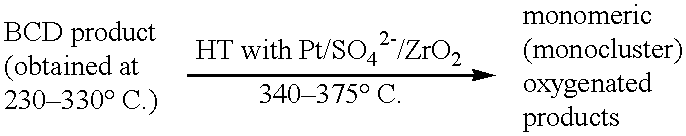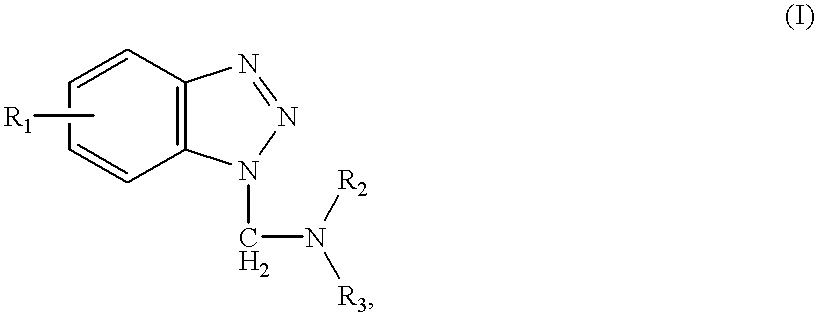Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4581 results about "Alkylbenzenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups of different sizes. They are a subset of the aromatic hydrocarbons. The simplest member is toluene, in which a hydrogen atom of the benzene was replaced by a methyl group.
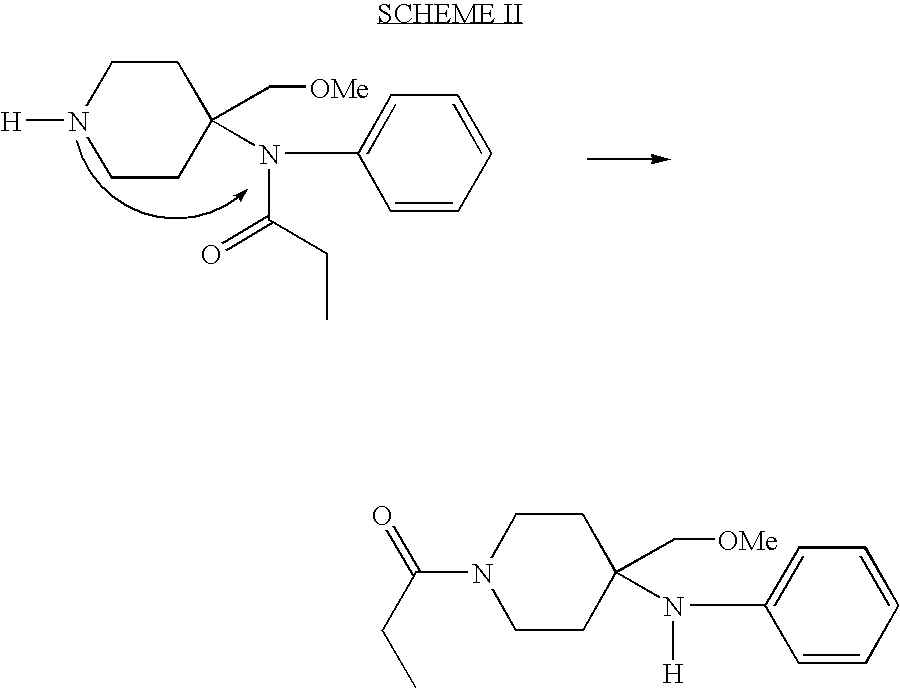
Self adaptive cement systems
InactiveUS20070137528A1Avoid seizuresInhibit migrationSolid waste managementDrilling compositionSelf-healingChemical products
A self-adaptive cement system includes cement, water and at least one additive that reacts or and expands in contact with oil and gas. Several chemical products have been identified including rubber alkylstyrene, polynorbornene, resins such precrosslinked substituted vinyl acrylate copolymers and diatomaceous earth. These additives have the effect of making the cement self-healing in the event of physical failure or damage such as micro-annuli. The self healing property is produced by the contact with subterranean hydrocarbon fluids, the potential repair mechanism is thus activated if and when needed in case of start of loss of zonal isolation. In another embodiment, the expansion is deliberately induced by pumping a hydrocarbon fluid in the vicinity of the set cement.
Owner:SCHLUMBERGER TECH CORP
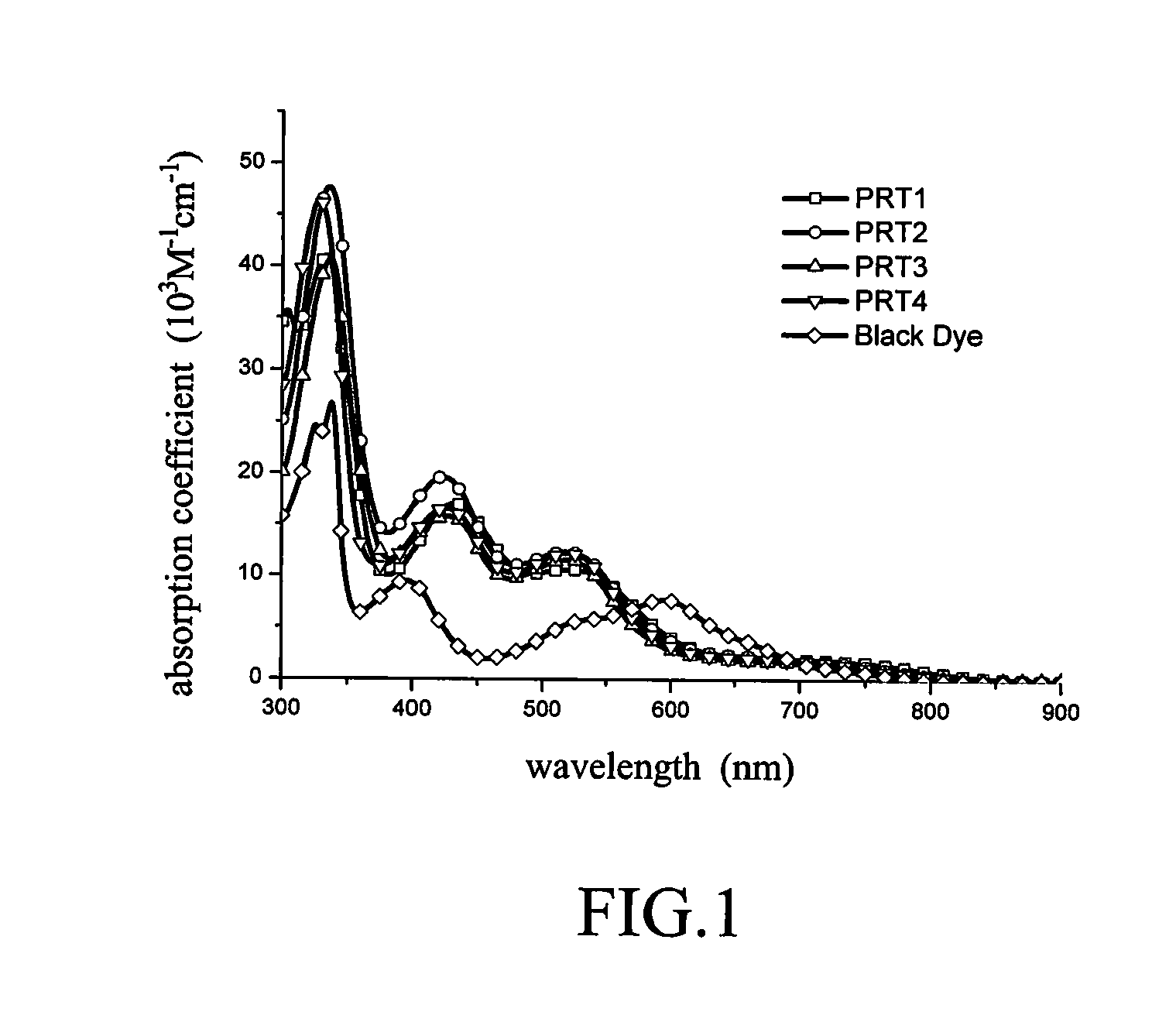
Panchromatic photosensitizers and dye-sensitized solar cell using the same
InactiveUS20100258175A1Improve photoelectric conversion efficiencyImprove response efficiencyRuthenium organic compoundsElectrolytic capacitorsPhosphoric acidCarboxylic acid
Panchromatic photosensitizers having a Formula of ML1L2X were synthesized, wherein M comprises ruthenium atom; X is a monodentate anion; L1 is heterocyclic bidentate ligand having one of formulae listed below:wherein G2 has one of formulae listed below:and L2 is a tridentate ligand having a formula listed below:The substituents R1, R2, R3, R4, R5, R6, R7 of L1 and L2 are the same or different, and represent alkyl, alkoxy, alkylthio, alkylamino, halogenated alkyl, phenyl or substituted phenyl group, carboxylic acid or counter anion thereof, sulfonic acid or counter anion thereof, phosphoric acid or counter anion thereof, amino-group, halogens, or hydrogen. The above-mentioned photosensitizers are suitable to use as sensitizers for fabrication of high efficiency dye-sensitized solar cell.
