A kind of new synthetic method of entecavir compound
A technology of entecavir and synthetic method, which is applied in the field of new synthesis of entecavir compounds, can solve the problems of high technical requirements of the method, rare raw materials, and expensive process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
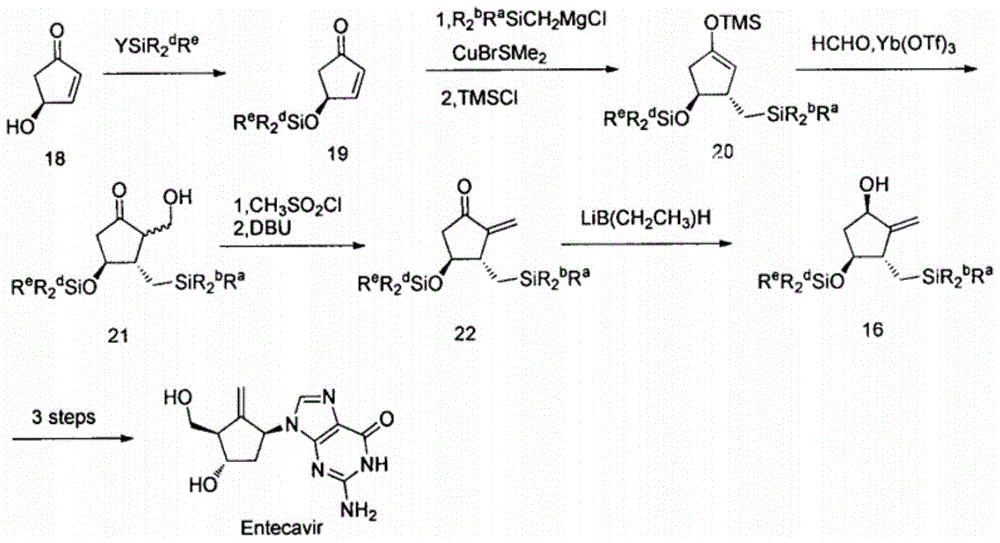
Image
Examples
Embodiment 1
[0101] 1) Synthesis of compound 2
[0102]
[0103] Dimethyl (S)-3-hydroxyadipate (190.2g, 1mol) was dissolved in 2000ml of dichloromethane, and imidazole (81.7g, 1.2mol) and TBSCl (165.8g, 1.1mol) were added sequentially. Stir at room temperature for 4 hours. The reaction was quenched by adding 1500 ml of water, and the liquid was separated. The organic phase was washed twice with 2000 ml of water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain compound 2 (289.25 g, yield 95%).
[0104] 2) Synthesis of compound 2
[0105]
[0106] Dimethyl (S)-3-hydroxyadipate (190.2g, 1mol) was dissolved in 2000ml tetrahydrofuran, and pyridine (103.1g, 1.3mol) and TBSCl (180.9g, 1.2mol) were added in sequence. The reaction solution was kept at room temperature. Stir for 3 hours. The reaction was quenched by adding 1500 ml of water, and the liquid was separated. The organic phase was washed twice with 2000 ml of water, dried over anhydrous sodium ...
Embodiment 2
[0108] 1) Synthesis of compound 3
[0109]
[0110] Compound 2 (228.35 g, 750 mmol) was dissolved in 12,500 ml of methanol, 25% sodium methoxide methanol solution (486 g, 2250 mmol) was added, and the mixture was heated to reflux for 3 hours. After the reaction, it was concentrated under reduced pressure to remove methanol. The residue was added with 5000ml 0.5M hydrochloric acid solution, extracted with 2500ml dichloromethane, the organic phase was washed twice with 2500ml water, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and crude product column chromatography (petroleum ether / ethyl acetate=80: 1) Compound 3 (147.1 g, yield 72%) was purified.
[0111] 2) Synthesis of compound 3
[0112]
[0113] Compound 2 (243.6 g, 0.8 mol) was dissolved in 12,500 ml of tert-butanol, and 25% potassium tert-butoxide (t-BuOK) solution (179.5 g, 1.6 mol) in tert-butanol was added, and heated to reflux for 2 hours. After the reaction, tert-butanol was concen...
Embodiment 3
[0115] 1) Synthesis of compound 4
[0116]
[0117] Compound 3 (136.2g, 500mmol) was dissolved in 280ml of toluene, added ethylene glycol (93.1g, 1500mmol) and p-toluenesulfonic acid monohydrate (7.6g, 40mmol), heated to reflux and separated water, and reacted for 8 hours. When there is no moisture, stop heating and cool to room temperature. Add 750 ml of saturated sodium bicarbonate solution and stir for 10 minutes, separate the organic phase, wash twice with 1000 ml of water, dry with anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain compound 4 (145.6 g, yield 92%).
[0118] 2) Synthesis of compound 4
[0119]
[0120] Dissolve compound 3 (136.2g, 500mmol) in 280ml of dichloromethane, add ethylene glycol (62.1g, 1000mmol) and pyridine hydrochloride (5.8g, 50mmol), heat to reflux and separate water, and react for 9 hours. After moisture is generated, stop heating and cool to room temperature. Add 750 ml of saturated sodium bicarbonate solution and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com