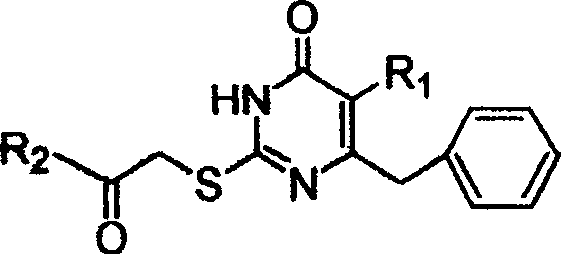
S-DABO compound, synthesizing method and usage
A compound and alkyl technology, applied in organic chemistry, antiviral agents, etc., can solve the problems of unfavorable binding, large electron cloud density, and large rigidity of naphthalene ring, and achieve the effect of strong inhibition, low cytotoxicity, and convenient synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The following examples will help to understand the present invention, but cannot limit the scope of the present invention.
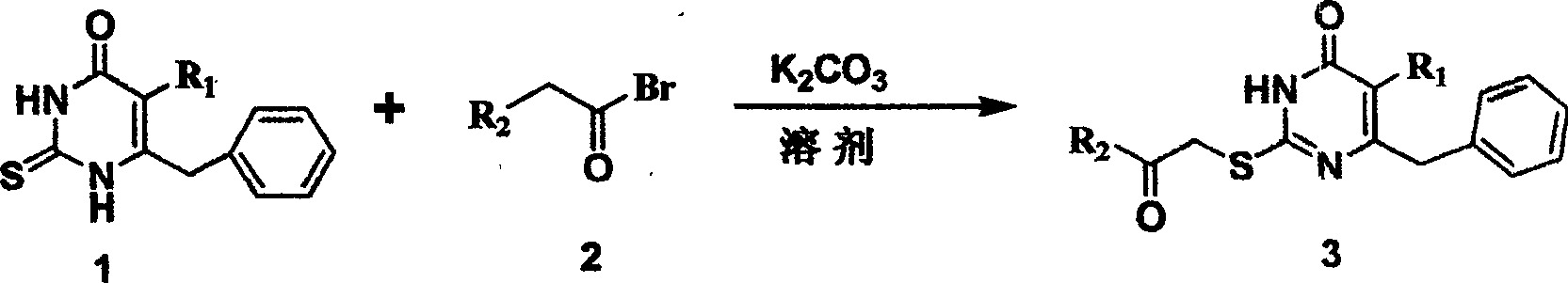
[0024] Synthesis of 5-alkyl-6-phenyl-2-(substituted arylcarbonylmethylthio)uracils. General operation of the reaction: 5-alkyl-6-phenyl-2-thiouracil (3mmol) and K 2 CO 3 Place in a flask, add 15ml of dry DMF, stir at room temperature for half an hour, add α-bromoketone R 1 COCH 2 Br (3.6mmol), continue to stir the reaction at a suitable temperature, TLC traces the disappearance of the raw material point to stop the reaction, pour the reaction solution into 30mL ice water, stir and precipitate the precipitate, filter, wash the precipitate with water, suction filter and dry to obtain the crude product, further Various white powders can be obtained by column chromatography purification. Recrystallization with an appropriate solvent can give white crystals of 5-alkyl-6-phenyl-2-(substituted arylcarbonylmethylthio)uracil.
[0025] Different 5-alky...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com