Preparation method of chiral 1, 4-diphenyl-2-hydroxy-1, 4-dibutanone compound
A technology of dibutyl ketone and diphenyl, which is applied in the field of preparation of chiral 1,4-diphenyl-2-hydroxy-1,4-dibutanone compounds, can solve the enantioselective selection of substrate applicability Sex needs to be further improved and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
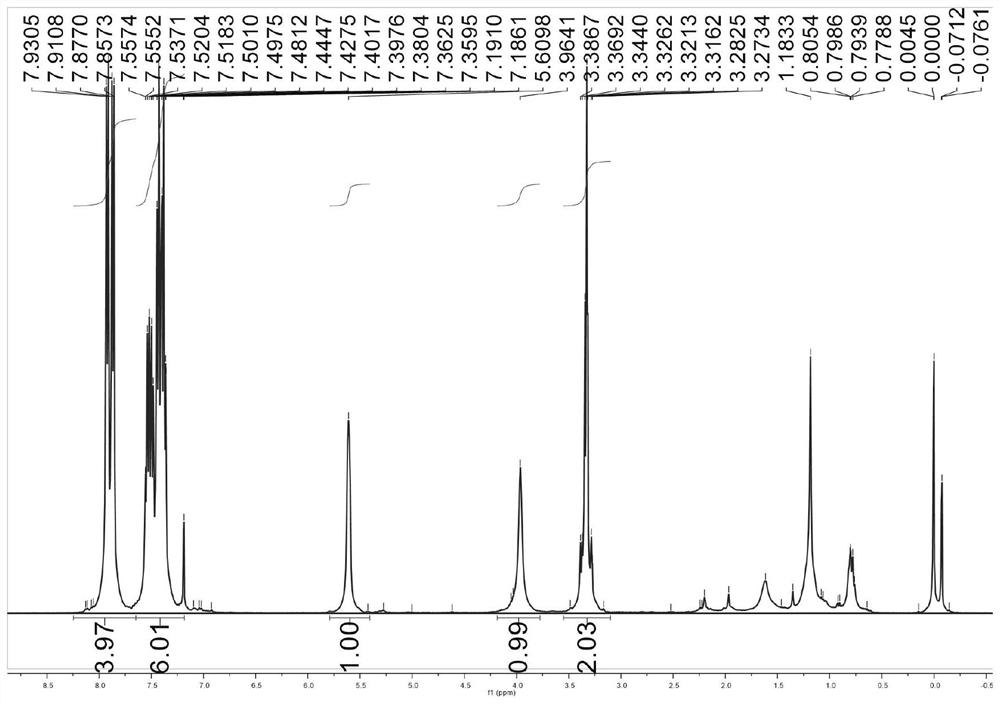
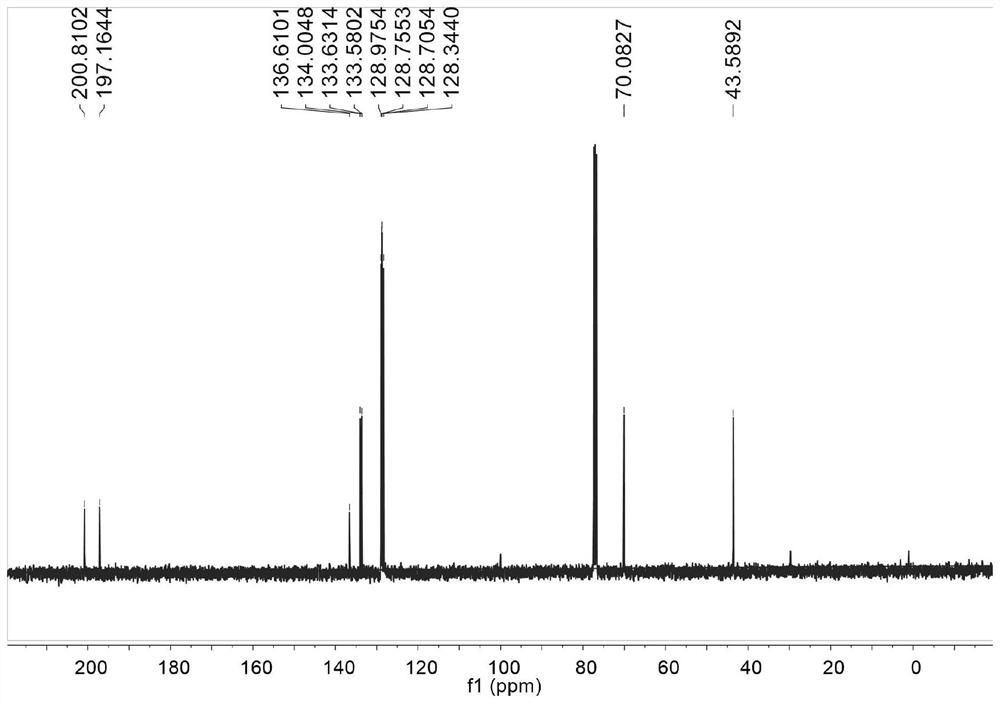
[0095]Therefore, the present invention also provides a simple and environmentally friendly preparation method of 1,4-diphenyl-2-hydroxyl-1,4-dibutanone compounds, the method comprising: using the chiral Metal complexes or chiral metal complexes prepared according to the present invention are mixed with enol silyl ethers and phenylglyoxal monohydrate or substituted phenylglyoxal monohydrate to obtain 1,4-di Phenyl-2-hydroxyl-1,4-dibutanone compounds, the R of the enol silyl ether 1 It is a phenyl or furan structure, the substituent R in the substituted phenylglyoxal monohydrate 2 One or more selected from hydrogen atom, halogen, methyl, methoxy, nitro and trifluoromethyl, the substituent R 2 In the ortho, meta or para position of the benzene ring.