Owner:NATIONAL TSING HUA UNIVERSITY
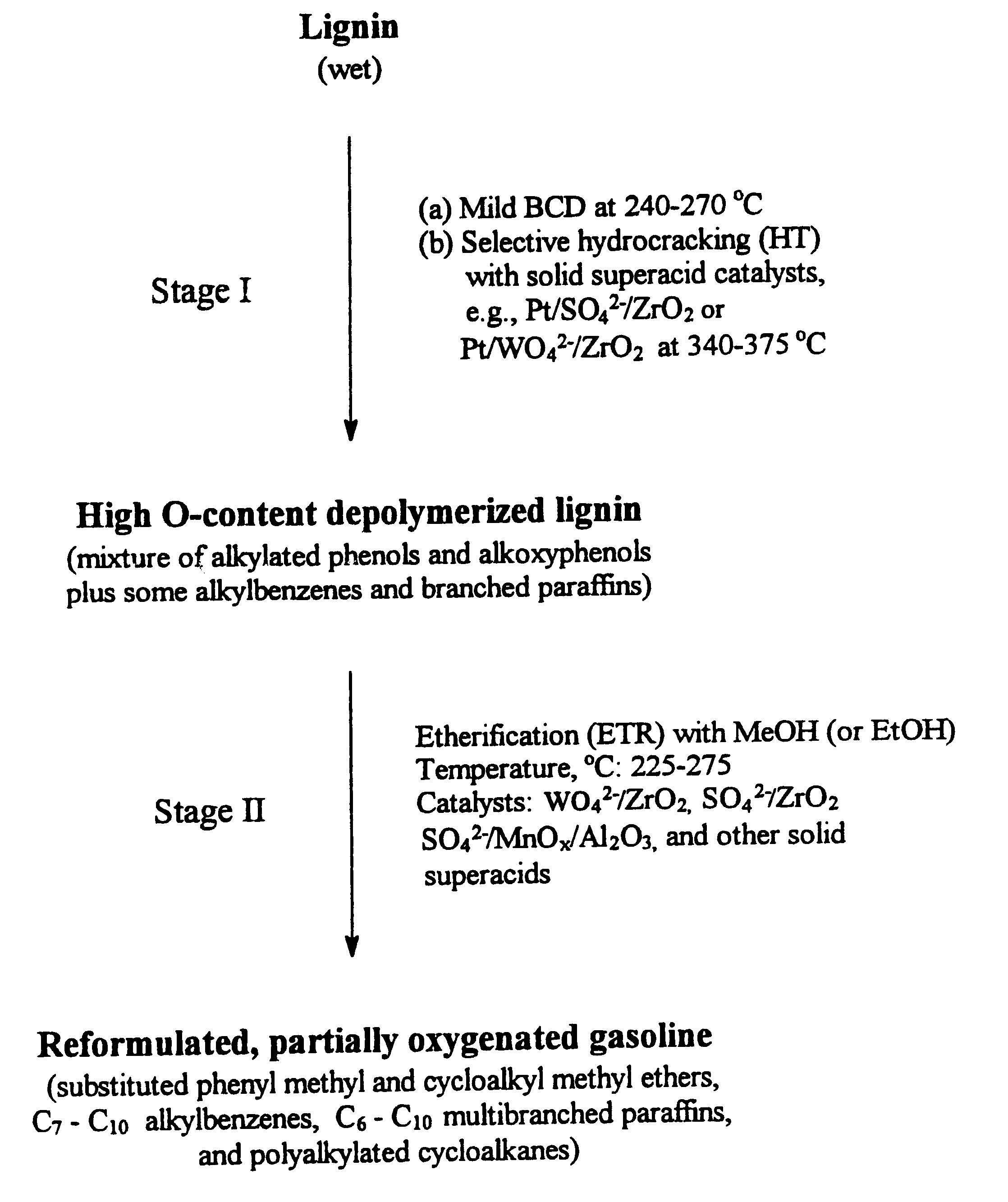
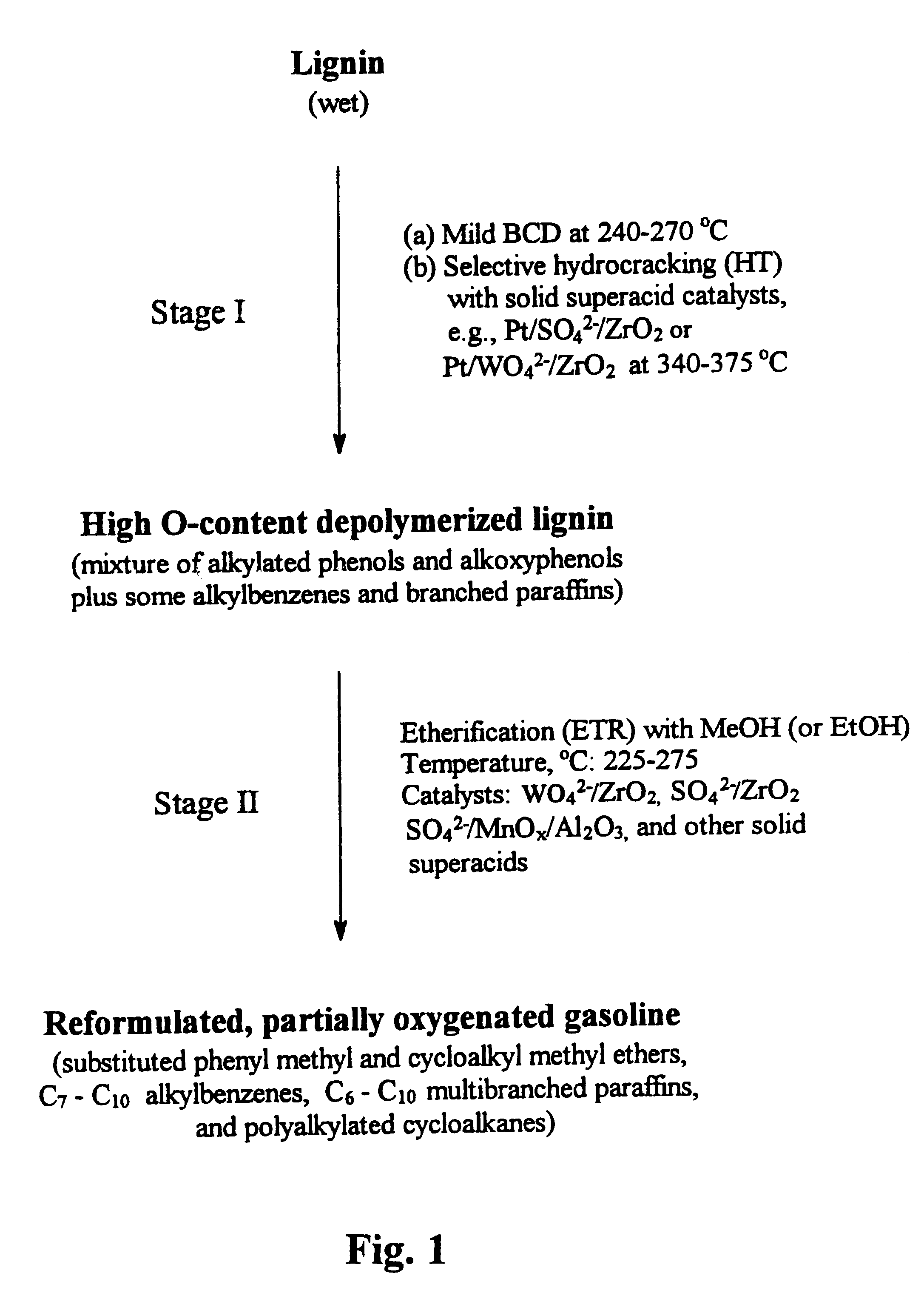
Process for conversion of lignin to reformulated, partially oxygenated gasoline
InactiveUS6172272B1Short reaction timeMaintain good propertiesOrganic compound preparationHydrocarbon from oxygen organic compoundsDepolymerizationMethyl group
A high-yield process for converting lignin into reformulated, partially oxygenated gasoline compositions of high quality is provided. The process is a two-stage catalytic reaction process that produces a reformulated, partially oxygenated gasoline product with a controlled amount of aromatics. In the first stage of the process, a lignin feed material is subjected to a base-catalyzed depolymerization reaction, followed by a selective hydrocracking reaction which utilizes a superacid catalyst to produce a high oxygen-content depolymerized lignin product mainly composed of alkylated phenols, alkylated alkoxyphenols, and alkylbenzenes. In the second stage of the process, the depolymerized lignin product is subjected to an exhaustive etherification reaction, optionally followed by a partial ring hydrogenation reaction, to produce a reformulated, partially oxygenated / etherified gasoline product, which includes a mixture of substituted phenyl / methyl ethers, cycloalkyl methyl ethers, C7-C10 alkylbenzenes, C6-C10 branched and multibranched paraffins, and alkylated and polyalkylated cycloalkanes.
