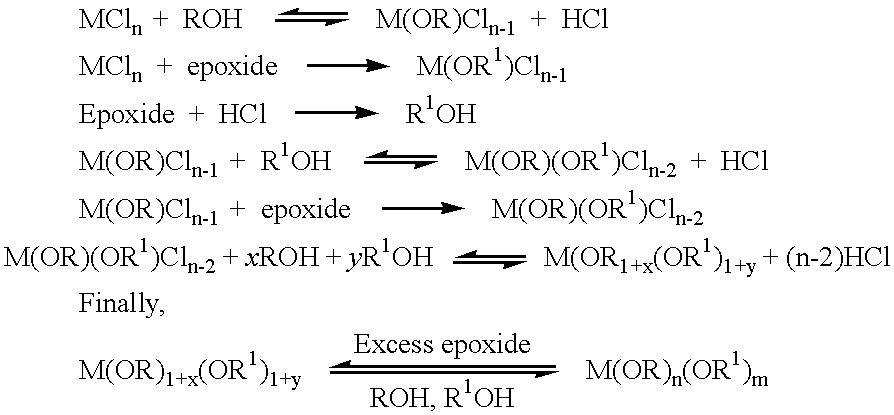Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
697results about "Group 2/12 organic compounds without C-metal linkages" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
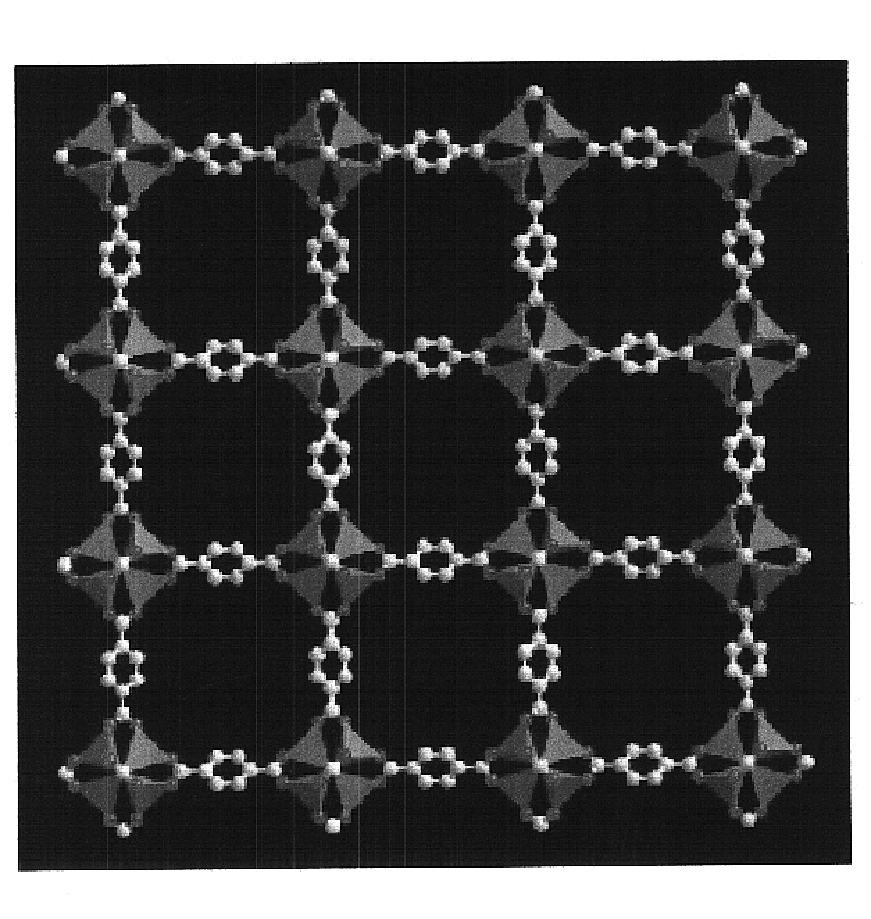
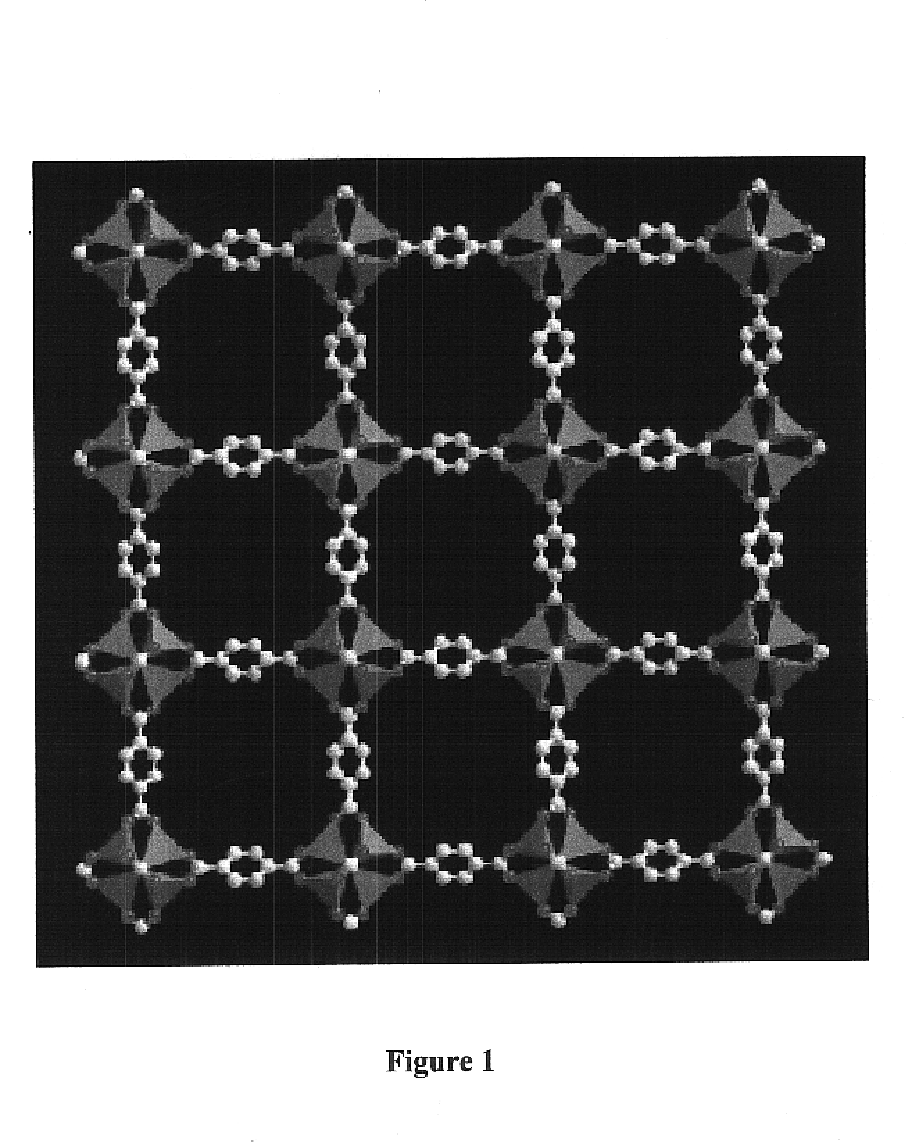
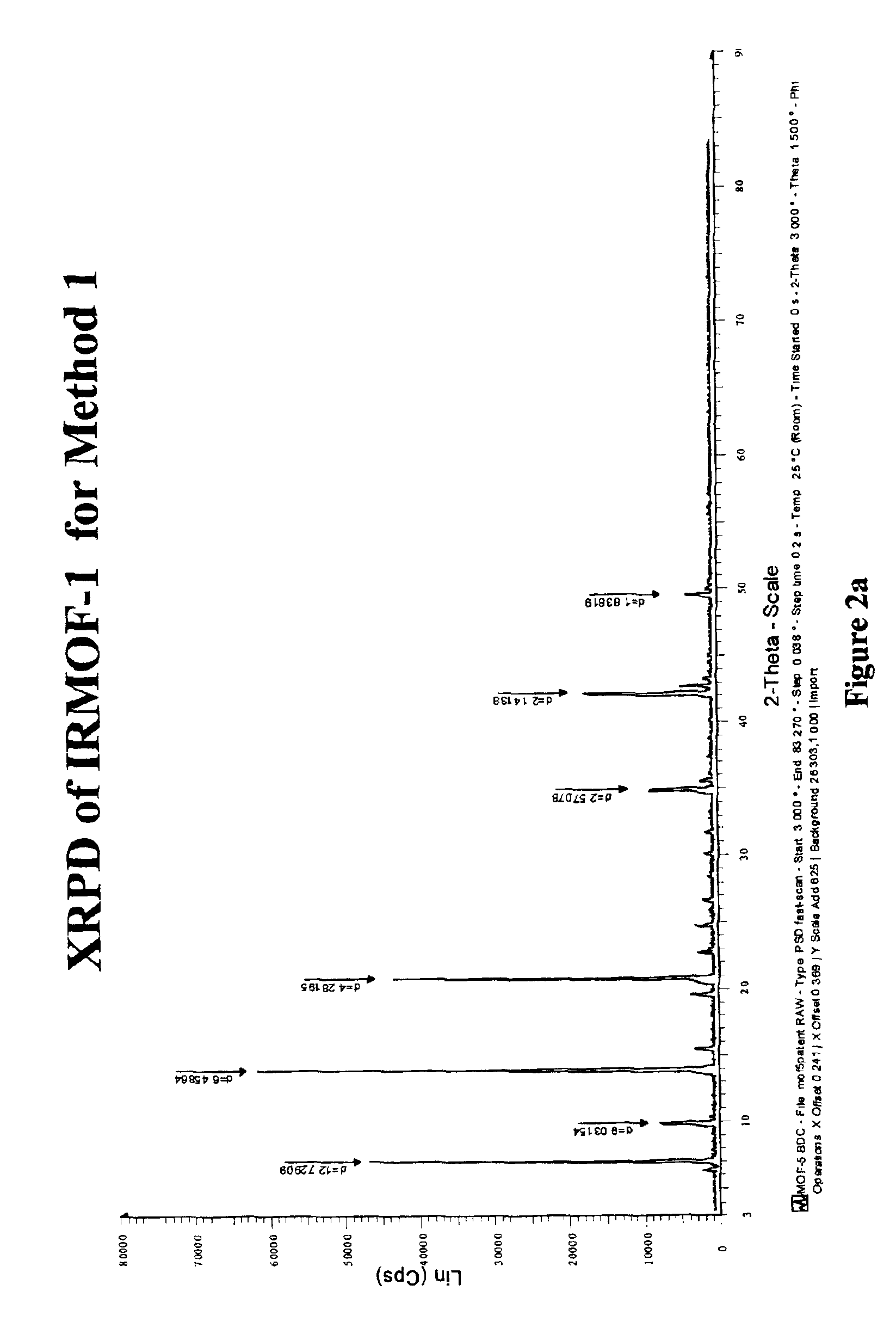
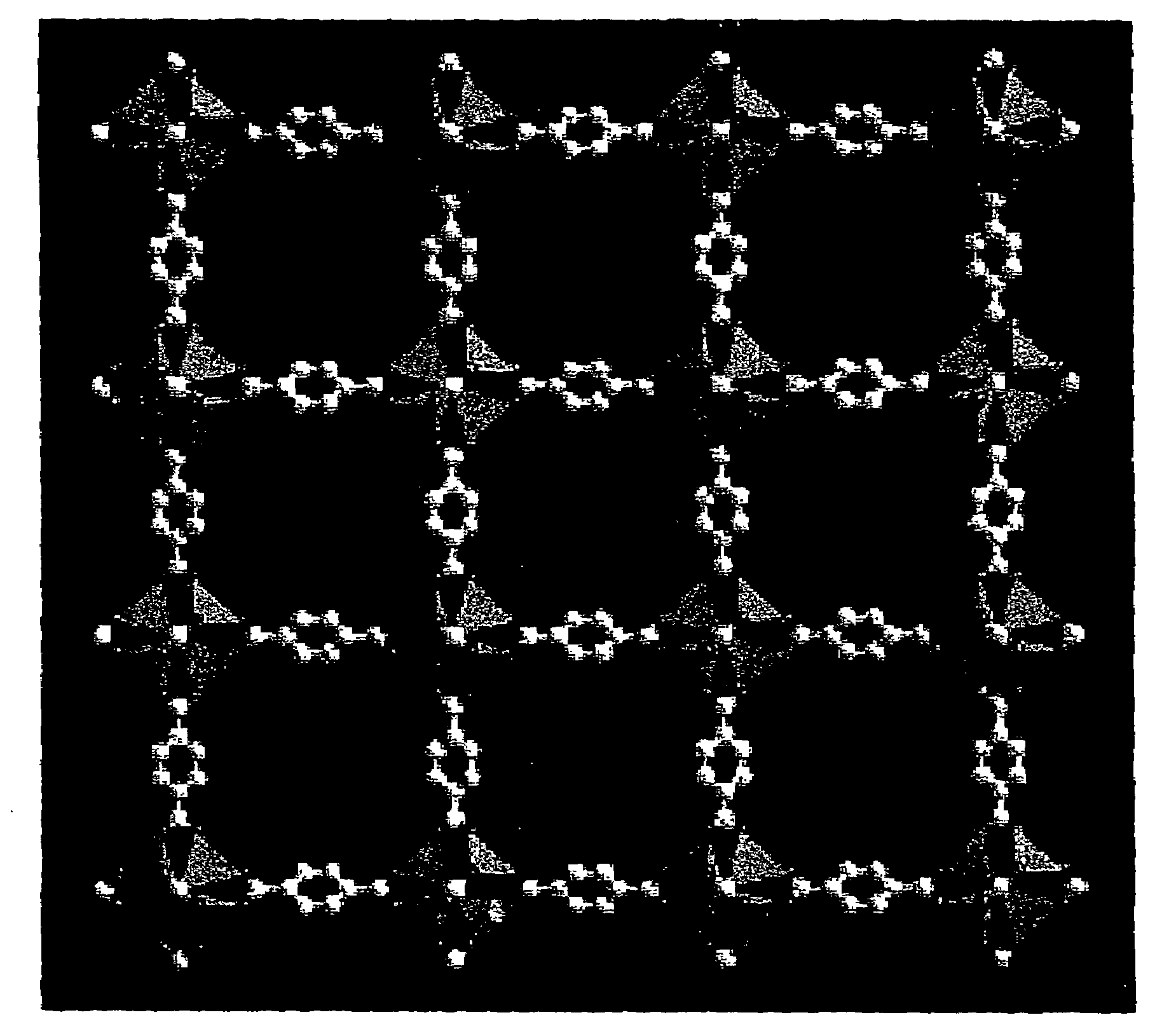
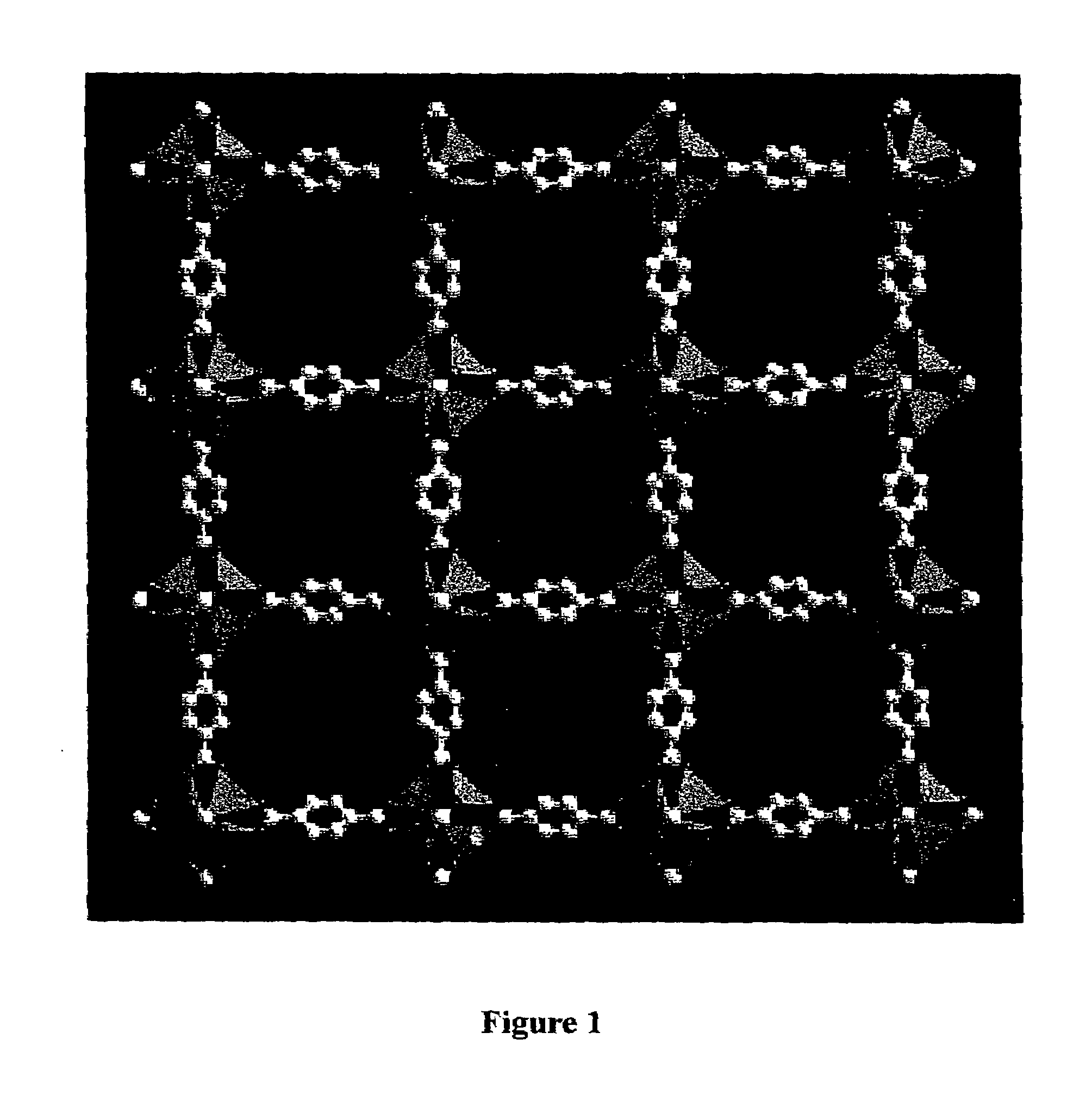
