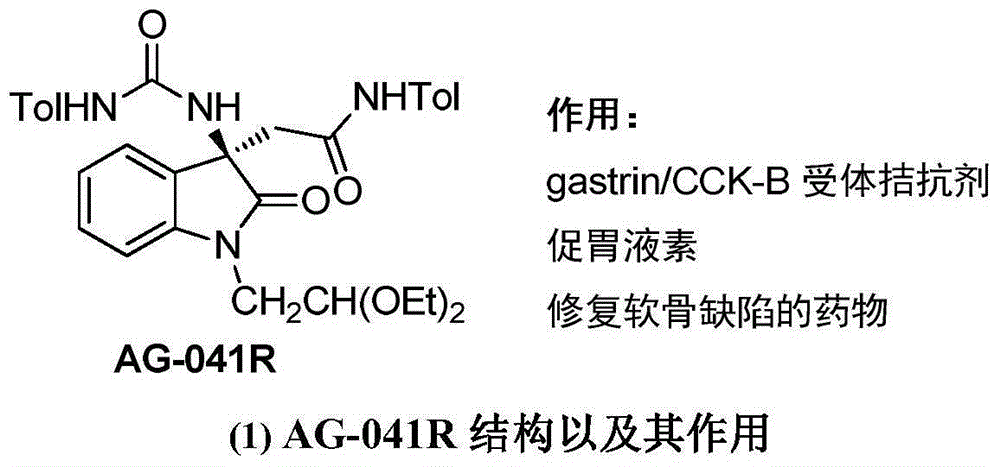
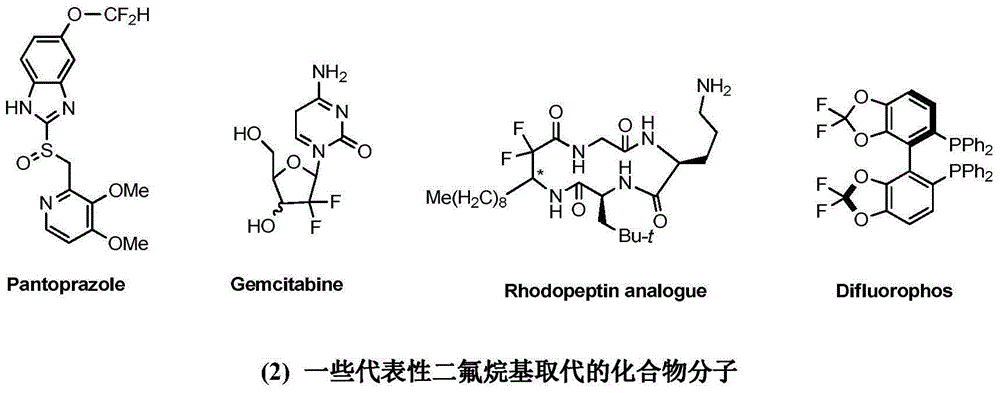
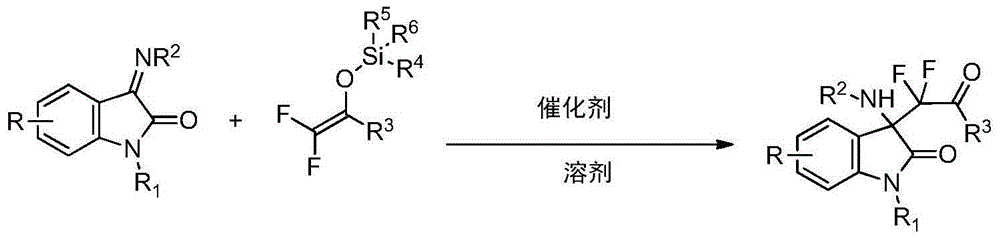
3-difluoroalkyl substituted amino oxindole derivative and synthesis method thereof
A technology of carbooxindole derivatives and substituents, applied in the field of aminoquaternary carbooxindole derivatives and their synthesis, achieving high yield, simple raw materials and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Under nitrogen, add Ph to the 25.0mL Schleck reaction flask 3 PAuCl (3.7mg, 0.0075mmol) and AgOTf (1.9mg, 0.0075mmol) were added, followed by anhydrous dichloromethane (5.0mL), and the reaction solution was stirred at room temperature for half an hour. join in turn Molecular sieve, indoimine I-1 (0.25mmol), difluoroenol silyl ether II-1 (2.5mmol), TLC detection raw materials have basically been reacted, stop the reaction. Direct column chromatography, eluent (CH 2 Cl 2 / EtOAc=50:1~30:1), the product III-1 was obtained as a white solid with a yield of 92%. 1 HNMR (400MHz, CDCl 3 ):δ7.82-7.80(m,2H),7.60-7.56(m,1H),7.42-7.33(m,4H),7.03-7.00(m,1H),6.84-6.82(m,1H),6.18 (s,1H),3.21(s,3H),1.27(s,9H); 19 FNMR (376MHz, CDCl 3 ): δ-107.17(d, J=275Hz, 1F), -108.29(d, J=275Hz, 1F), -119.81(s, 1F); 13 CNMR (100MHz, CDCl 3): δ188.73(t, J=28Hz), 170.73, 153.24, 144.85, 134.43, 132.70, 130.46, 129.94(t, J=3.6Hz), 128.41, 125.08, 123.71, 122.75, 115.32(t, J=265Hz ...