Owner:ALLIANCE FOR SUSTAINABLE ENERGY +1
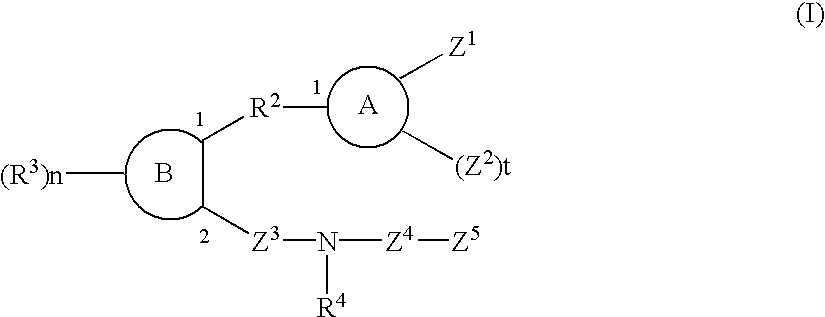
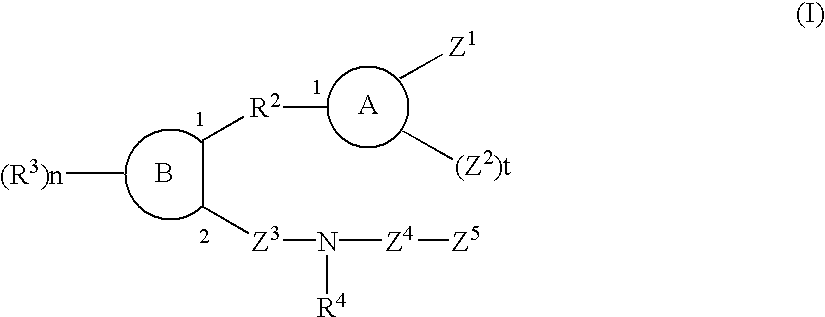
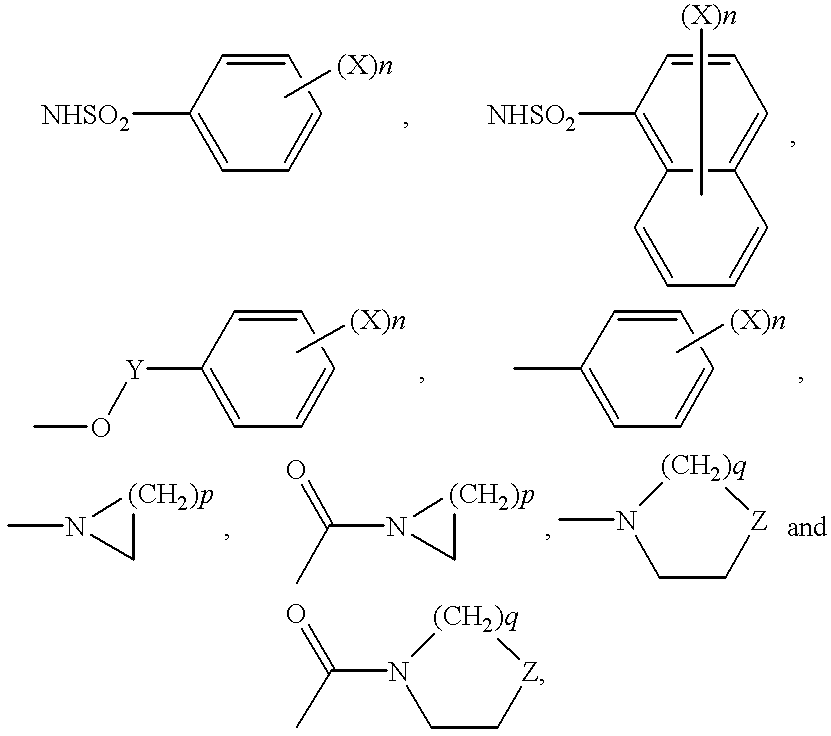
Sulfonamide and carboxamide derivatives and drugs containing the same as the active ingredient
InactiveUS6448290B1Low toxicityEasy to useBiocideOrganic chemistryBULK ACTIVE INGREDIENTAnalgesic agents
The sulfonamide or carboamide derivatives of the formula (I) and a pharmaceutical composition which comprise them as an active ingredient:(wherein A ring, B ring is carbocyclic ring, heterocyclic ring; Z1 is -COR1, -CH=CH-COR1 etc.; Z2 is H, alkyl etc.; Z3 is single bond, alkylene; Z4 is SO2, CO; Z5 is alkyl, phenyl, heterocyclic ring etc.; R2 is CONR8, O, S, NZ6, Z7-alkylene, alkylene etc.; R3 is H, alkyl, halogen, CF3 etc.; R4 is H, (substituted) alkyl etc.; n, t is 1-4).The compounds of the formula (I) can bind to receptors of PGE2 and show antagonistic activity against the action thereof or agonistic activity. Therefore, they are considered to be useful as medicine for inhibition of uterine contraction, analgesics, antidiarrheals, sleep inducers, medicine for increase of vesical capacity or medicine for uterine contraction, cathartic, suppression of gastric acid secretion, antihypertensive or diuretic agents.
Owner:ONO PHARMA CO LTD
Anionic surfactants based on alkene sulfonic acid
InactiveUS6043391AIncrease productionImprove yieldGroup 3/13 element organic compoundsFlushingAlkylphenolAlpha-olefin
New anionic surfactants and methods of preparation which are derived from aromatic or substituted aromatic molecules and alkenesulfonic acid. Wherein the aryl compound is alkylated and sulfonated in one-step with an alkene sulfonic acid prior to sulfonic acid neutralization. The methods allow the functional sulfonate group to be attached to the end of the alkyl chain rather than to the aromatic ring thus allowing for selective substituted groups, either branched, linear or alkoxylated or combinations thereof to be placed on the aryl compound prior to sulfonation and alkylation. The invention uses the alkene sulfonic acid produced from thin-film sulfonation of an alpha-olefin to alkylate benzene, mono-substituted aromatic, poly-substituted aromatic, alkylbenzene, alkoxylated benzene, polycyclic aromatic, mono-substituted polycyclic aromatic, poly-substituted polycyclic aromatic, naphthalene, alkylnaphthalene, phenol, alkylphenol, alkoxylated phenol, and alkoxylated alkylphenolalkyl substituted or polysubstituted cyclic or polycyclic compounds to produce the corresponding sulfonic acid having an additional alkyl group derived from the alpha-olefin used during the thin-film sulfonation which is either linear or branched.
Owner:OIL CHEM TECH
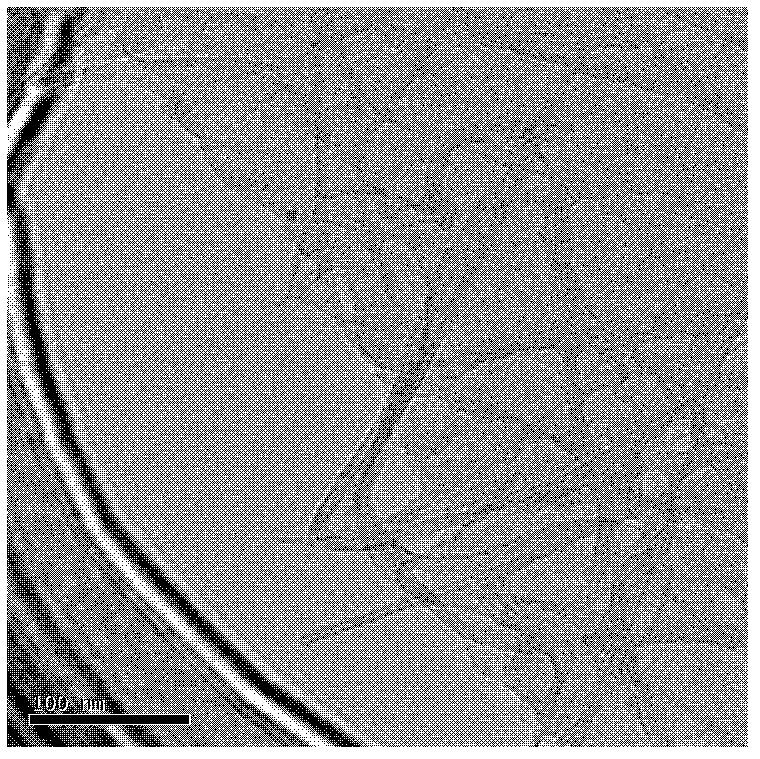
Preparation method of graphene-carbon nanotube compound film based on three-dimensional network appearance
InactiveCN102417176AImprove uniformityReduce surface resistanceNanotechnologyPolyethylene terephthalate glycolMeth-
The invention discloses a preparation method of a graphene-carbon nanotube compound film based on a three-dimensional network appearance. The method comprises the step of: transferring and stamping graphene and a carbon nanotube onto glass, a tantalum sheet, a silicon chip, a stainless steel plate or a polyethylene glycol terephthalate substrate in the mass ratio (1-10):1 through spraying deposition or vacuum suction filtration, wherein the grapheme is graphene oxide prepared by using an improved Hummers method; and a preparation method of a carbon nanotube solution comprises the following steps of: mixing acids; dispersing surfactants such as sodium lauryl sulfate, sodium dodecyl benzene sulfonate and hexadecyl trimethyl ammonium bromide in an auxiliary way, and the like. The graphene-carbon nanotube compound film prepared by adopting the method has the advantages of adjustable transmission and surface resistance, high uniformity, high stability, simple preparation method process and the like, and can be loaded on a rigid substrate as well as a flexible substrate.
Owner:TIANJIN UNIV
Stabilized hydrotreated and hydrowaxed lubricant compositions
InactiveUS6410490B1Meet the requirementsLiquid carbonaceous fuelsAdditivesSarcosinePhenolic antioxidant
The instant invention relates to a lubricant composition stabilized against the deleterious effects of heat and oxygen. The composition comprises a hydrotreated or hydrodewaxed oil and an effective antioxidant stabilizing amount of a mixture of a phenolic antioxidant; an N,N-disubstituted aminomethyl-1,2,4-triazole; an aromatic amine antioxidant; an alkyl phenoxy alkanoic acid; and an N-acyl sarcosine derivative. Optionally, further additives are added to the subject lubricant compositions.
Owner:CIBA SPECIALTY CHEM CORP
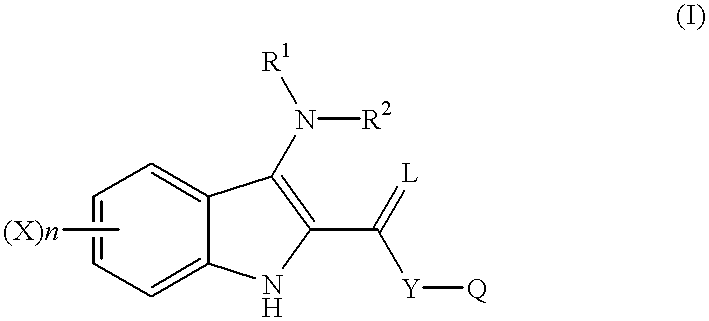
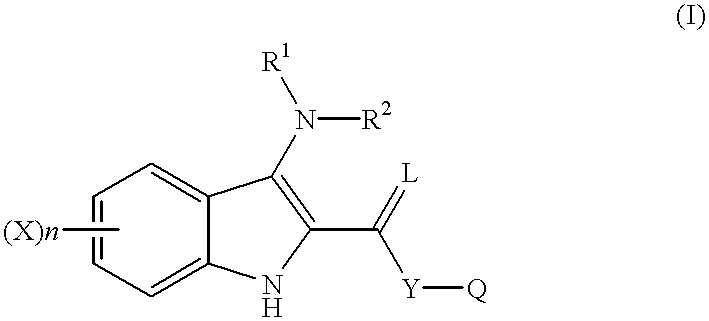
Indole compounds as COX-2 inhibitors
InactiveUS6300363B1Inhibit prostaniod-induced smooth muscle contractionInhibit synthesisBiocideOrganic chemistryDiseaseEthyl group
This invention provides a compound of the following formula:and the pharmaceutically acceptable salts thereof, wherein L is oxygen or sulfur; Y is a direct bond or C1-4 alkylidene; Q is C1-6 alkyl, C3-7 cycloalkyl, phenyl, naphthyl, heteroaryl or the like; R1 is hydrogen, C1-6 alkyl or the like; R2 is hydrogen, C1-4 alkyl, C(O)R5 wherein R5 is C1-22 alkyl or C2-22 alkenyl, halosubstituted C1-8 alkyl, halosubstituted C2-8alkenyl, -Y-C3-7 cycloalkyl, -Y-C3-7 cycloalkenyl, phenyl, naphthyl, heteroaryl or the like; X is halo, C1-4 alkyl, hydroxy, C1-4 alkoxy or the like; and n is 0, 1, 2 or 3, with the proviso that a group of formula -Y-Q is not methyl or ethyl when X is hydrogen; L is oxygen; R1 is hydrogen; and R2 is acetyl.This invention also provides a pharmaceutical composition useful for the treatment of a medical condition in which prostaglandins are implicated as pathogens.