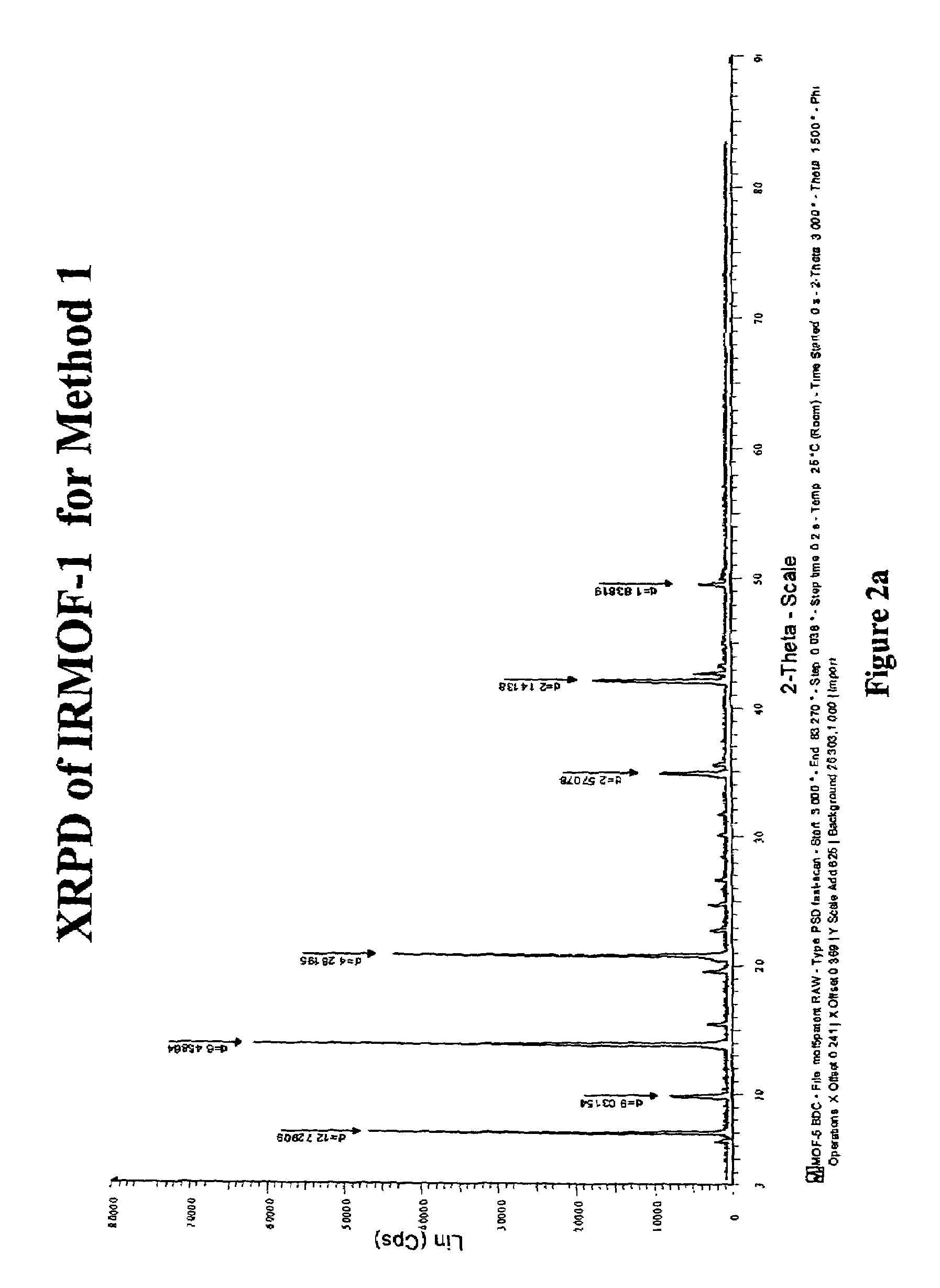
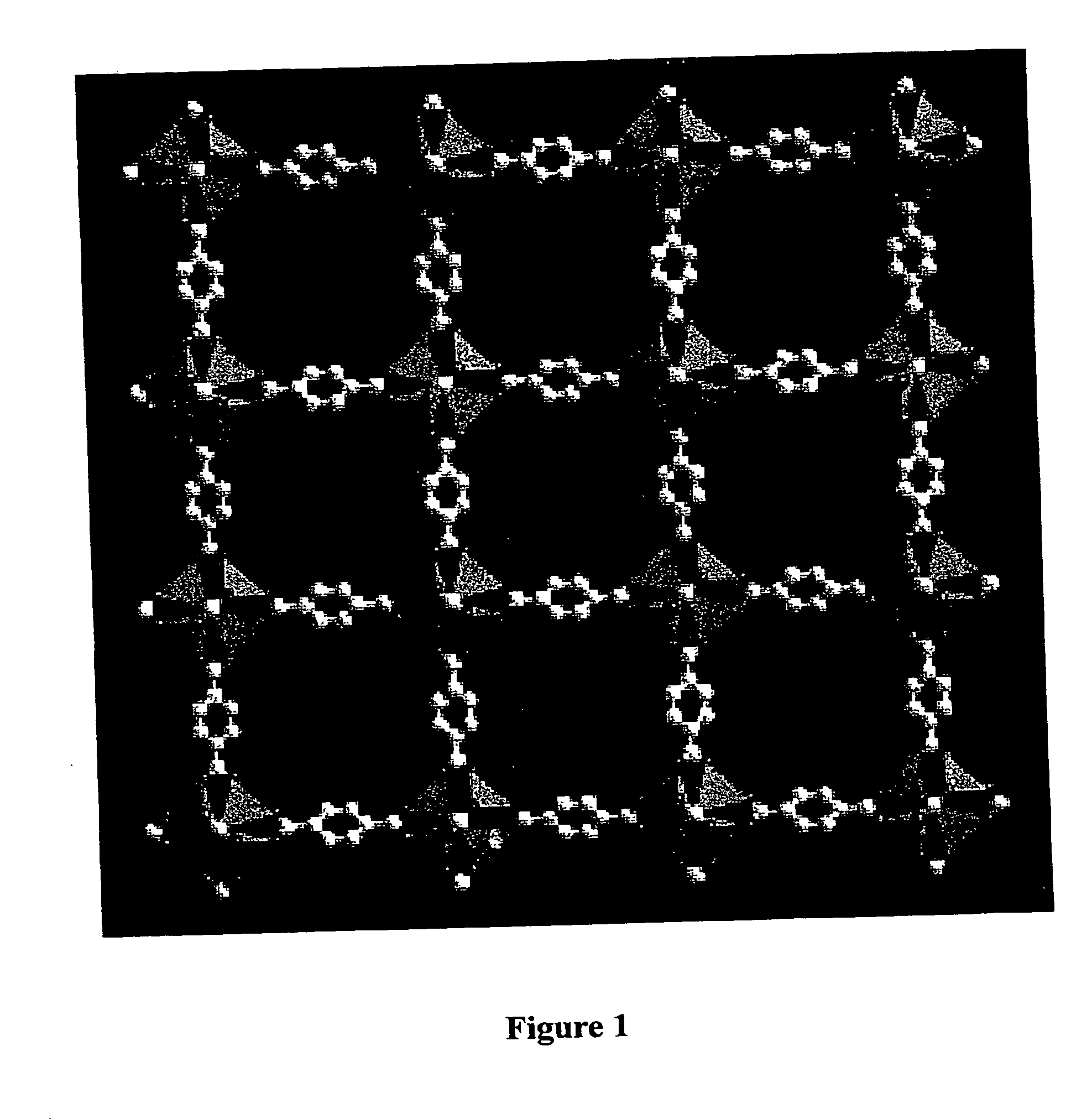
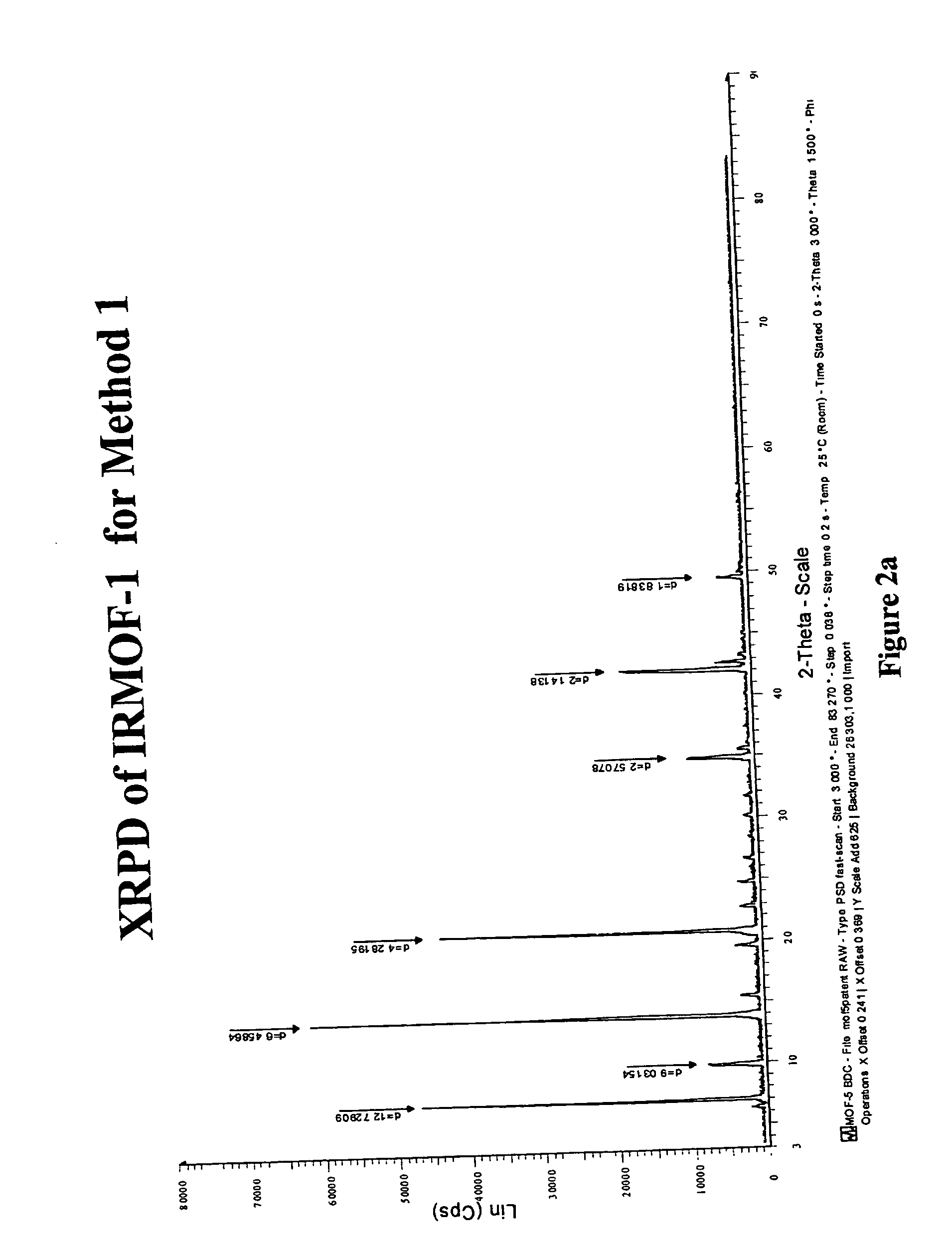
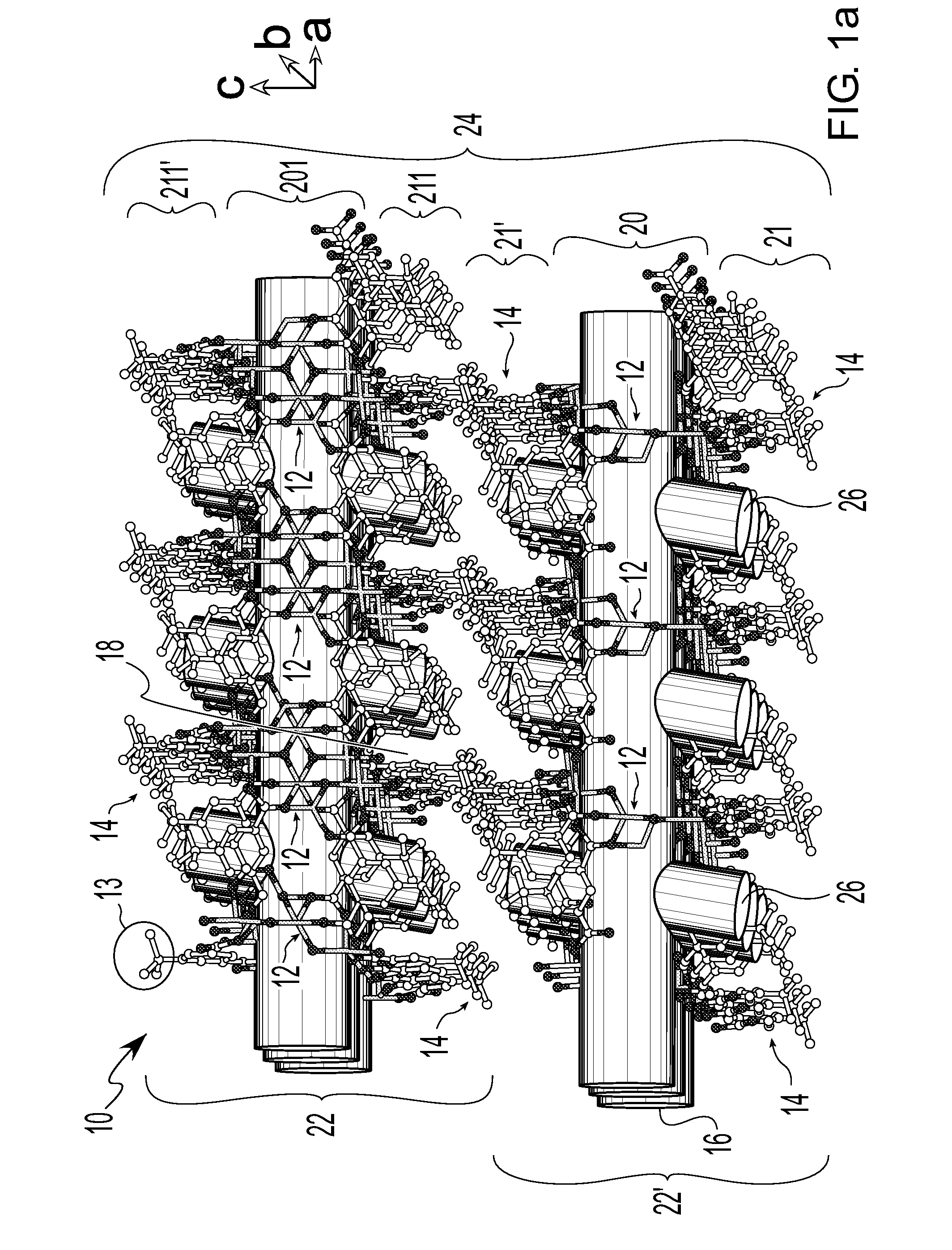
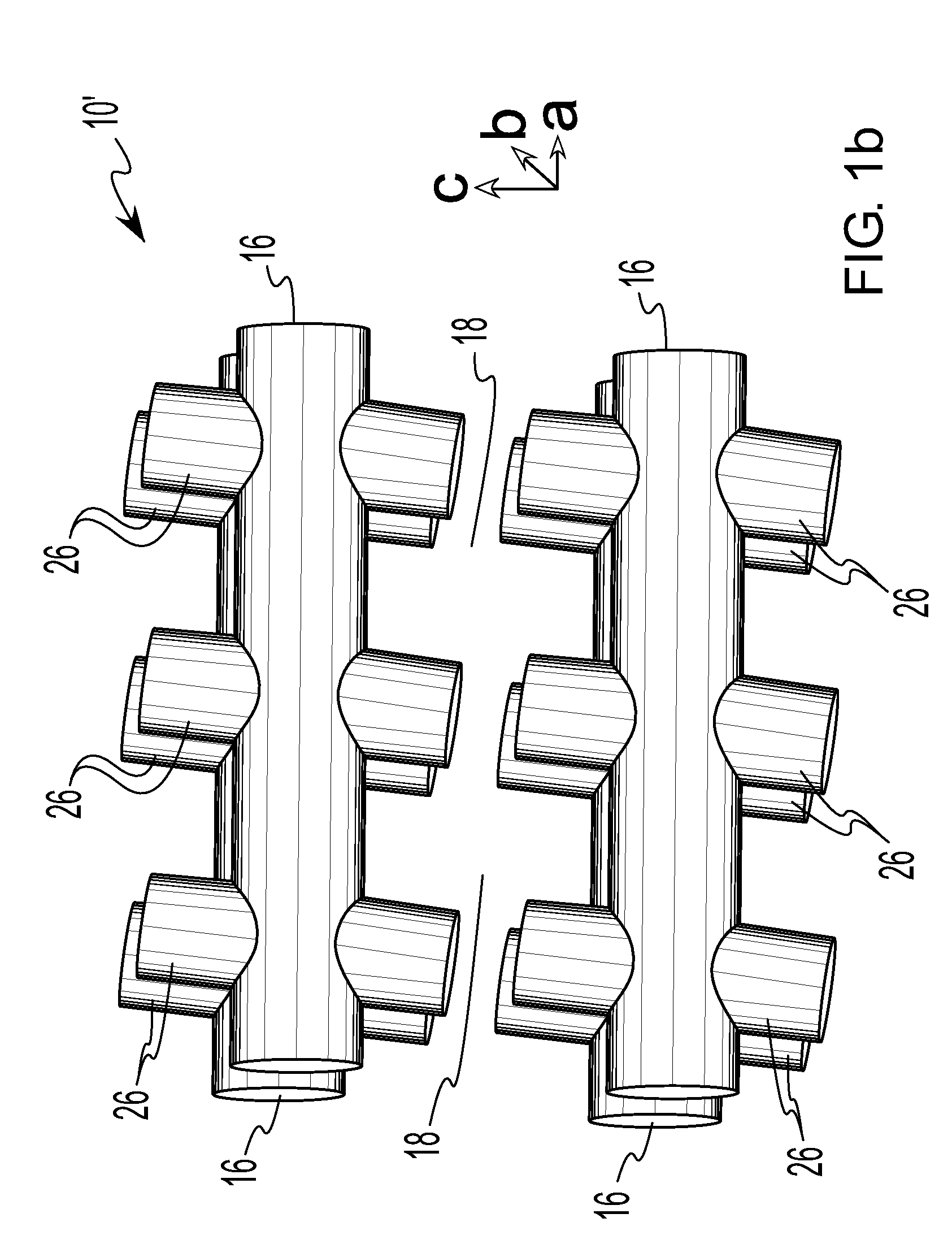
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS6930193B2High methane storage capacityIncrease storage capacityGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsOrganic linkingSystems design
An isoreticular metal-organic framework (IRMOF) and method for systematically forming the same. The method comprises the steps of dissolving at least one source of metal cations and at least one organic linking compound in a solvent to form a solution; and crystallizing the solution under predetermined conditions to form a predetermined IRMOF. At least one of functionality, dimension, pore size and free volume of the IRMOF is substantially determined by the organic linking compound.
