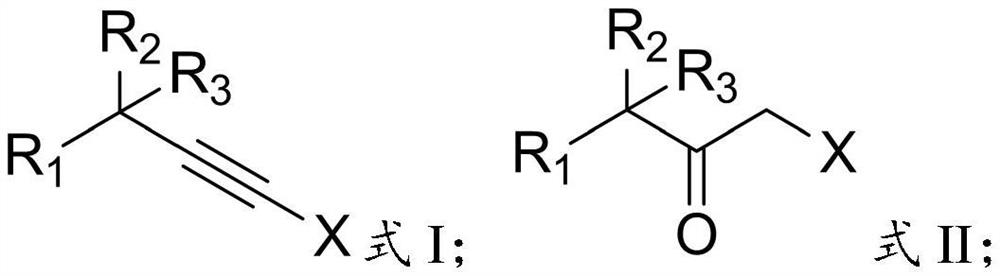
[0027] In the present invention, the terminal alkyne halide has the structure shown in formula I:
[0028]
[0029] In formula I: R 1 is hydrocarbyl, substituted hydrocarbyl, hydrocarbyl formyl, aryl or substituted aryl, R 2 and R 3 are independently chain hydrocarbon groups, cyclohydrocarbyl groups or substituted hydrocarbon groups, and X is a halogen atom.
[0030] Specifically, when the R 1 When it is a hydrocarbon group, the hydrocarbon group is preferably an alkyl group, and the number of carbon atoms of the alkyl group is preferably 1 to 6, more preferably 2 to 4, and the alkyl group preferably includes chain alkyl and cycloalkyl, specifically preferably Methyl, ethyl, isopropyl or cyclohexyl; said R ...
Embodiment 1
[0044] The synthesis of embodiment 1 (4-chloro-2-methyl-butan-3-ketone-2-yl) benzene
[0045] Reaction formula:
[0046]
[0047] The amount of reaction raw materials is shown in Table 1:
[0048] Table 1 reaction raw material consumption
[0049] raw material molecular weight moles The molar ratio of quality (4-Chloro-2-methyl-but-3-yn-2-yl)benzene 178.66 1mol 1 178.66g 36.5% hydrochloric acid solution 36.5 5mol 5.0 500.00g Dichloroethane 98.97 10mol 10 989.7g
[0050] The reaction steps are as follows:
[0051] Dissolve 176.88 g of (4-chloro-2-methyl-but-3-yn-2-yl)benzene in 989.7 g of dichloroethane, add 500 g of 36.5% hydrochloric acid, raise the temperature to 66°C, and stir for 10 h , after all the raw materials have been converted, the reaction mixture is cooled to room temperature, washed with 2500 mL of aqueous sodium bicarbonate solution and 500 mL of brine, the organic phase is separated, the organic phase is ...
Embodiment 2
[0052] Embodiment 2: the synthesis of (4-chloro-2-methyl-butan-3-ketone-2-yl) benzene
[0053] Reaction formula:
[0054]
[0055] The amount of raw materials is shown in Table 2:
[0056] Table 2 reaction raw material consumption
[0057] raw material molecular weight moles The molar ratio of quality (4-Chloro-2-methyl-but-3-yn-2-yl)benzene 178.66 1mol 1 178.66g 36.5% hydrochloric acid solution 36.5 5mol 5.0 500.00g ethanol 46.07 10mol 10 460.7g
[0058] The preparation steps are as follows:
[0059] Dissolve 178.66 g of (4-chloro-2-methyl-but-3-yn-2-yl)benzene in 460.7 g of ethanol, add 500 g of 36.5% hydrochloric acid to it, raise the temperature to 78°C, stir and react for 10 h, and wait for the raw materials to After all conversions, the reaction mixture was cooled to room temperature, washed with 2500 mL of aqueous sodium bicarbonate solution and 500 mL of brine, separated the organic phase, concentrated under redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com