Owner:PFIZER INC +1
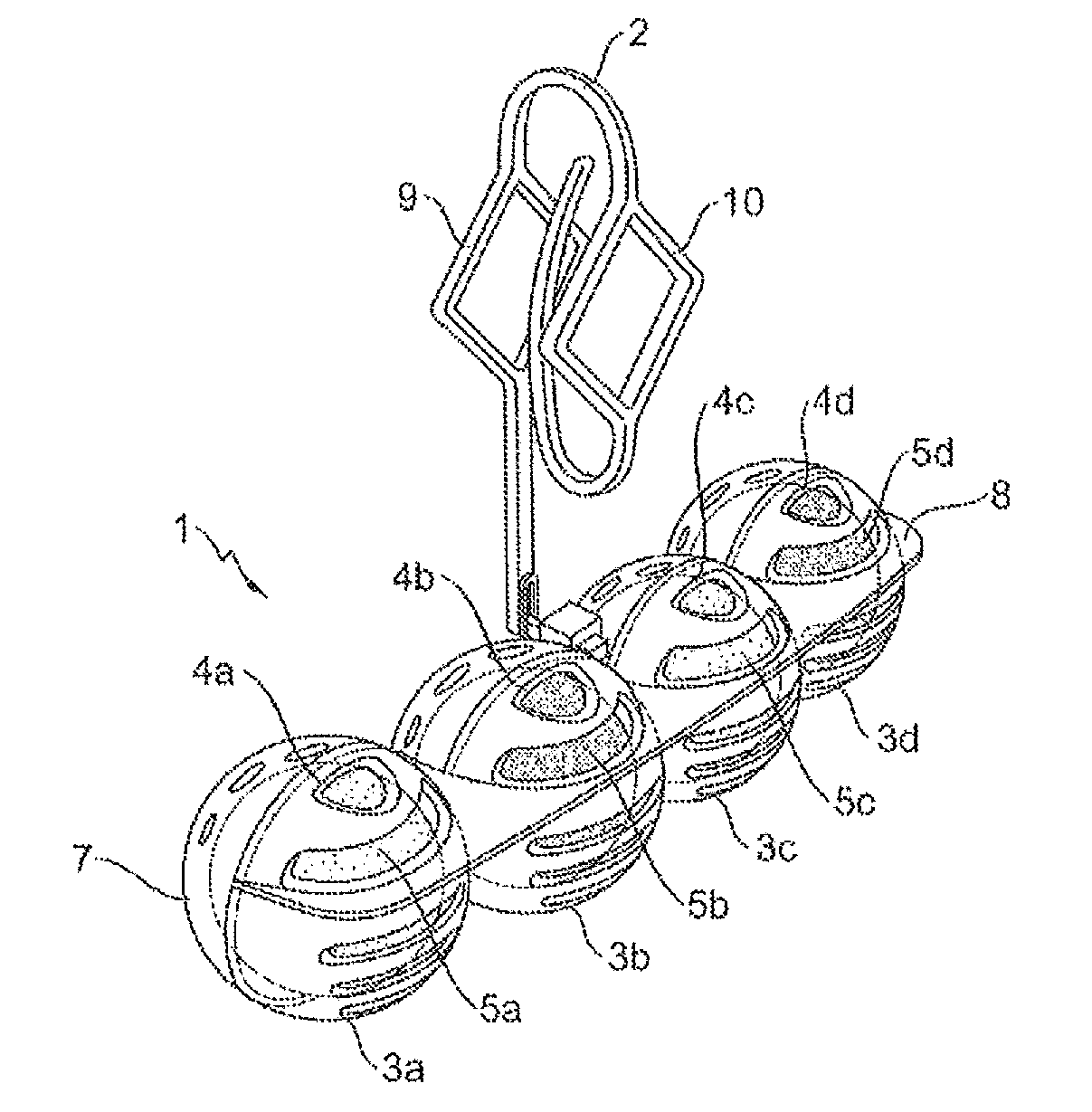
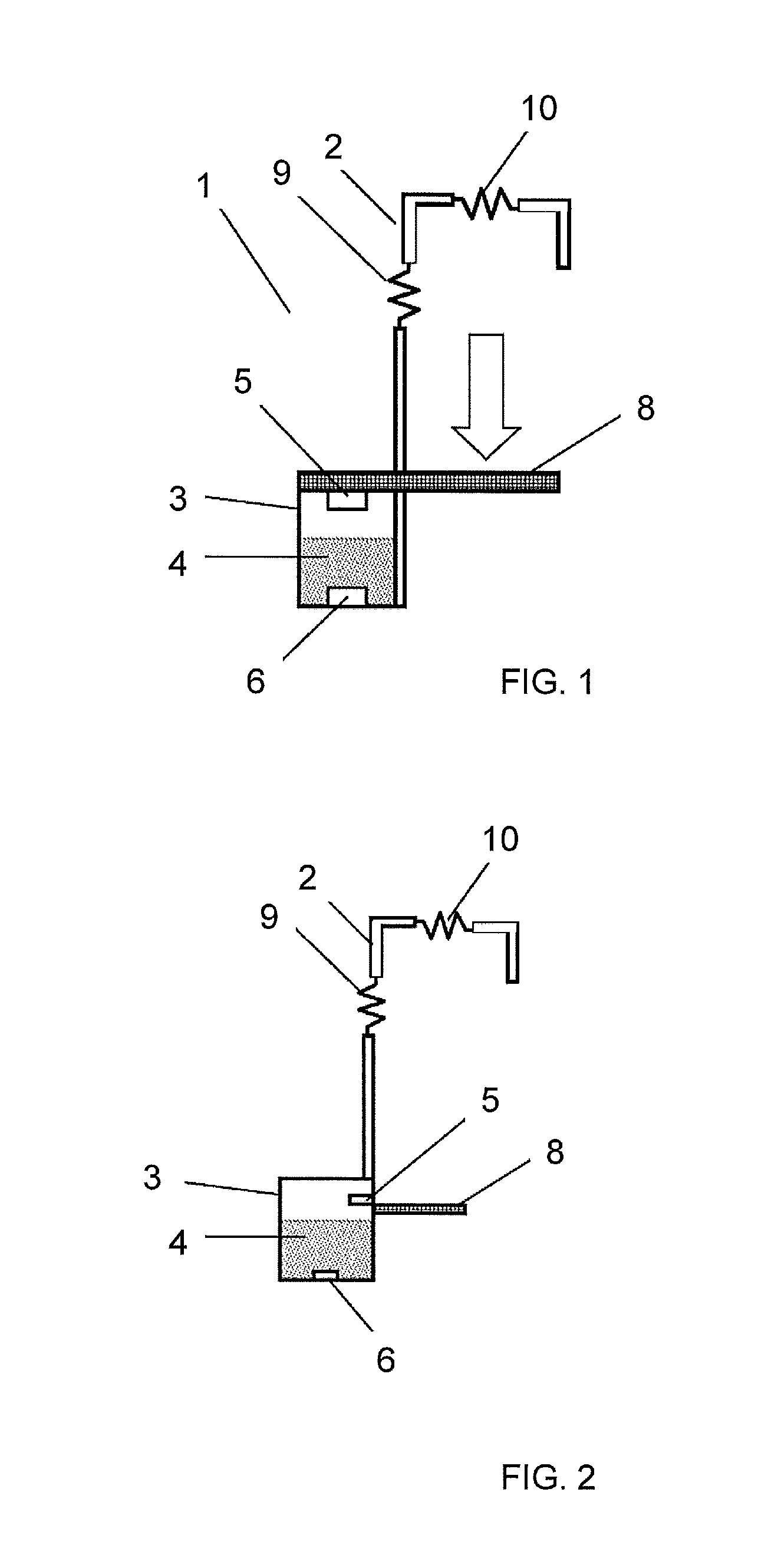
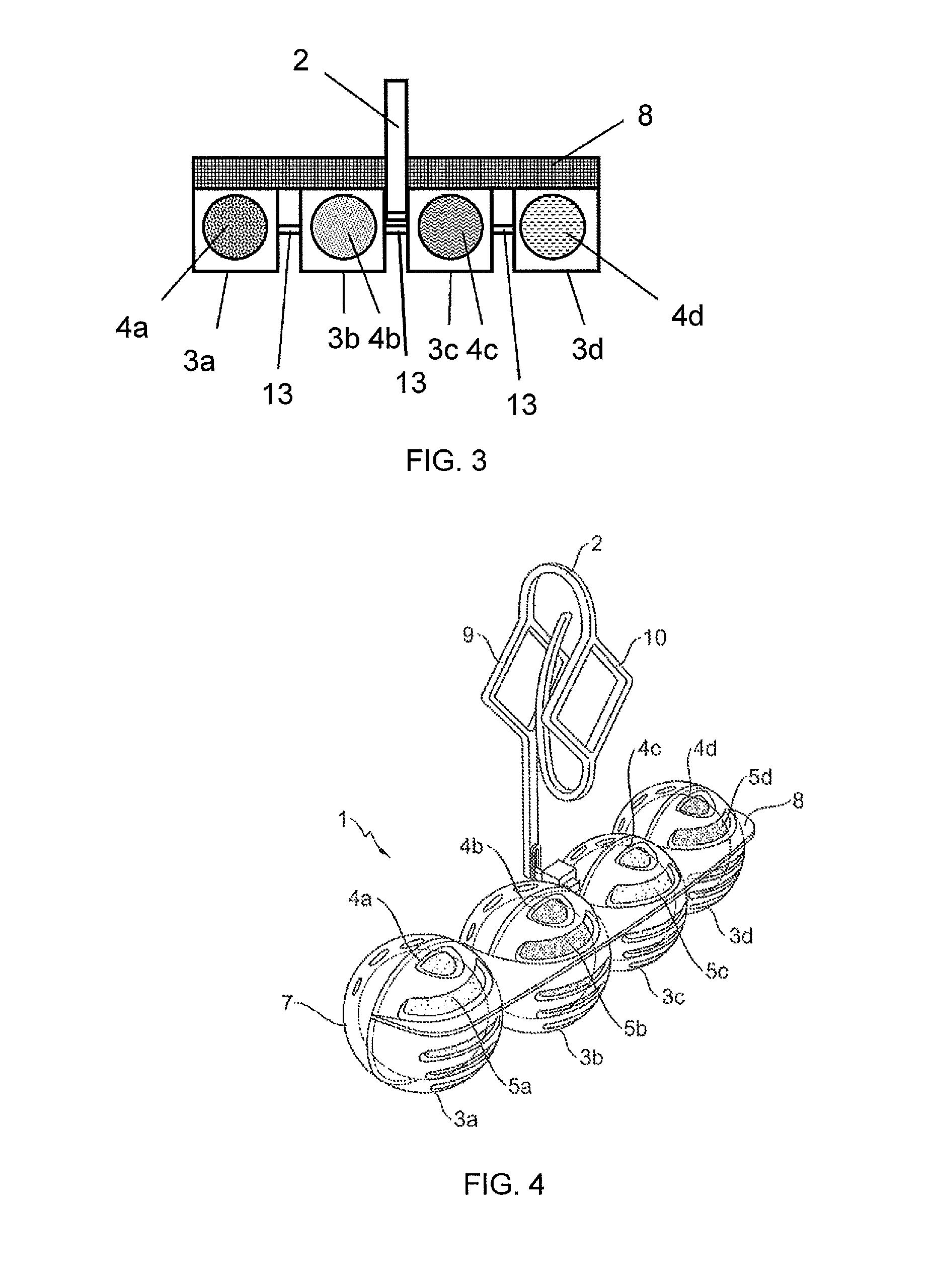
Spherical toilet cleaner blocks, method for the production thereof, and cleaning holder comprising spherical toilet cleaner blocks
ActiveUS20120047640A1Minimal exposed surface areaRetain its shapeNon-ionic surface-active compoundsLavatory sanitorySulfonateEngineering
A toilet cleaner composition containing perfume, at least one non-ionic surfactant, at least one alkylbenzene sulfonate, and at least one olefin sulfonate, is sufficiently malleable that it may be shaped into rotationally symmetrical toilet cleaner blocks, and in particular into spherical blocks, using a rolling machine or a press. The resulting rotationally-symmetrical toilet cleaner block may be part of a toilet cleaning system comprising at least one toilet cleaner block and at least one dispenser device for use in a toilet.
Owner:HENKEL KGAA
Nonaqueous electrolytic solution and lithium secondary batteries
ActiveUS20040121239A1Not inhibit overchargeImprove battery performanceOrganic electrolyte cellsLi-accumulatorsSolventBiphenyl compound
In order to manufacture a lithium secondary battery having excellent performances in safety under overcharge condition, cycle property, electric capacity, and storage endurance, 0.1 wt. % to 10 wt. % of a tert-alkylbenzene compound is favorably incorporated into a non-aqueous electrolytic solution comprising a non-aqueous solvent and an electrolyte, preferably in combination with 0.1 wt. % to 1.5 wt. % of a biphenyl compound.
Owner:UBE IND LTD
Method for preparing high-alkali value (TBN400) synthesized calcium alkyl benzene sulfonate
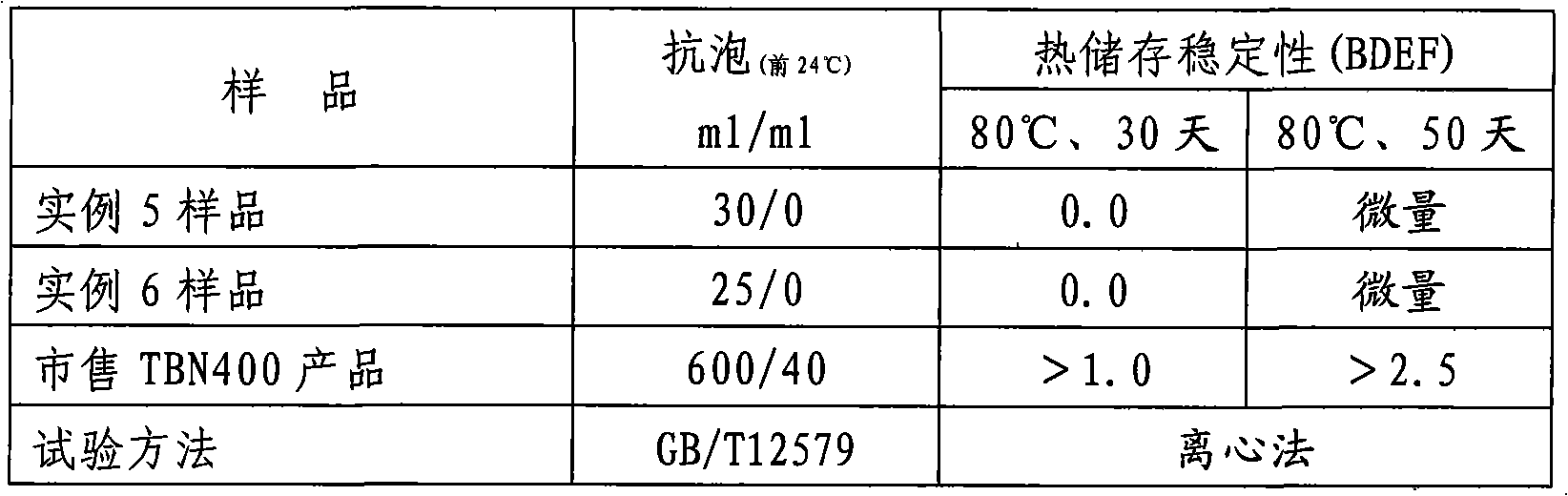
ActiveCN101318915AImprove cleanlinessGood dispersionAdditivesSulfonic acid preparationTotal Base NumberAlkaline earth metal
The invention provides a method for preparing high base number (TBN400) synthetic calcium alkyl-benzene sulfonate. The method comprises the following steps of: adopting a mixed acid of long-chain linear alkyl-benzene sulfonic acid and high-boiling heavy alkyl-benzene sulfonic acid, calcium oxide and / or calcium hydroxide, low-carbon alcohol, alkaline-earth metal halide or nitrate, and a mixture of alkaline-earth metal alkylphenol or alkaline-earth metal alkylphenate and polyisobutylene succinic anhydride for a neutralization reaction in the presence of a solvent and cutback oil at a temperature of between 40 and 80 DEG