Owner:RGT UNIV OF MICHIGAN
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS7196210B2Group 8/9/10/18 element organic compoundsGroup 5/15 element organic compoundsSystems designMetal-organic framework
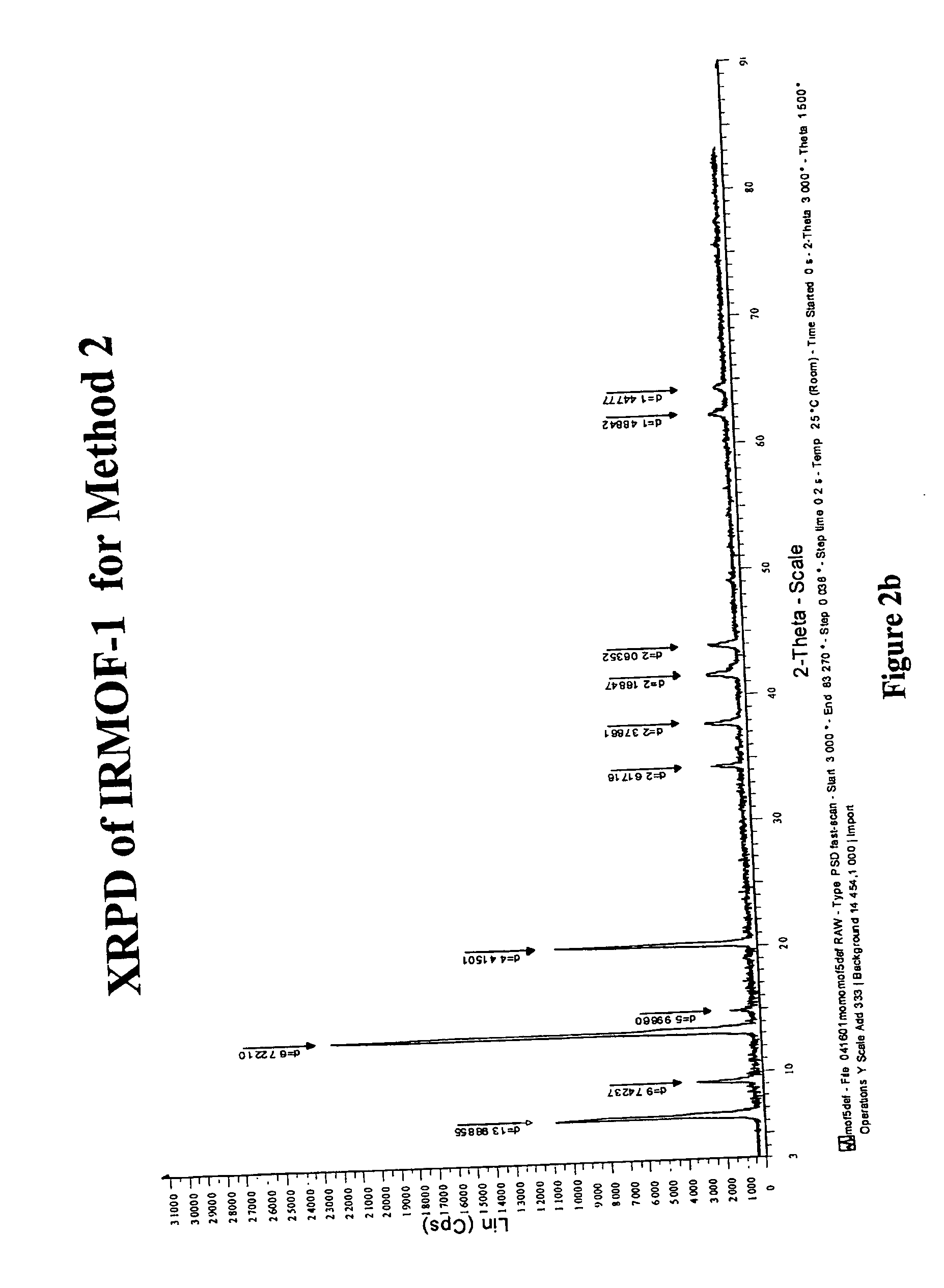
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS20050192175A1Catalyst protectionMolecular sieve catalystsSystems designMetal-organic framework
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
Zinc amino acid chelates having ligands comprised of glycine and a sulfur-containing amino acids
InactiveUS6166071AInterfere with absorptionHigh weight percentageOrganic active ingredientsBiocideGlycineSulfur containing
A zinc amino acid chelate formulation is disclosed comprising zinc ions being chelated by an amino acid ligand mixture comprising glycine and a sulfur-containing amino acid wherein the ligand to zinc molar ratio is from about 1:1 to 2:1 and wherein the glycine to sulfur-containing amino acid molar ratio is between about 1:6 to 6:1.
Owner:ALBION LAB
Implementation of a strategy for achieving extraordinary levels of surface area and porosity in crystals
InactiveUS7652132B2Large specific surface areaIncrease the number ofGroup 1/11 element organic compoundsGroup 5/15 element organic compoundsMetal clustersPorosity
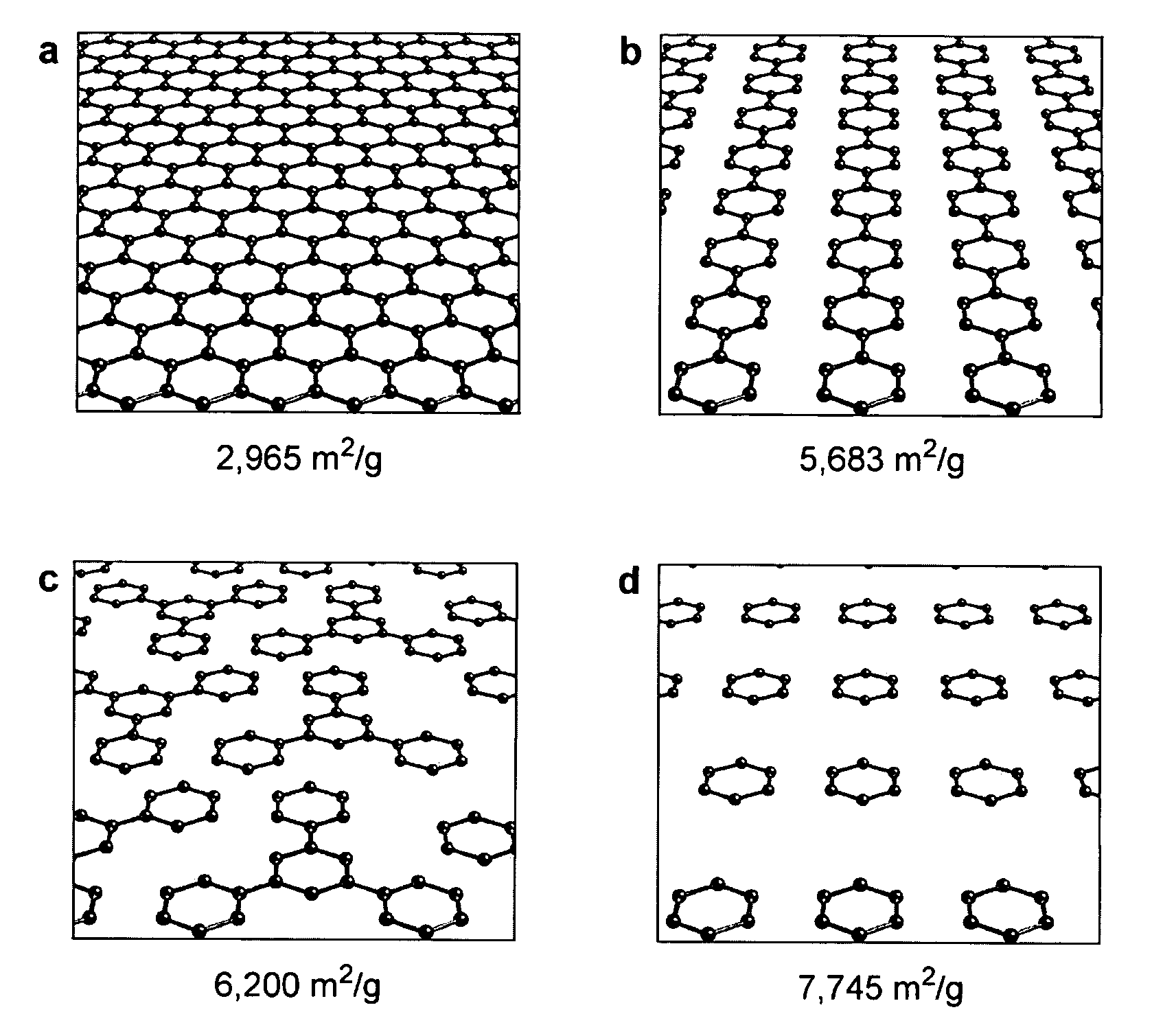
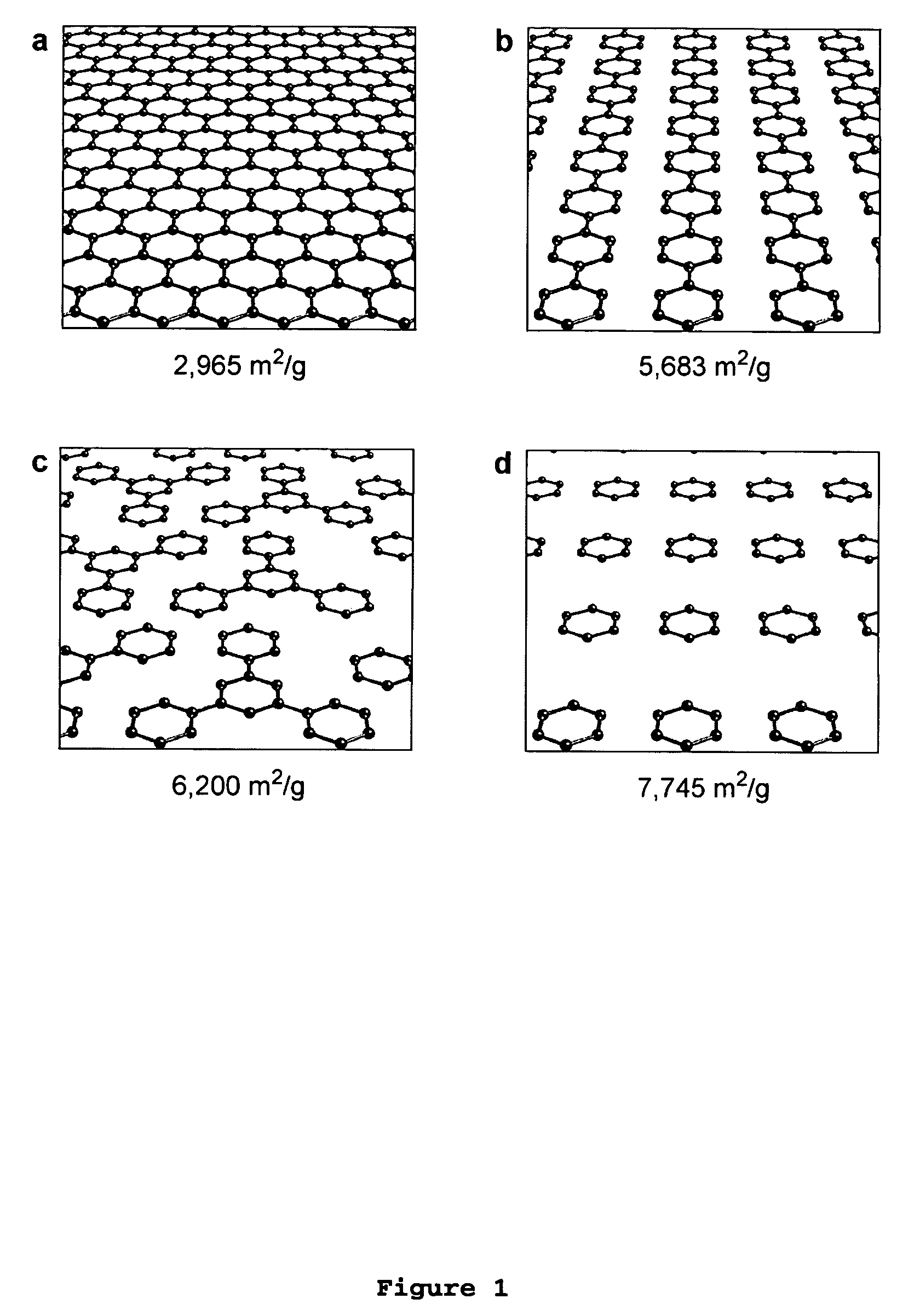
The present invention provides a metal-organic framework (“MOF”) comprising a plurality of metal clusters and a plurality of multidentate linking ligands. Each metal of the plurality of metal clusters comprises one or more metal ions. Each ligand of the plurality of multidentate linking ligands connects adjacent metal clusters. The present invention also provides a method of forming the metal-organic framework. The method of the invention comprises combining a solution comprising one or metal ions with a multidentate linking ligand having a sufficient number of accessible sites for atomic or molecular adsorption that the surface area of the resulting metal-organic framework is greater than 2,900 m2 / g.
