Activity-controllable (gamma-methyl)-alpha-methylene-gamma-butyrolactone polymerizing method
A methylene and butyrolactone technology is applied in the field of active controllable polymerization catalyst systems, which can solve the problems of long reaction time, difficult to achieve complete conversion of monomers, etc., and achieves easy availability of raw materials, good chain extension, and high conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 A kind of specific synthetic method of enol silyl ether described in the present invention
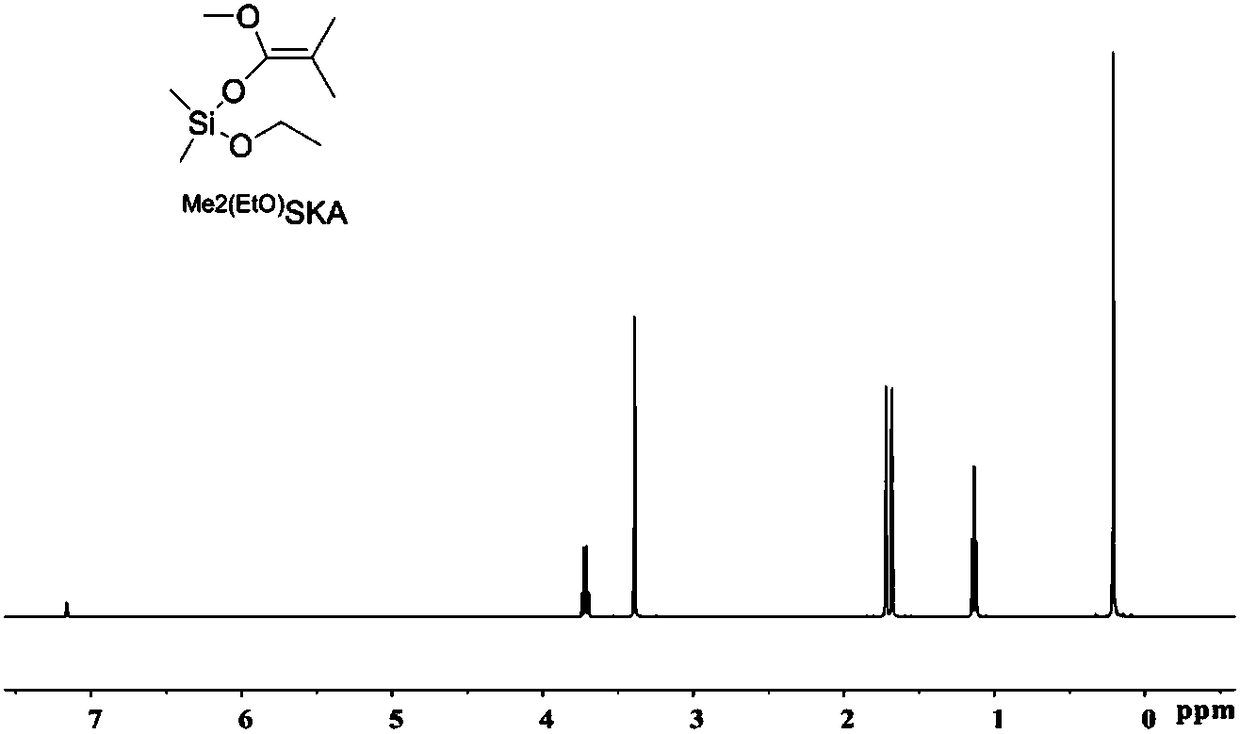
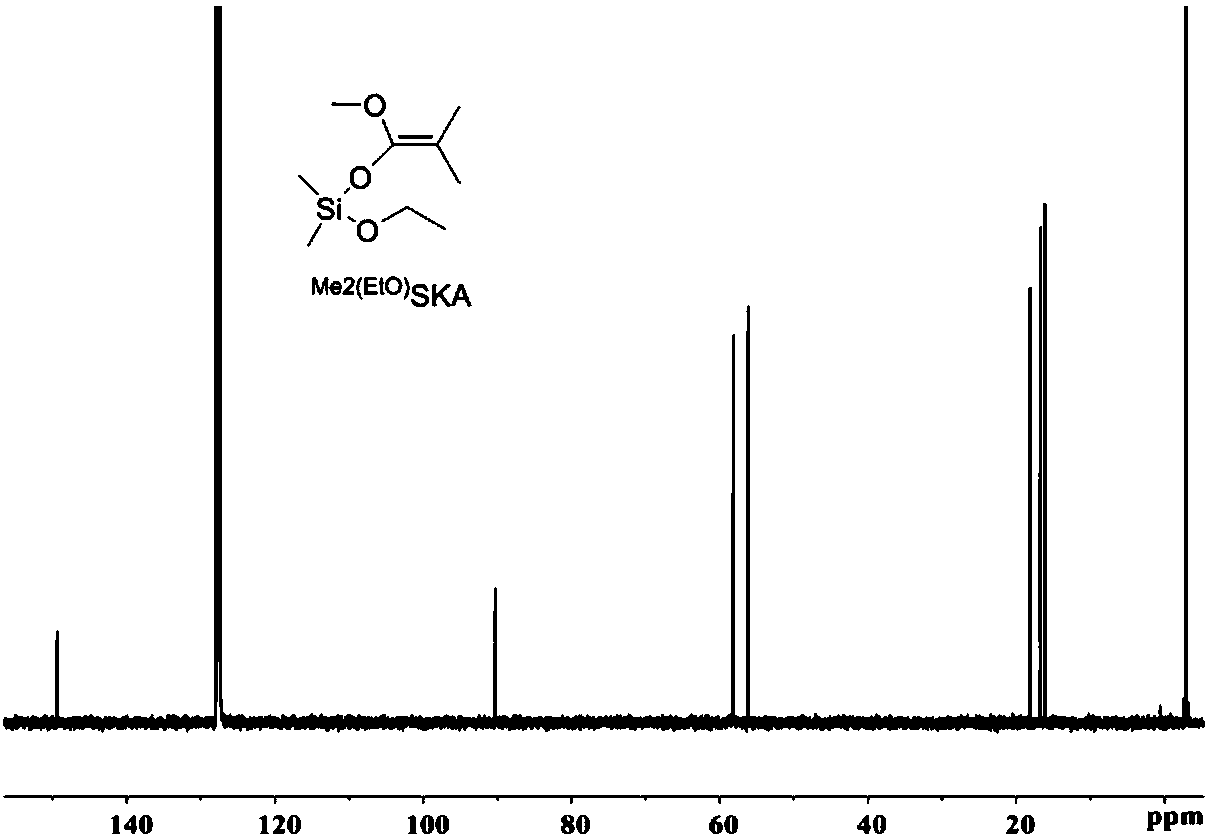
[0046] Synthesis of dimethylketene-methyl-dimethylethoxy acetal Me 2 C=C(OMe)OSiMe 2 (EtO) ( Me2 (EtO) SKA).
[0047] 1) Synthesis of dimethylethoxychlorosilane (Me 2 (EtO)SiCl)
[0048]
[0049] In a glove box filled with nitrogen, take ethanol (5.08mL, 90.0mmol) and B(C 6 f 5 ) 3 (461mg, 0.90mmol) into a 200mL Schlenk bottle, add dichloromethane (100mL) and seal it with an inversion plug, take out the glove box and connect it to the Schlenk line, cool to -78°C and keep it for 20min. Dimethylchlorosilane (10.0 mL, 90.0 mmol) was slowly added dropwise into the system via a syringe. Slowly warm up to room temperature (over 30 min), dichloromethane was removed in vacuo (be careful not to extract the product), and distillation under reduced pressure gave a colorless oily product. 12.48 g of the product was obtained with a yield of 91%. 1 H NMR (500MHz, Be...
Embodiment 2
[0053] Example 2 Polymerization of γ-methyl-α-methylene-γ-butyrolactone (MMBL) and α-methylene-γ-butyrolactone (MBL)