Embodiment 2
[0039]
[0040] Under nitrogen, add AgOTf (1.9 mg, 0.0075 mmol), and anhydrous dichloromethane (3.0 mL) to a 25.0 mL Schleck reaction flask, followed by Molecular sieve, indimine I-2 (0.25mmol), difluoroenol silyl ether II-1 (0.5mmol), TLC detection raw materials have basically been reacted, stop the reaction. Direct column chromatography, eluent (CH 2 Cl 2 / EtOAc=50:1~30:1), the product III-2 was obtained as a white solid with a yield of 80%. 1 HNMR (400MHz, CDCl 3 ):δ7.87-7.85(m,2H),7.63-7.59(m,1H),7.45-7.42(m,2H),7.17-7.14(m,1H),7.08-7.03(m,1H),6.79 -6.76(m,1H),6.21(s,1H),3.22(s,3H),1.31(s,9H); 19 FNMR (376MHz, CDCl 3 ): δ-106.52(d, J=279Hz, 1F), -108.11(d, J=279Hz, 1F), -119.81(s, 1F); 13 CNMR (100MHz, CDCl 3 ), 128.57, 125.33(d, J=7.2Hz), 116.71(d, J=23.4Hz), 115.20(t, J=266Hz), 113.47(d, J=25.6Hz), 108.94(d, J=7.9Hz) ,81.22,65.34(t,J=22.9Hz),28.00,26.83.MS(EI):434(M + ,6),334(3),205(3),179(100),105(15),77(15),57(10),44(5).HRMS(EI):ExactmasscalcdforC 22 h ...
Embodiment 3
[0042]
[0043] Add Hg(OTf) to a 25.0mL Schleck reaction flask under nitrogen 2 (0.0075mmol), and anhydrous dichloroethane (3.0mL), followed by adding Molecular sieve, indoimine I-3 (0.25mmol), difluoroenol silyl ether II-1 (0.75mmol), TLC detection raw materials have basically been reacted, stop the reaction. Direct column chromatography, eluent (CH 2 Cl 2 / EtOAc=50:1~30:1), the product III-3 was obtained as a white solid with a yield of 70%. 1 HNMR (400MHz, CDCl 3 ):δ7.87-7.85(m,2H),7.63-7.59(m,1H),7.46-7.42(m,2H),7.36-7.31(m,2H),6.79-6.76(m,1H),6.19 (s,1H),3.22(s,3H),1.31(s,9H); 19 FNMR (376MHz, CDCl 3 ): δ-106.38(d, J=280Hz, 1F), -107.87(d, J=280Hz, 1F); 13 CNMR (100MHz, CDCl 3 ): δ188.20(t, J=28Hz), 170.44, 153.24, 143.57, 134.75, 132.36, 130.36, 130.06(t, J=4Hz), 128.58, 128.15, 125.55, 125.48, 115.22(t, J=266Hz) ,109.35,81.29,65.09(t,J=23Hz),28.02,26.83.MS(EI):452[M( 37 Cl) + ,2.6],450[M( 35 Cl) + ,7.7],350(3),221(3),195(100),197(33),105(20),77(20),57(1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com