Owner:MICHIGAN UNIV OF RGT
Metal complexes produced by Maillard Reaction products
InactiveUS6992203B2Increase productionMore stable metal chelateGroup 1/11 organic compounds without C-metal linkagesIron group organic compounds without C-metal linkagesMaillard reactionMetal chelate
Owner:J H BIOTECH
Overbased magnesium deposit control additive for residual fuel oils
An overbased magnesium composition deposit control additive for residual fuel oils and turbine fuels is an overbased magnesium sulfonate, carboxylate or phenate or mixtures thereof containing at least 14% and upwards to about 18% by weight of magnesium and containing a succinic anhydride and lower carboxylic acid co-promoter reaction product. The additive when added to fuel oils, such as residual fuel oils containing high asphaltenes, reduces, if not eliminates, magnesium / asphaltene deposits or sediment and the consequential plugging of filters. The additive also reduces, if not eliminates, vanadium caused corrosion in the turbine. The invention is also the process for preparing the overbased composition or deposit control additive, wherein the overbasing reaction incorporates the combination of a lower carboxylic acid, preferably acetic acid and a succinic anhydride, preferably dodecenyl succinic anhydride (DDSA), as the co-promoter.
Owner:CK WITCO
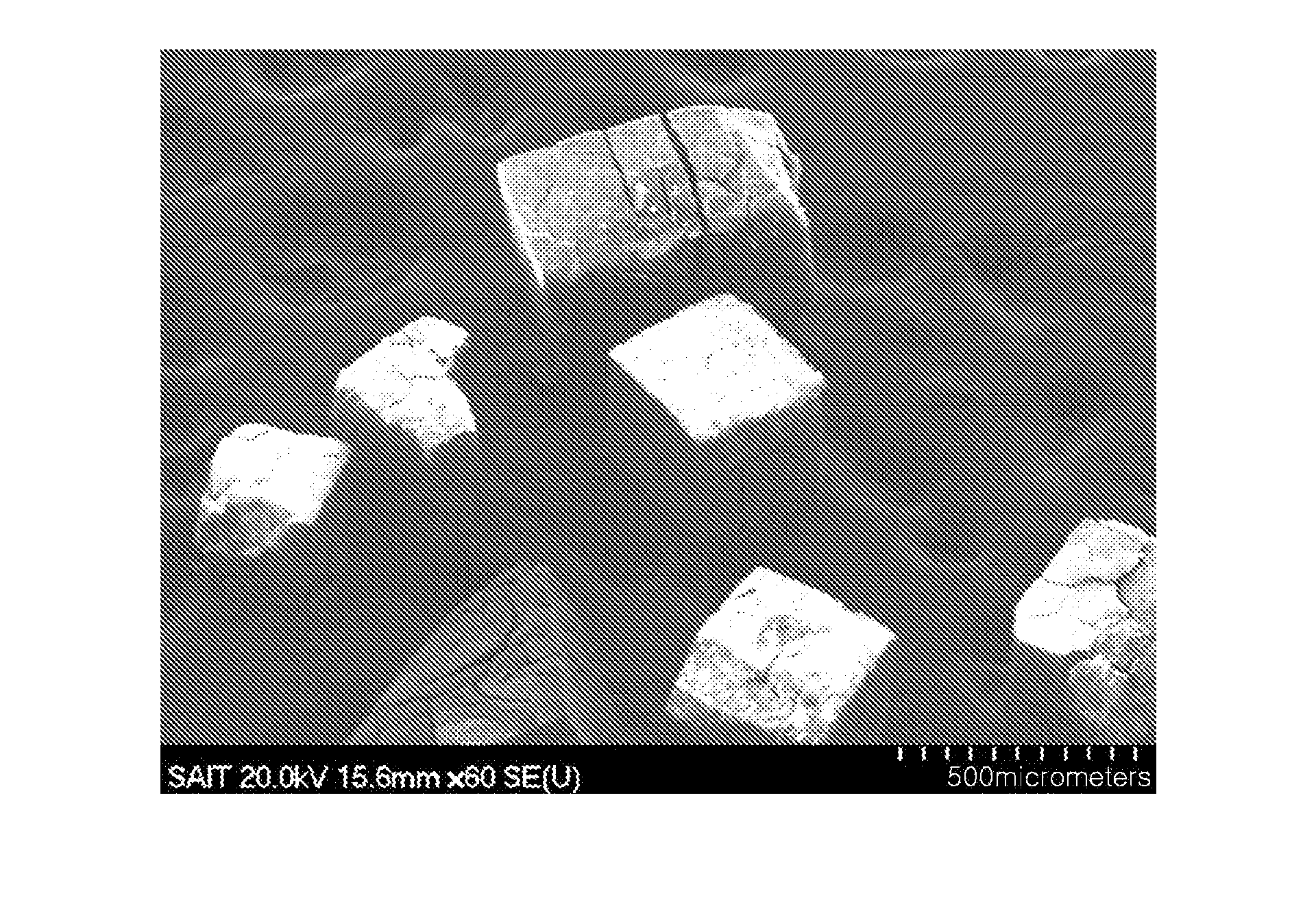
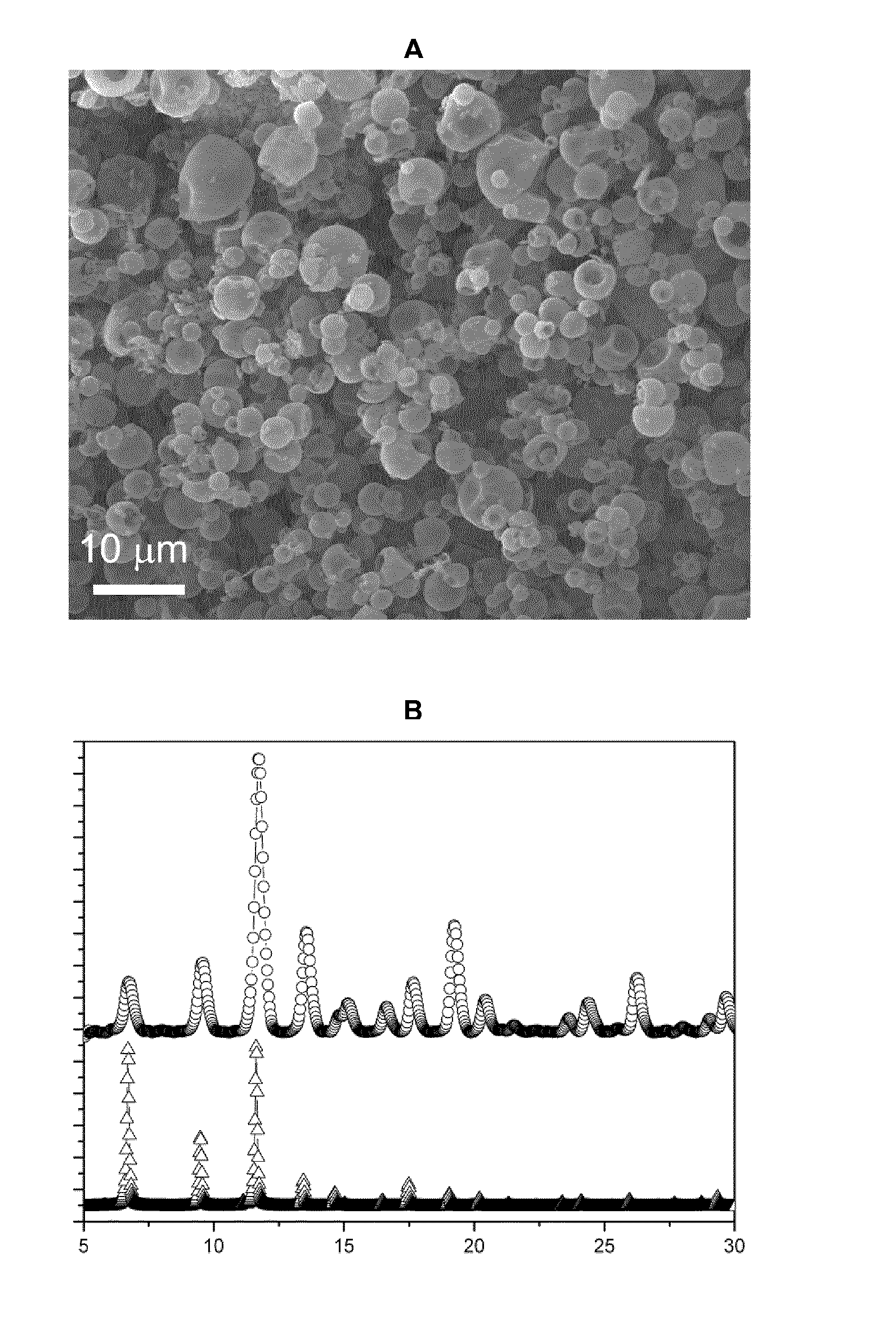
Method for regulating ZIF-8 crystal morphology by utilizing surfactant
InactiveCN107722046ARaw materials are cheap and easy to getLow costOrganic chemistry methodsGroup 2/12 organic compounds without C-metal linkagesCrystal morphologyRhombic dodecahedron
The invention provides a method for regulating ZIF-8 crystal morphology by utilizing a surfactant and belongs to the technical field of synthesis of porous materials. The method disclosed by the invention is mainly characterized in that the morphology and size of the ZIF-8 crystal are controlled by adding a certain amount of a surfactant. The specific preparation method comprises the following steps: respectively dissolving a zinc salt, a surfactant and dimethyl imidazole into a certain amount of water, stirring to enable the components to be fully dissolved, and preparing a solution; mixing adimethyl imidazole solution and a surfactant solution, fully stirring, finally adding a zinc salt solution, and mixing and stirring for 10 minutes; then transferring the mixed solution into a high temperature reactor, and reacting at a constant temperature for several hours; cooling, centrifuging, washing and drying, thereby obtaining the ZIF-8 crystal. The ZIF-8 crystal prepared by the method has excellent crystallinity and has five morphologies such as rhombic dodecahedron, cube, sheet, interpenetration twin and rod. The invention provides a novel method for controlling the morphology and size of MOFs materials.