[0054] The polymerization reaction was carried out in a glove box, and the Lewis acid was weighed in a 20 ml reaction bottle, and MMBL (0.5ml, 4.68mmol) or MBL (0.41mL, 4.68mmol) was added. After the monomer reacted fully with the Lewis acid, two Chloromethane solvent (the total volume of the solution after adding is 5mL), add the enol silyl ether that has been weighed ( R SKA), and start timing, stir for a period of time until the monomer is completely converted, the reaction bottle is taken out from the glove box, and 5% HCl / methanol solution is added to terminate the polymerization reaction. The polymer was filtered off, washed thoroughly with methanol, and dried in vacuum at 60°C until constant weight. The molecular weight and molecular weight distribution of the resulting polymers were determined by gel permeation chromatography.
[0055] The characteri...
Embodiment 3
[0064] Example 3 Chain extension of MMBL
[0065] The polymerization reaction was carried out in the glove box, and the Al(C 6 f 5 ) 3 In a 20 ml reaction bottle, add MMBL (0.5 ml, 4.68 mmol), after the monomer and Lewis acid have fully reacted, add tetrahydrofuran solvent (the total volume of the solution after adding is 5 mL), add the weighed iBu SKA, and start timing, stir for a period of time until the monomers are completely converted, then add MMBL (0.5ml, 4.68mmol), and then add MMBL (0.5ml, 4.68mmol) after a period of time to make the monomers completely converted, and wait until all the monomers are completely converted After complete conversion of the body, the reaction bottle was taken out of the glove box, and 5% HCl / methanol solution was added to terminate the polymerization reaction. The polymer was filtered off, washed thoroughly with methanol, and dried under vacuum at 60°C to constant weight. The molecular weight and molecular weight distribution of the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com