C; then, passing through carbon dioxide to a product of the neutralization reaction at a temperature of between 40 and 60 DEG C for a carbonation reaction; and producing high base synthetic alkyl-benzene sulfonate with a total base number (TBN) of 400mgKOH / g by adopting a process of a one-step method. The product is divided into high-base number (TBN400) synthetic alkyl-benzene sulfonate containing chlorine and high-base number (TBN400) synthetic alkyl-benzene sulfonate without the chlorine. The product produced by adopting the method with low viscosity, small turbidity, easy filtration, light color and no skin formation has the advantages of excellent high-temperature detergency, excellent anti-foaming property and excellent heat storage stability.
Owner:JINZHOU DPF TH CHEM CO LTD
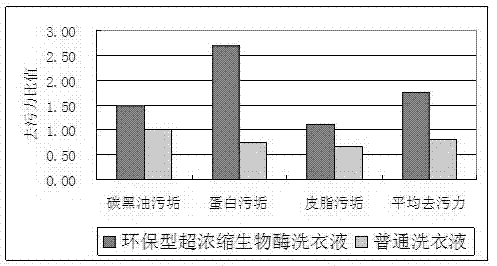
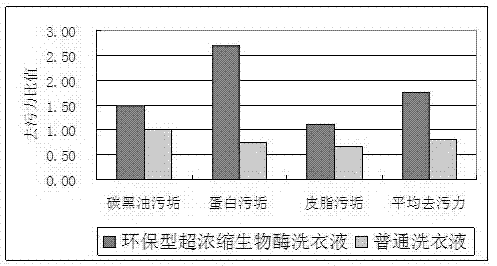
Environment-friendly type hyperconcentration biological enzyme liquid laundry detergent and preparation method thereof
ActiveCN102242022ANo pollution in the processReduce consumptionNon-ionic surface-active compoundsDetergent mixture composition preparationHydrotropePhosphate
The invention relates to a detergent for daily use, and specifically relates to an environment-friendly type hyperconcentration biological enzyme liquid laundry detergent and a preparation method thereof. The prepared product in the invention is mobile liquid with low viscosity, contains no phosphates, fluorescent brightening agents or alkylphenol polyoxyethylene, and has no pollution to environment. The production process for the product is simple; preparation and mass production of the product are easy; the product can be widely used for washing of various fabrics and clothing and is both applicable to hand wash and machine wash. The environment-friendly type hyperconcentration biological enzyme liquid laundry detergent comprises, by weight, 0.5 to 50 parts of fatty alcohol polyoxyethylene ether sodium sulfate-70, 0.5 to 50 parts of fatty alcohol polyoxyethylene ether-9, 0.5 to 30 parts of sodium alkylbenzene sulfonate-60, 0.2 to 15 parts of triethanolamine, 0.1 to 10 parts of sodium citrate, 0 to 5 parts of citric acid, 0 to 5 parts of aliphatic acid, 0.02 to 8 parts of biological enzyme, 0.1 to 20 parts of an enzyme stabilizer, 0.5 to 30 parts of a solvent, 0 to 15 parts of a hydrotropic agent, 0 to 5 parts of a bactericide, 0.001 to 5 parts of an antiseptic, 0 to 3 parts of essence and 1.0 to 40 parts of softened water.