Owner:NANJING UNIV OF TECH
Metal organic framework film and preparation method therefor
The present invention discloses a method for preparing a metal organic framework film. The method comprises a step of contacting a metal source with an organic ligand to form the metal organic framework film on a substrate by a hot-pressing method. By means of the method provided by the present invention, a large number of metal organic framework films with quite high purity can be obtained conveniently and rapidly, so that industrialized production and application are implemented, and the method has the advantages of low costs, simplicity in operation, rapid production, mass production of products, high purity and the like.
Owner:理工清科(北京)科技有限公司
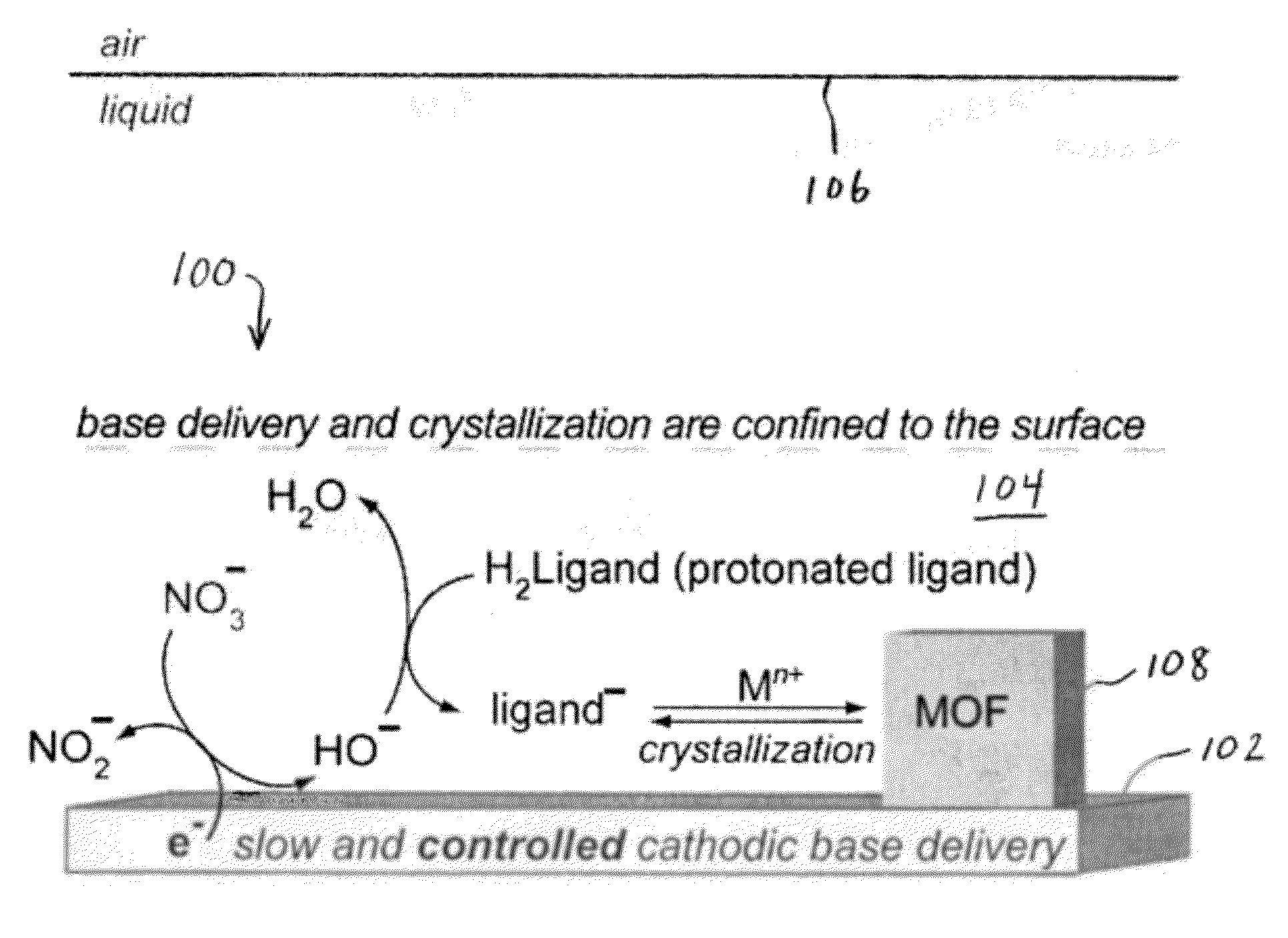
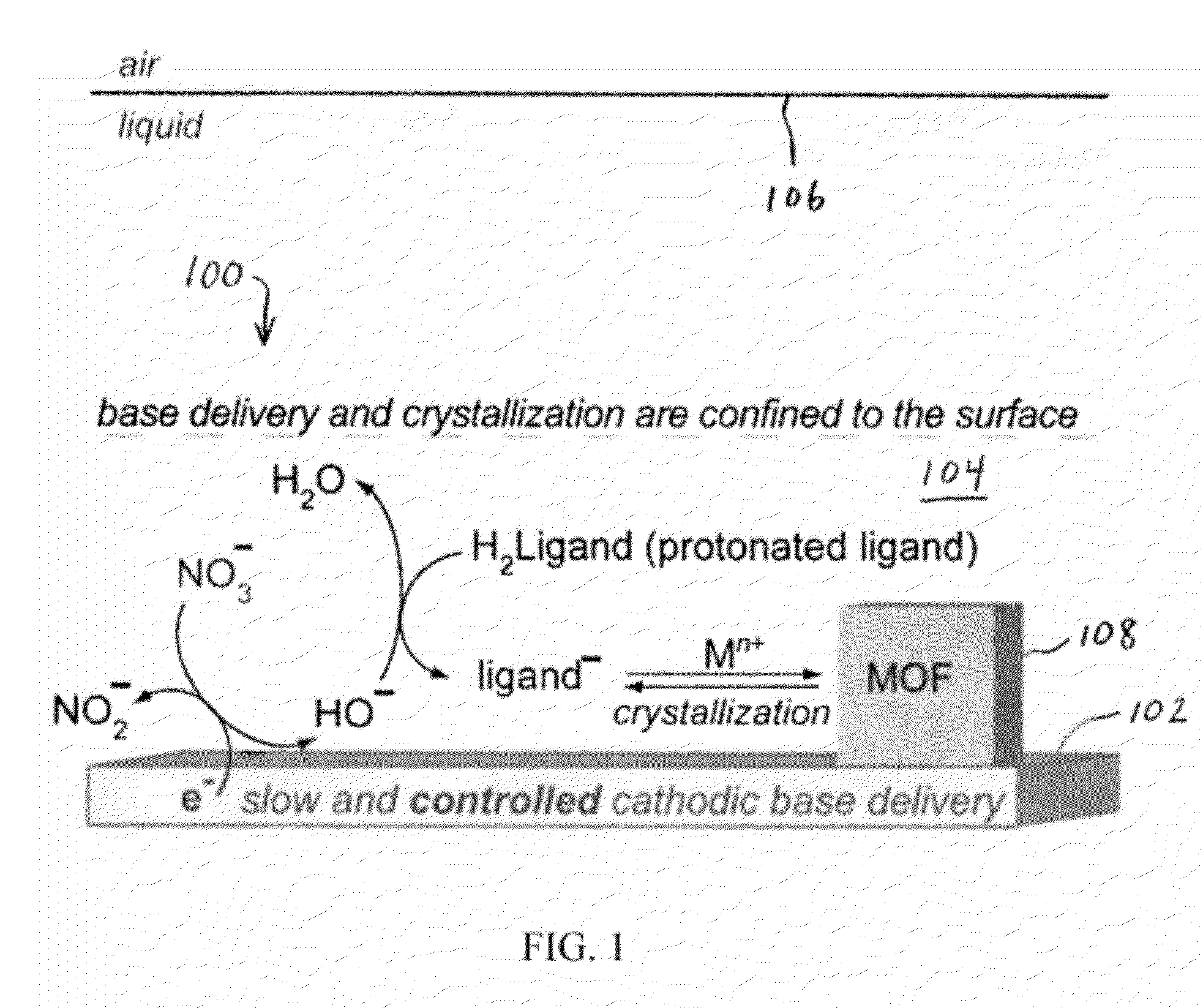
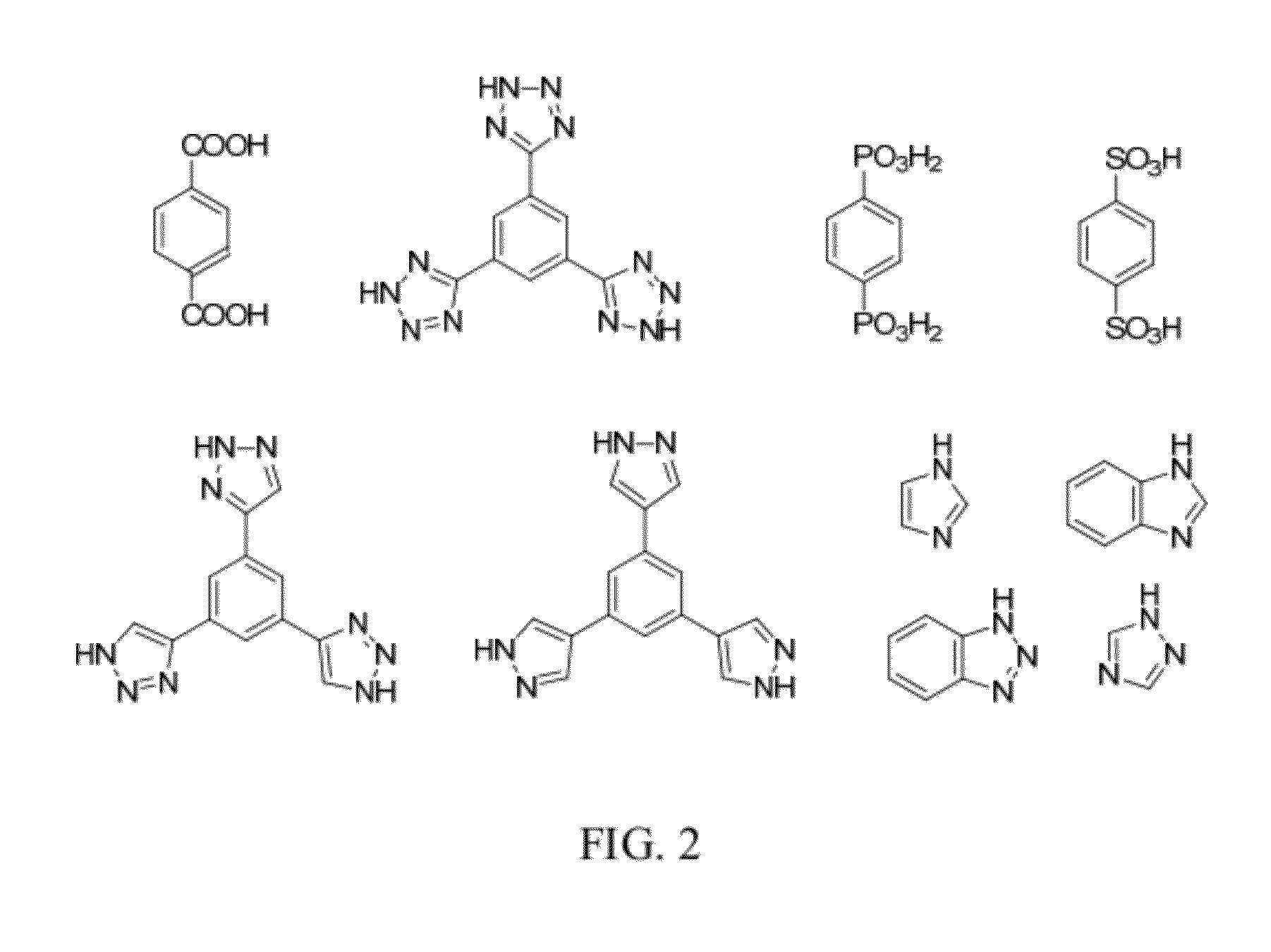
Methods for Electrochemically Induced Cathodic Deposition of Crystalline Metal-Organic Frameworks
InactiveUS20120297982A1Fast reaction timeActive material electrodesIsotope separationPower flowMetal-organic framework
Embodiments of the invention relate to a method for preparing crystalline metal-organic frameworks (MOFs). The method includes the steps of providing an electrolyte solution in contact with a conductive surface, and applying a current or potential to the conductive surface in contact with the electrolyte solution. The electrolyte solution includes a protonated organic ligand, a metal ion, and a probase. Application of the reductive current or potential to the conductive surface produces the crystalline metal-organic framework (MOF) deposited on the conductive surface. The MOFs produced by the method may be incorporated into a gas separation membrane, a purification filter, and / or a sensor.