[0096]
[0097] The chiral metal complex described in the present invention can participate in the reaction at a concentration of 0.5-5mmol / L; the temperature of the Mukaiyama aldol reaction is -10-25°C; the time of the Mukaiya...
Embodiment 1
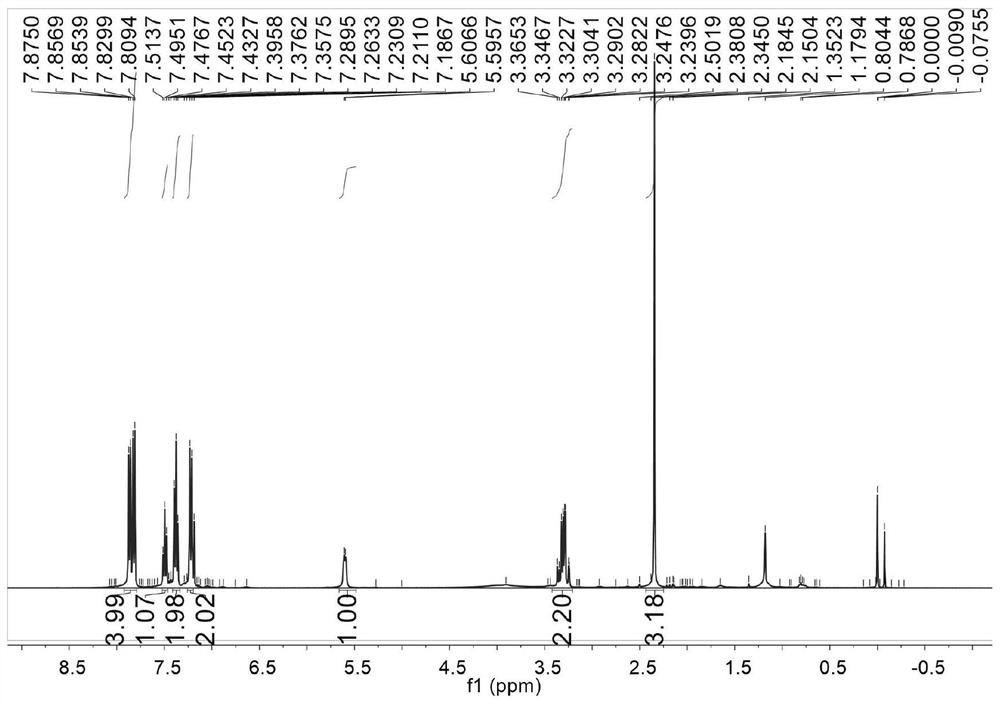
[0115] Preparation of proline-derived chiral ligands, taking L-proline with a protecting group to prepare chiral ligand L2 as an example;
[0116] The synthetic route is as follows:
[0117]
[0118] Add solvent tetrahydrofuran (20 mL) into a three-necked flask filled with nitrogen atmosphere, add magnesium strips (0.36 g, 15 mmol) and p-methylbromobenzene (1.3 g, 7.5 mmol), and heat with a hair dryer until the reaction is initiated. The remaining p-methylbromobenzene (1.3 g, 7.5 mmol) was added dropwise over 20 min. After the dropwise addition, continue to heat to 70°C and reflux for 2h. A tetrahydrofuran solution (20 mL) of benzyl-protected L-proline A (3.3 g, 15 mmol) was added dropwise to the reaction system, and continued to reflux at 70° C. for 7 h. The completion of the reaction was monitored by thin layer chromatography and quenched with saturated aqueous ammonium chloride. Extract with ethyl acetate, back extract with saturated brine. After drying over anhydrou...
Embodiment 2
[0122] Preparation of proline-derived chiral ligands, taking L-proline with a protecting group to prepare chiral ligand L3 as an example;
[0123] The synthetic route is as follows:
[0124]
[0125] Add solvent tetrahydrofuran (20 mL) into a three-necked flask filled with nitrogen atmosphere, add magnesium strips (0.36 g, 15 mmol) and p-methoxybromobenzene (1.4 g, 7.5 mmol), and heat with a blower until the reaction is initiated. The remaining p-methoxybromobenzene (1.4 g, 7.5 mmol) was added dropwise over 20 min. After the dropwise addition, continue to heat to 70°C and reflux for 2h. A tetrahydrofuran solution (20 mL) of benzyl-protected L-proline A (3.3 g, 15 mmol) was added dropwise to the reaction system, and continued to reflux at 70° C. for 7 h. The completion of the reaction was monitored by thin layer chromatography and quenched with saturated aqueous ammonium chloride. Extract with ethyl acetate, back extract with saturated brine. After drying over anhydrous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com