Owner:上海开米科技有限公司
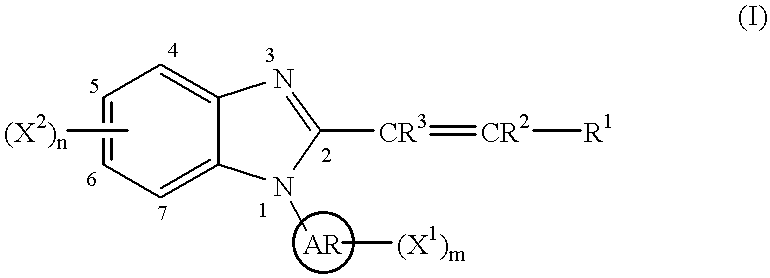
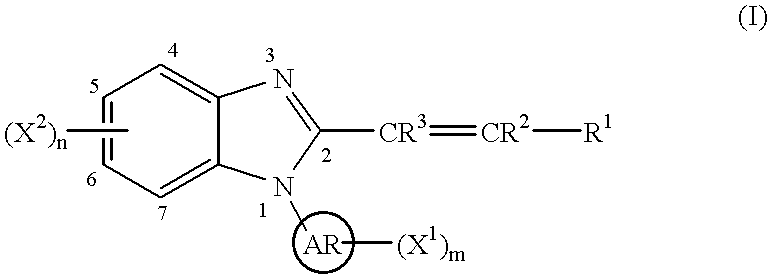
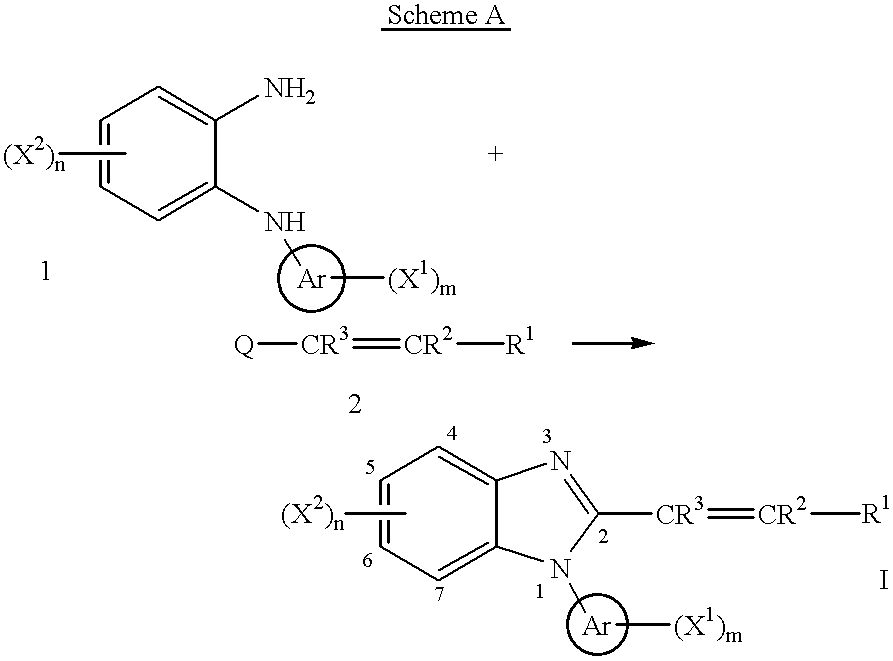
Benzimidazole cyclooxygenase-2 inhibitors
This invention provides a compound of the following formula:or the pharmaceutically acceptable salts thereof, whereinAr is heteroaryl; X1 and X2 are independently selected from halo, C1-C4 alkyl, hydroxy, C1-C4 alkoxy, amino, C1-C4 alkanoyl, carboxy, carbamoyl, cyano, nitro, mercapto, (C1-C4 alkyl)thio, (C1-C4 alkyl)sulfinyl, (C1-C4 alkyl)sulfonyl, aminosulfonyl, or the like; R1 is selected from hydrogen, straight or branched C1-C4 alkyl, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, phenyl , heteroaryl and the like; R2 and R3 are independently selected from hydrogen, halo, C1-C4 alkyl, phenyl and the like; or R1 and R2 can form, together with the carbon atom to which they are attached, a C5-C7 cycloalkyl ring; and m and n are independently 0, 1, 2 or 3.These compounds and pharmaceutical compositions containing such compounds are useful as analgesics and anti-inflammatory agents.
Owner:PFIZER INC
Coal slime flotation collector and preparation method thereof
The invention discloses a coal slime flotation collector and a preparation method thereof. The coal slime flotation collector comprises the following matters in percentage by weight: 20-50 percent of kerosene and / or light diesel oil, 1-5 percent of primary emulsion, 0.006-0.015 percent of auxiliary emulsion and the balance of water; wherein the primary emulsion is a mixture of polyoxyethylene sorbitan fatty acid ester and dehydrated sorbitol fatty acid ester, and the hydrophile-lipophile balance (HLB) value of the primary emulsion is within 12.8-14.3; the auxiliary emulsion is selected from the following (1) or (2), wherein the (1) is sodium dodecyl benzene sulfonate, and the (2) is a mixture obtained by mixing fatty alcohol polyoxyethylene ether sulfate and the sodium dodecyl benzene sulfonate according to the mass ratio of 1: 0.5-2. The coal slime flotation collector has good stability, simple preparation process and 40-60 percent of the oil-saving ratio on the premise of improving the float yield and the tail coal ash proportion. The collector is beneficial to saving the energy, reducing the emission and improving the economical benefit when being used for floating the coal slime.
Owner:SHANXI MEDICAL UNIV
Completely fused paper soap and its making process
InactiveCN1357613AImprove solubilityImprove decontamination abilityDetergent materialsPhenolFatty alcohol
The component of the paper soap include carboxymethyl cellulose sodium, fatty alcohol polyioxymethyl ethyleneethere sodium sulfate, sodium dodecyl benzene sulfonate, lauryl sodium sulfate, cocinin diethanolamide, nonly phenol polyoxyethyleneether, glycerine, quaternary ammonium salt and citric acid. Its preparation includes preparing carboxymethyl cellulose sodium mother liquor, adding the mixed solution of other components to prepare solution through stirring, spraying the prepared solution of PVC plate and drying in a sealed room and moisture extract at 35-40 deg.c to form film.
Owner:成都洁利康实业发展有限公司
Thermoplastic elastomer composition
A thermoplastic elastomer composition comprising (I) 100 parts by weight of at least one block copolymer formed by addition polymerization which is selected among block copolymers which comprise a polymer block (A) consisting of aromatic vinyl units and a polymer block (B) consisting of conjugated diene compound units and which have been crosslinked in the polymer block (A) preferably with a structural unit derived from a (C1-C8 alkyl)styrene and / or functional group and among products of hydrogenation of the copolymers, (II) 10 to 300 parts by weight of a polyolefin, and (III) 0 to 300 parts by weight of a softener (III) for rubbers; and a process for producing the composition. The composition has excellent strain recovery at high temperatures and satisfactory moldability, is flexible, and has satisfactory rubber properties. It is effectively useable in various applications.
Owner:KURARAY CO LTD
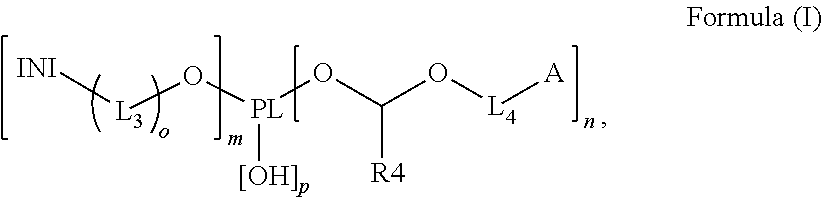
Polymerizable polymeric photoinitiators and radiation curable compositions
InactiveUS20120046376A1Simple and cost efficient procedureAvoiding unecological removal of solventInksVinyl etherPhosphine oxide
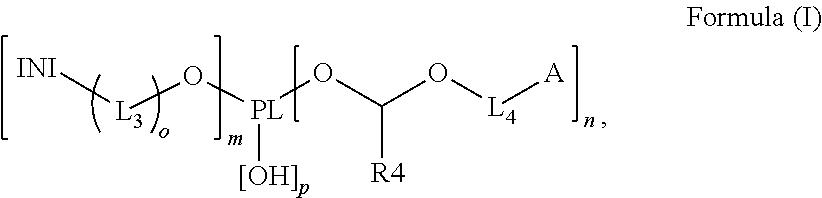
A polymerizable polymeric photoinitiator according to Formula (I):wherein:PL represents an n+m+p-functional polymeric core;n and m independently represent an integer from 1 to 30;p represents an integer from 0 to 10;o is 0 or 1;INI represents a group selected from the group consisting of a benzophenone, a thioxanthone, a carbazole, a anthraquinone, a camphor quinone, an α-hydroxyalkylphenone, an α-aminoalkylphenone, an acylphosphine oxide, a bisacyl phosphine oxide, an acylphosphine sulfide, a phenyl glyoxalate, a benzoin ether, a benzyl ketal, an α-dialkoxyacetophenone, a carbazolyl-O-acyl-oxime, an α-haloarylketone and an α-haloaryl sulfone;L3 and L4 represent a substituted or unsubstituted divalent linking group comprising 1 to 14 carbon atoms;A represents a radically polymerizable functional group selected from the group consisting of an acrylate, a methacrylate, a styrene, an acryl amide, a methacryl amide, a maleate, a fumarate, an itaconate, an vinyl ether, an allyl ether, an allyl ester, a maleimide, a vinyl nitrile and a vinyl ester; andR4 represents a substituted or unsubstituted alkyl group.Radiation curable compositions containing the polymerizable polymeric photoinitiator and methods for preparing the polymerizable polymeric photoinitiator are also disclosed.