Owner:MASSACHUSETTS INST OF TECH
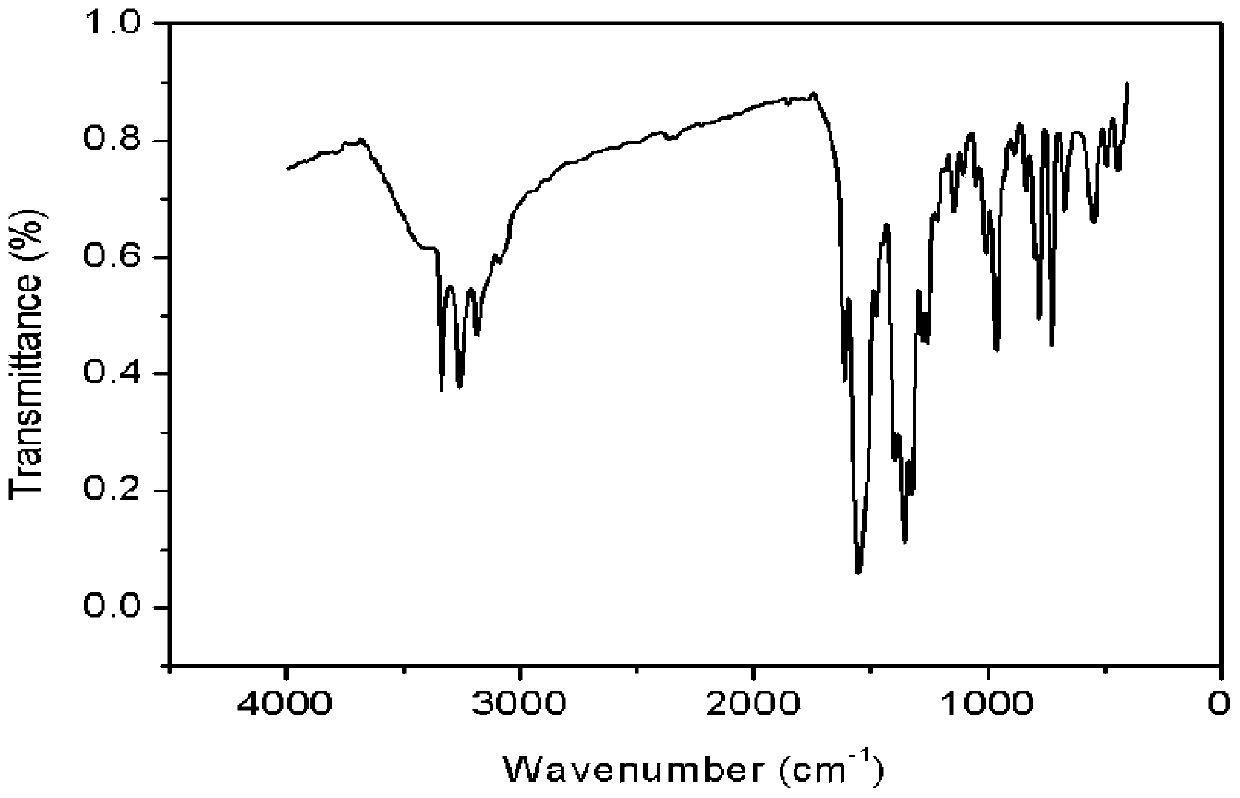
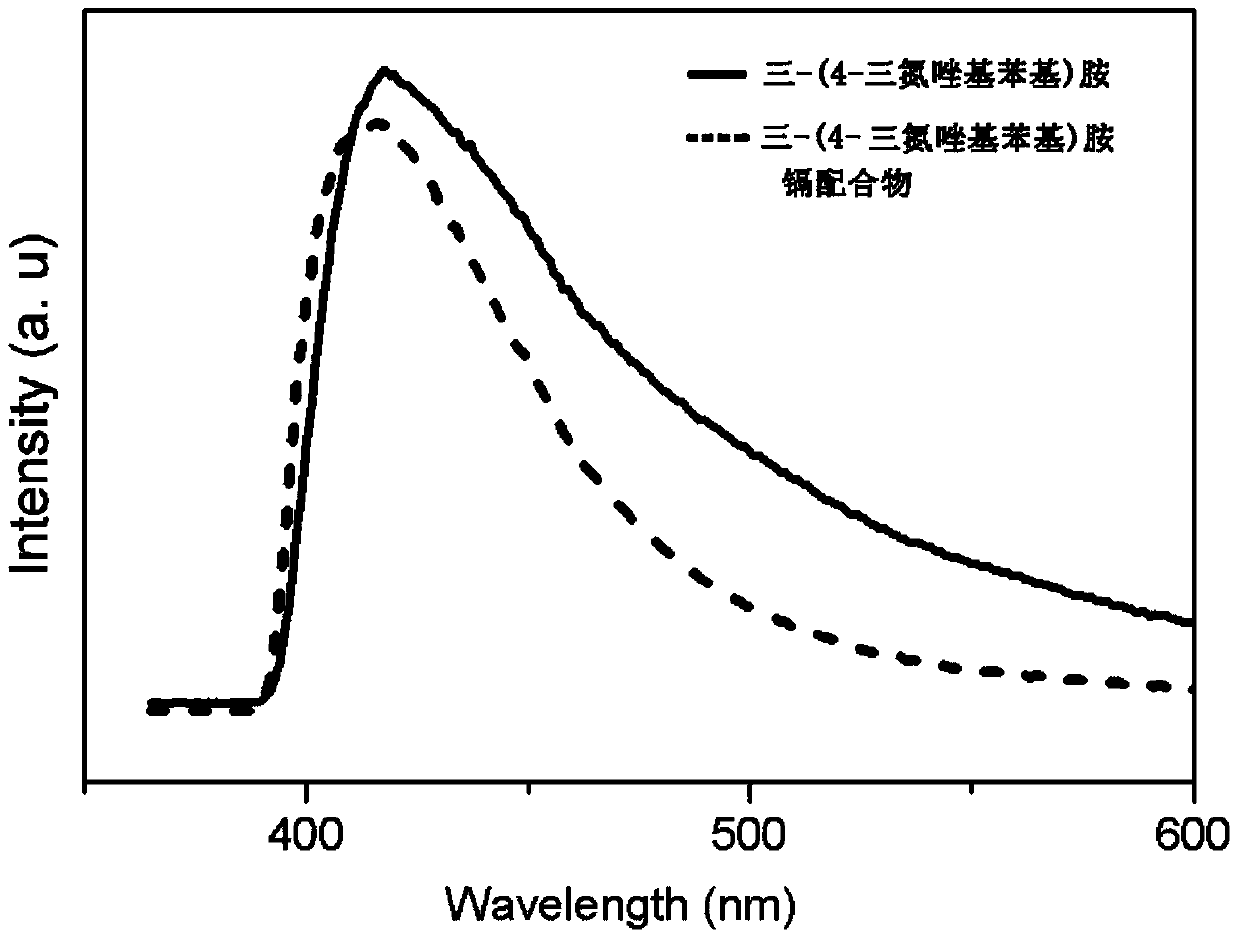
Synthesis methods of tri-(4-triazolyl phenyl) amine and tri-(4-triazolyl phenyl)amine cadmium complex
InactiveCN104193691AThe detection method is simpleHigh selectivityFluorescence/phosphorescenceGroup 2/12 organic compounds without C-metal linkagesSynthesis methodsCopper oxide
The invention discloses synthesis methods of a tri-(4-triazolyl phenyl) amine and a tri-(4-triazolyl phenyl) amine cadmium complex, and relates to the field of luminescent materials. The synthesis method of the tri-(4-triazolyl phenyl) amine takes copper oxide as a catalyst and dimethylsulfoxide as a solvent, tri-(4-iodobenzene) amine and triazole react at the temperature of 120-170 DEG C, then the mixture is diluted by the solvent, filtered, decolored, separated and purified sequentially to obtain the tri-(4-triazolyl phenyl) amine; the synthesis method of the cadmium complex is carried out by mixing 5-aminoisophthalic acid, 3-(4-triazolyl phenyl) amine and cadmium acetate into a mixed solution of water and alcohol, then the mixture is uniformly stirred under the acid or neutral condition to obtain a reaction liquid, and the reaction liquid is put into a reaction kettle to crystalize and react to obtain the tri-(4-triazolyl phenyl) amine cadmium complex. The synthesis methods obtain the tri-(4-triazolyl phenyl) amine and the tri-(4-triazolyl phenyl) amine cadmium complex, whose crystal purities are more than 95% and whose yields are more than 85%, through the simple synthesis route.