Owner:AGFA NV
Method for producing hydrocarbon fractions
InactiveUS20090314683A1Reduced activityReduce environmental loadMolecular sieve catalystsHydrocarbon oil crackingPolycyclic aromatic hydrocarbonHydrogen
A method for producing an LPG fraction, a gasoline fraction, a kerosene fraction, a gas oil fraction, monocyclic aromatic hydrocarbon and a non-aromatic naphtha fraction from hydrocracked oil includes hydrocracking hydrocarbon oil containing polycyclic aromatic hydrocarbon to convert into a light hydrocarbon fraction, and efficiently and selectively producing monocyclic aromatic hydrocarbon with higher valuable alkylbenzenes. The method for producing hydrocarbon fraction comprises subjecting hydrocarbon feedstock containing polycyclic aromatic hydrocarbon and in which the ratio of carbons constituting an aromatic ring to the total carbons in the hydrocarbon oil (the aromatic ring-constituting carbon ratio) is 35 mole % or more to catalytic cracking in the presence of hydrogen. 40% or more of a fraction with a boiling point of 215° C. or higher in the hydrocarbon feedstock is converted into a fraction with a boiling point lower than 215° C., producing hydrocracked oil containing 30 vol % or more of monocyclic aromatic hydrocarbon.
Owner:JAPAN ENERGY CORP
Method of producing alkylbenzene and catalyst used therefor
InactiveUS20120000819A1Increase added valueInhibit productionAluminium compoundsMolecular sieve catalystsAlkaneAcid strength
A method that efficiently produces an alkylbenzene with a high added value from a 1.5-cyclic aromatic hydrocarbon while suppressing excessive hydrocracking and nuclear hydrogenation, and preventing a decrease in catalytic activity due to deposition of carbon during a hydrocracking reaction, and a catalyst used therefor, are disclosed. A method of producing an alkylbenzene includes causing a hydrocarbon oil feedstock containing an alkylbenzene content of less than 20 vol %, a bicyclic aromatic hydrocarbon content of less than 30 vol %, and a 1.5-cyclic aromatic hydrocarbon content of 25 vol % or more to come in contact with a hydrocracking catalyst that includes a solid acid having a maximum acid strength of Brönsted acid of 110 kJ / mol or more and less than 140 kJ / mol.
Owner:GASOLINEEUM ENERGY CENT FOUND +1
Grinding aid for slag cement
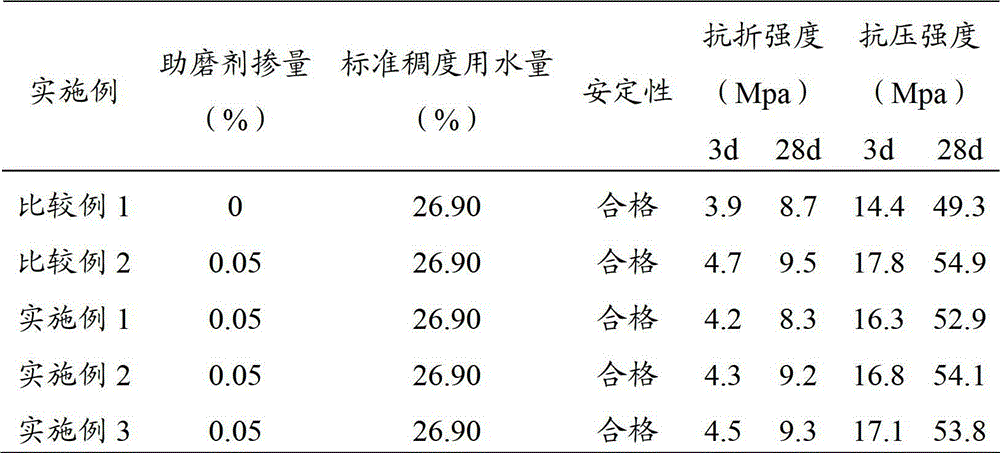
The invention provides a grinding aid for slag cement. The grinding aid provided by the invention comprises the following components by weight percent: 5-20wt% of alcohol amine compound, 0.1-5wt% of hydroxypropyl methyl cellulose, 5-30wt% of polyhydric alcohol compound, 0.5-10wt% of sodium hexametaphosphate, 1-10wt% of soluble sulfate, 1-8wt% of sodium dodecyl benzene sulfonate and the balance of water. In the grinding agent provided by the invention, the hydroxypropyl methyl cellulose is beneficial for stimulating the activity of the slag, forming stable hydrous products in the reaction process, improving the property of the cement, improving the strength of the cement and improving the stability of the liquid grinding aid at the same time. Experimental result shows that the grinding aid is capable of increasing the breaking strength of the cement to 9.5MPa and increasing the compressive strength to about 55MPa.
Owner:SHANDONG HONGYI TECH +1
Multilayer imageable elements
InactiveUS7186482B2Increase press run lengthIncrease the lengthPhotosensitive materialsRadiation applicationsN-phenylmaleimideHydrogen
Multilayer, positive working, thermally imageable, bakeable imageable elements have a substrate, an underlayer, and a top layer. The underlayer comprises a polymeric material that comprises, in polymerized form from about 5 mol % to about 30 mol % of recurring units derived from an ethylenically unsaturated polymerizable monomer having a carboxy group; from about 20 mol % to about 75 mol % of recurring units derived from N-phenylmaleimide, N-cyclohexylmaleimide, N-benzylmaleimide, or a mixture thereof; and from about 3 mol % to about 50 mol % of recurring units derived from a compound represented by the formula:CH2═C(R2)—C(O)—NH—CH2—OR1,in which R1 is C1 to C12 alkyl, phenyl, C1 to C12 substituted phenyl, C1 to C12 aralkyl, or Si(CH3)3; and R2 is hydrogen or methyl. Other materials, such as a resin or resins having activated methylol and / or activated alkylated methylol groups, such as a resole resin, may be present in the underlayer. The elements can be used to produce bakeable lithographic printing plates that are resistant to press chemistries.
Owner:EASTMAN KODAK CO
Boiler coal combustion-improving desulfurizing and denitrifying agent composition and preparation method thereof
The invention provides a boiler coal combustion-improving desulfurizing and denitrifying agent composition. The composition comprises the following raw materials in parts by weight: 2-7 parts of sodium carbonate, 1-3 parts of alumina, 2-8 parts of aluminium hydroxide, 2-5 parts of ferric trichloride, 2-6 parts of ferric oxide, 3-10 parts of potassium permanganate, 3-10 parts of potassium chlorate, 10-35 parts of activated attapulgite clay, 15-30 parts of urea, 2-4 parts of ammonium formate, 2-4 parts of ammonium chloride, 6-23 parts of ammonium acetate, 3-9 parts of manganese oxide, 9-12 parts of copper chloride, 1-3 parts of copper oxide, 2-4 parts of zinc sulfate, 1-3 parts of zinc nitrate, 7-18 parts of potassium dichromate, 1.0-1.5 parts of titanium dioxide, 0.5-1.0 part of barium molybdate, 0.5-1.5 parts of cobalt sulfate, 0.5-1.5 parts of vanadium pentoxide, 0.3-0.7 part of cerium oxide, 0.1-0.2 part of sodium dodecyl benzene sulfonate and 0.1-0.2 part of alkyl glyceryl ether. The composition is convenient to use, has stable properties, plays roles of combustion improving, desulfurization and denitrification, has coal saving rate of 8-25% and can remove fixed sulfur by 50-70%.
Owner:兰州熙瑞化工科技有限公司
Silane composition, silicon film forming method and solar cell production method
InactiveUS20060185712A1Increase the areaFormed with easeSilicon organic compoundsPV power plantsSilyleneSilanes
A silane composition for preparing a semiconductor thin films of a solar cell is disclosed. The silane composition contains a polysilane compound represented by the formula SinRm (n is an integer of 3 or more, m is an integer of n to (2n+2) and an m number of R's are each independently a hydrogen atom, alkyl group, phenyl group or halogen atom, with the proviso that when all the m number of R's are hydrogen atoms and m=2n, n is an integer of 7 or more), and at least one silane compound selected from cyclopentasilane, cyclohexasilane and silylcyclopentasilane.
Owner:JSR CORPORATIOON
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
Panchromatic photosensitizers and dye-sensitized solar cell using the same
InactiveUS20120111410A1Improve photoelectric conversion efficiencyImprove response efficiencyRuthenium organic compoundsElectrolytic capacitorsHydrogenPhotosensitizer
Panchromatic photo sensitizers having a Formula of ML1L2X were synthesized, wherein M represents ruthenium atom; X represents a monodentate anion; L1 is heterocyclic bidentate ligand having one of formulae listed below:wherein G2 has one of formulae listed below:and L2 is a tridentate ligand having a formula listed below:Substituents R1, R2, R3 and R4 of L1 are the same or different and are selected from the group consisting of hydrogen, halogens, amino-group alkyl, alkoxy, alkylthio, alkylamino, halogenated alkyl, phenyl and substituted phenyl group. Substituents R5, R6 and R7 of L2 are the same or different and are selected from the group consisting of carboxylic acid and counter anion thereof, sulfonic acid and counter anion thereof, phosphoric acid and counter anion thereof. The above-mentioned photosensitizers are suitable to use as sensitizers for fabrication of high efficiency dye-sensitized solar cell.
Owner:NATIONAL TSING HUA UNIVERSITY
Non-toxic anti-pollution paint for sea and method for preparing same
InactiveCN101333348AEasy to buyEasy to synthesizeAntifouling/underwater paintsPaints with biocidesHypochloriteElectrolysis
Nontoxic marine anti-fouling dope and a preparation method thereof are disclosed, belonging to technical fields of anti-fouling dope and underwater dope. The nontoxic marine anti-fouling dope contains film forming matter, nontoxic antifouling compound, dye, addition agent and water. The nontoxic antifouling compound is pre-dispersed slurry prepared by adding super-fine tourmaline powder and nano zinc oxide powder into 10 times amount of water, coating with sodium alkylbenzene sulfonate while heating and stirring. The nontoxic marine anti-fouling dope can be prepared through the steps of adding the nontoxic antifouling compound into ethanol aqueous solution, adjusting pH value to be 9-12, adding silicon ester ethanol solution to react for 2-4h, bathing in water and separating, and drying. The tourmaline of the nontoxic anti-fouling dope automatically develops an electric field on the surface of the dope in the seawater to enable the seawater to electrolyse to produce hypochlorite ions, which can prevent halobios to adhere or grow on the boat and the structure. The ion membrane is approximately 10 microns and causes no environment pollution. The nontoxic marine anti-fouling dope is mainly suitable for underwater fouling prevention.
Owner:DALIAN MARITIME UNIVERSITY +1
Methods for the syntheses of alfentanil, sufentanil and remifentanil
Synthetic pathways are disclosed for synthesizing derivatives or analogs of fentanyl. Specifically set out are pathways for synthesizing alfentanil, sufentanil and remifentanil. The disclosed methods require fewer steps and produce a greater yield of product than methods reported in the prior art. The pathways to all these compounds begin with a common pathway of condensing a piperidone with a primary amine so as to form a 4-amino carboxyamino-piperidine, wherein N of said piperidone is a —N—COO—(CH2)nCH3, alkylating an N of said primary amine which was condensed with said piperidone thereby producing an N-alkyl-anilide, and hydrolyzing said —COO—(CH2)nCH3? group of said 4-amino-4-carboxyamino-piperidine following the condensation reaction so as to form a piperidine hydrolysis product. This product can then be convened to remifentanil in a 4 step reaction. Also, this hydrolysis product can be treated with a hydride to yield a 4-hydroxymthyl-piperidine which can be converted to alfentanil in 3 further steps, to sufentanil in 3 more steps, or to a variety of remifentanil analogs in two steps.
Owner:MALLINCKRODT INC
Metallographic etched process for displaying G Cr15 original austenite grain border
InactiveCN101187606AShorten the timeGood reproducibilitySurface/boundary effectPreparing sample for investigationAlcoholMetallography
A metallographic corrosion method for displaying a GCr15 original austenitic grain boundary comprises adding picric acid 5g into distilled water100ml and mixing continuously, then adding sodium dodecyl benzene sulfonate 5ml 50% and mixing, finally adding ferric chloride 2g, and using after placing for 24 hours. A sample is grinded roughly, grinded finely, polished, cleared, dried, immersed into caustic erodent for 2-5 minutes according to normal method under a quenching tempering condition until etched surface is changed into silver grey, cleaned up through flowing water, cleaned with alcohol 95%, and dried. If the sample is over-corroded, polishing paste W0.5-1.0 or metallographic polishing egent0.5-1.0 is added on silk polishing cloth, the sample is polished slightly with hands, then cleaned with alcohol 95%, and dried. According to practical condition, grain granularity measurement can adopt methods of picture contrast, grid, intercept, quantitative metallography, and the like, to assess according to relevant standards.
Owner:LUOYANG BEARING SCI & TECH CO LTD
Oligomeric organosilicon compounds, their use in rubber mixtures and for the production of shaped articles
Oligomeric organosilicon compounds are disclosed of the formula I wherein R1, R2, R3 independently of one another denote H, (C1-C4)alkyl, (C1-C4) alkoxy, (C1-C4) haloalkoxy, (C1-C4)haloalkyl, phenyl, aryl or aralkyl and Z denotes an alkylidene radical having 0-6 carbon atoms, x can be a statistical average of 1-6 and n=1-150 and . . . means that z can be bonded either to the one or the other C atom, and the particular free valency is occupied by a hydrogen. and their use in rubber mixtures and for the production of shaped articles, in particular pneumatic tires.
Owner:DEGUSSA AG
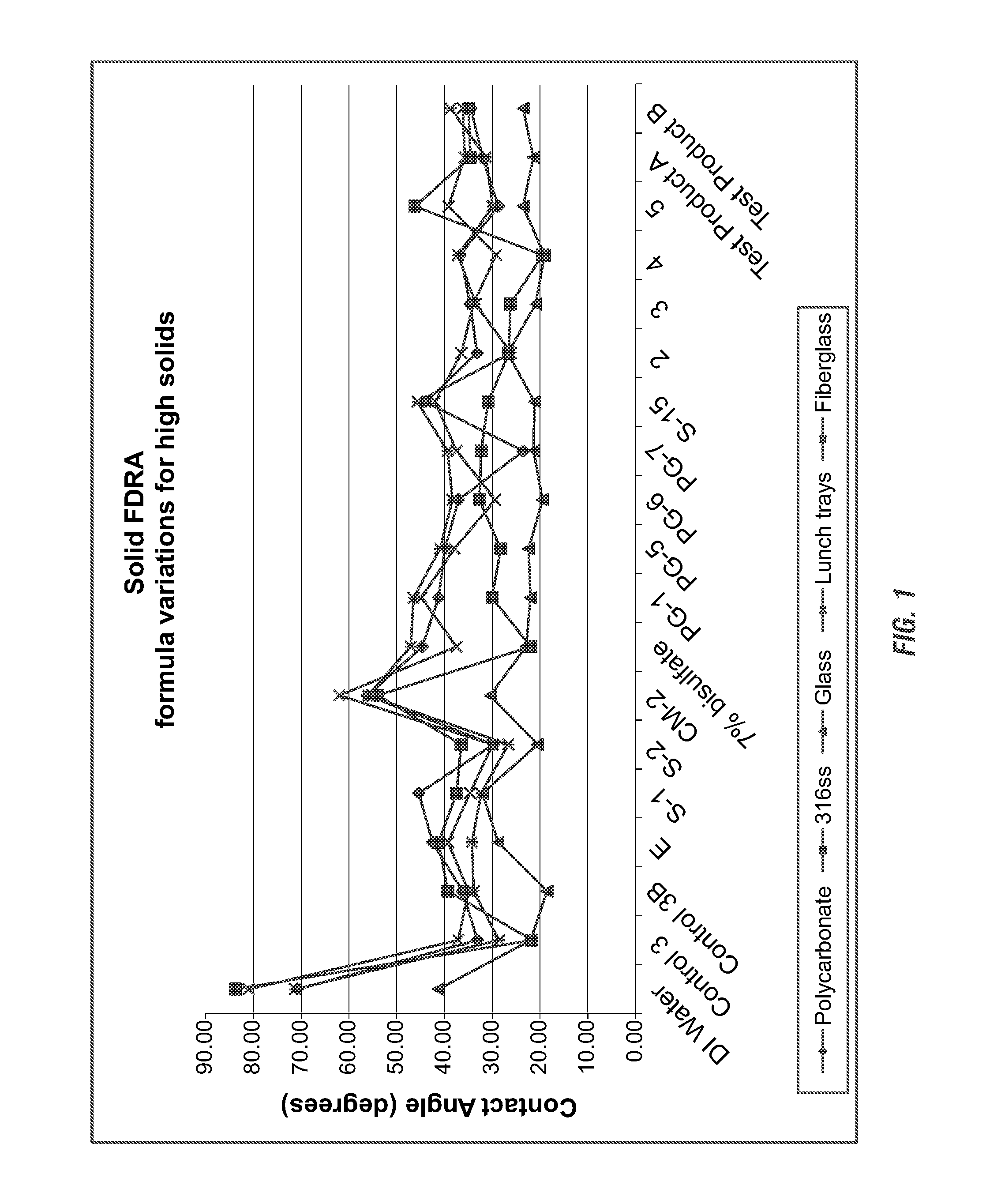
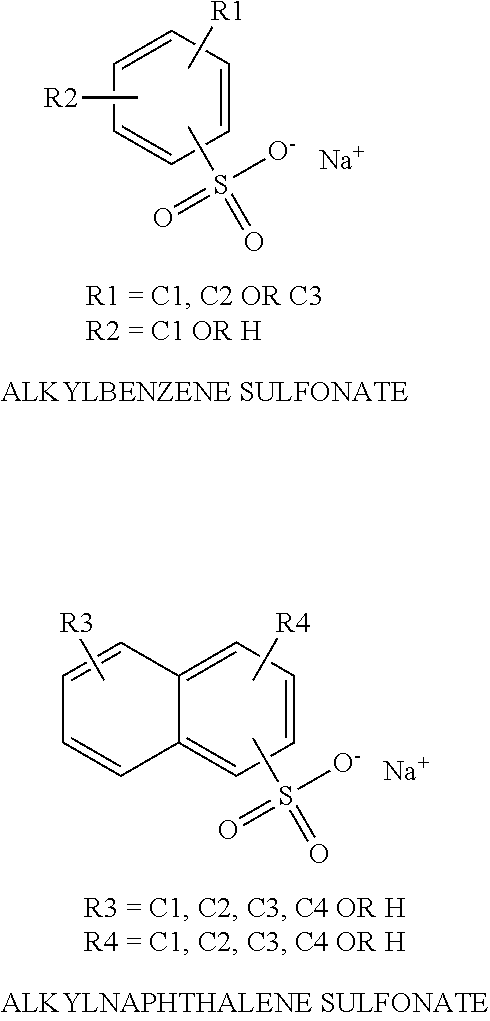
Solid fast draining/drying rinse aid for high total dissolved solid water conditions
ActiveUS20130345111A1Reducing stability of foamReduced stabilitySurface-active detergent compositionsDetergent mixture composition preparationSulfonateTotal dissolved solids
The present invention is a solid rinse aid composition and methods of making and using the same. Applicants have surprisingly found that the crystal modifier sodium xylene sulfonate (short chain alkyl benzene or alkyl naphthalene sulfonates) at higher percentage can act as a solidification agent. The solid rinse aid composition generally includes an short chain alkyl benzene or alkyl naphthalene sulfonates solidification agent and an effective amount of a surfactant which can include a sheeting agent component, defoamer component and / or association disruption agent. The solid rinse aid composition may be phosphate-free, aminocarboxylate-free, and GRAS if desired.
Owner:ECOLAB USA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com