Owner:NANJING AGRICULTURAL UNIVERSITY
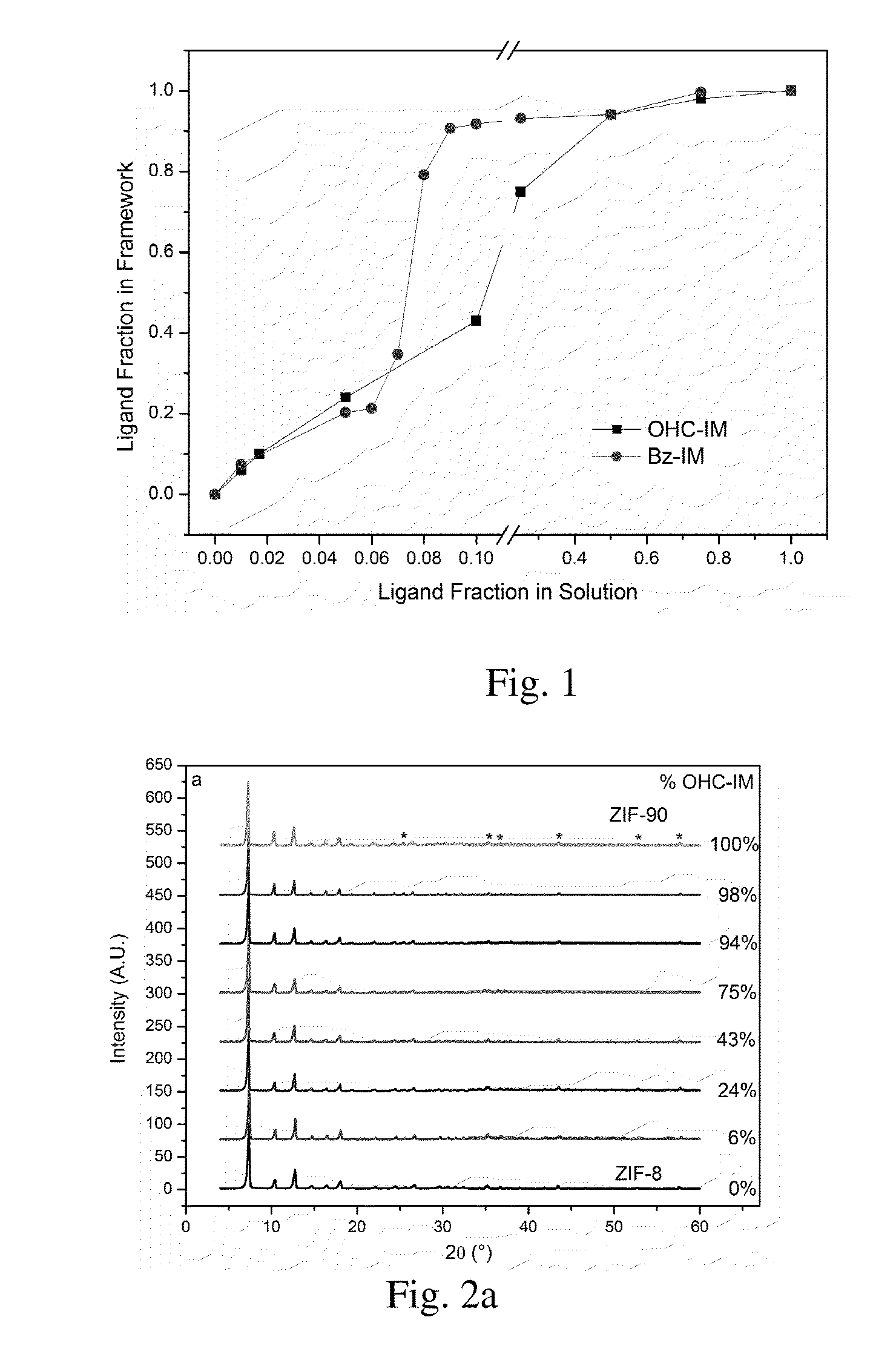
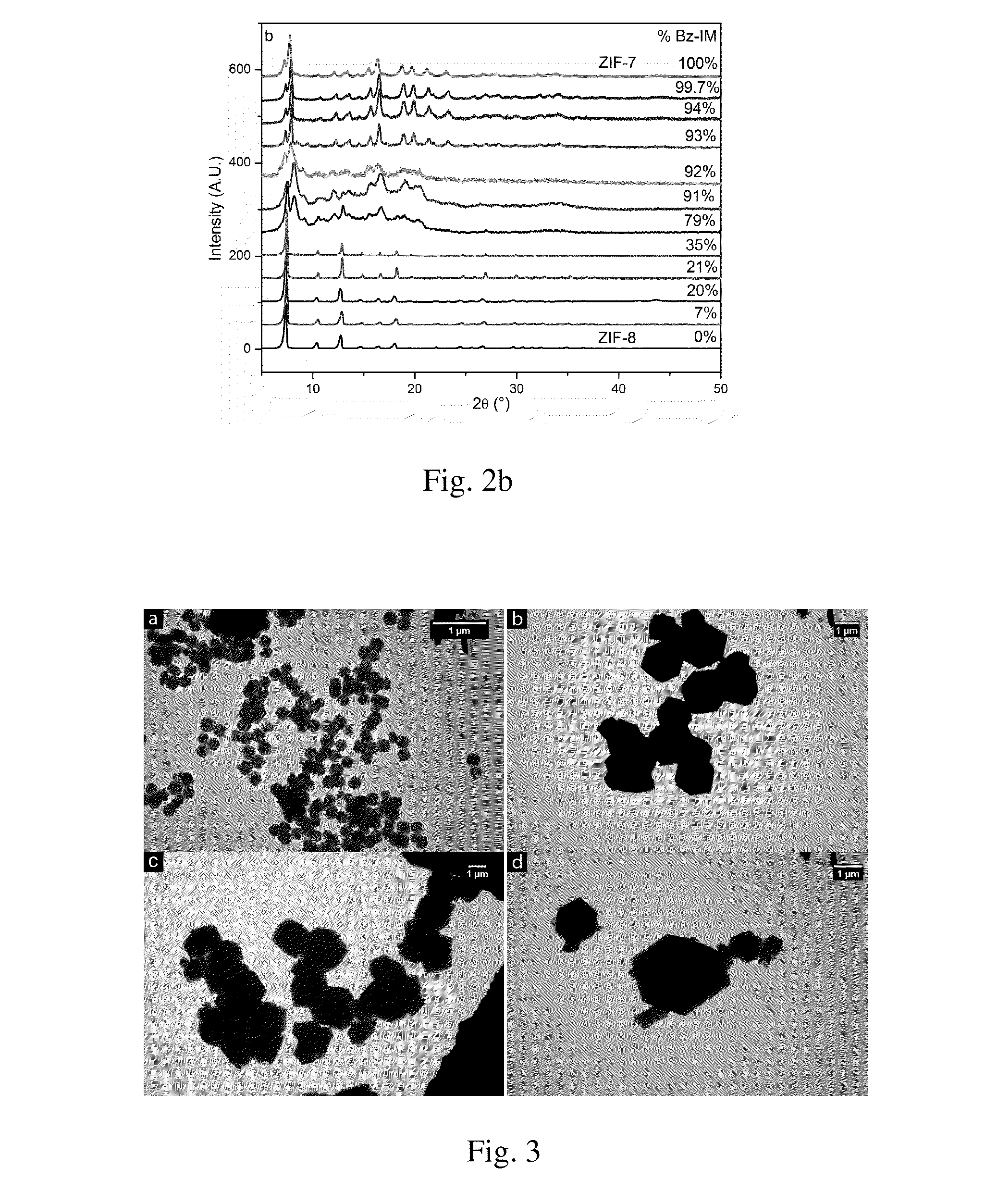
Hybrid Zeolitic Imidazolate Frameworks: Controlling Framework Porosity and Functionality by a Mixed-Ligand Synthetic Approach
Metal-organic frameworks, in particular hybrid zeolitic imidazolate frameworks (ZIFs), devices having hybrid ZIFs, and methods for preparing hybrid ZIFs are disclosed herein. In some embodiments, the method includes preparing a first solution comprising a first imidazolate and a second imidazolate, preparing a second solution comprising a metal ion, and combining the first solution and the second solution to form the hybrid ZIF.
Owner:GEORGIA TECH RES CORP
Metal Complexes Produced by Maillard Reaction Products
InactiveUS20050033037A1Enhanced Maillard ReactionIncrease productionGroup 1/11 organic compounds without C-metal linkagesIron group organic compounds without C-metal linkagesMaillard reactionMetal chelate
A method is disclosed for the formation of metal chelates which are able to remain stable in high alkaline environments when compared to metal chelates produced from a reaction with amino acids. The method involves the reaction of sugars, amino groups, and metal components for a sufficient period of time and temperature in a water solution. Additionally, the stability of metal chelates can be enhanced by oxidation of the sugars with an oxidizing agent such as hydrogen peroxide which form an MRP which will react with the metal component to form a more stable metal chelate than if oxidation were not utilized.
Owner:J H BIOTECH
Bimetallic zinc complex and process of producing polycarbonate using the same as polymerization catalyst
ActiveUS7244805B2Reduce the amount requiredEconomically beneficialCosmetic preparationsMake-upPolycarbonatePolymerization catalysts
Provided are a bimetallic zinc complex and a method of producing polycarbonate, including polymerizing an epoxy compound and carbon dioxide using the bimetallic zinc complex. The bimetallic zinc complex according to the present invention has a distance between zinc-zinc atoms, which is maintained in a limited range regardless of its concentration in a reaction medium for the polymerization. Thus, the bimetallic zinc complex can have a polymerization activity even at a high ratio of monomer / catalyst, thereby reducing a catalyst amount to be used, which is economically advantageous. Further, the bimetallic zinc complex can produce a high molecular weight polycarbonate.
Owner:LG CHEM LTD
Adsorption systems using metal-organic frameworks
InactiveUS20150291870A1High mass loadingReduce heat of adsorptionGroup 1/11 organic compounds without C-metal linkagesComplex cyanidesAdsorption chillerSorbent
The present invention relates to sorbants such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs), porous aromatic frameworks (PAFs) or porous polymer networks (PPNs) for separations of gases or liquids, gas storage, cooling, and heating applications, including, but not limited to, adsorption chillers.
Owner:BATTELLE MEMORIAL INST +1
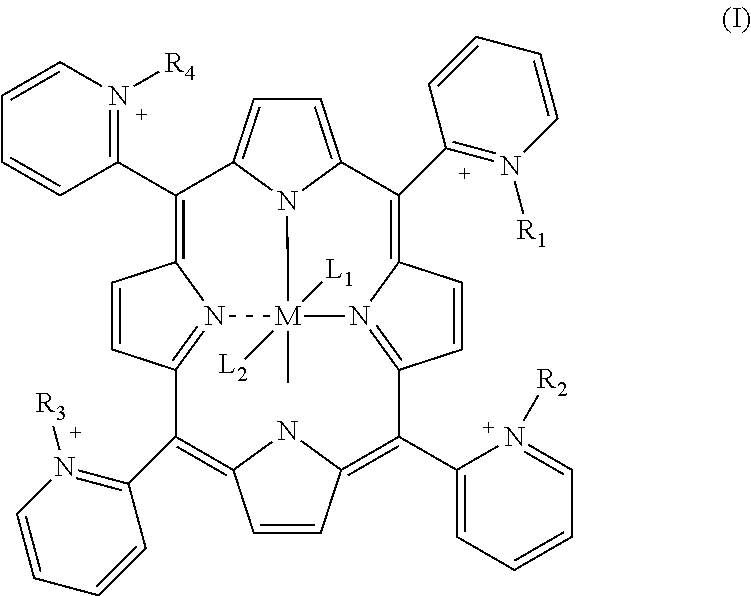
Porphyrin catalysts and methods of use thereof
InactiveUS8232267B2Extended service lifeHigh catalytic activityBiocideNervous disorderPeroxynitriteDecomposition
This invention provides a novel class of substituted macrocyclic porphyrin compounds. The compounds are useful as peroxynitrite decomposition catalysts. Pharmaceutical compositions, and methods of making and using the compounds, or a pharmaceutically acceptable salt, hydrate, or prodrug thereof are also described.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Hybrid porous material and methods of preparing the same
ActiveUS20110144365A1Silicon organic compoundsOxygen/ozone/oxide/hydroxideMaterials scienceChemical bond
A hybrid porous material including at least a first and a second porous material portion which are chemically bonded to each other and are each a different type of material.
Owner:SAMSUNG ELECTRONICS CO LTD
Tetrahydrofuran-adducted group II beta-diketonate complexes as source reagents for chemical vapor deposition
InactiveUS6218518B1Simple methodGroup 3/13 element organic compoundsGroup 2/12 organic compounds without C-metal linkagesHolographic storageBarium strontium titanate
Owner:ADVANCED TECH MATERIALS INC
Metal-containing azo compound and optical recording media
InactiveUS6551682B1Improve performanceGroup 1/11 organic compounds without C-metal linkagesLayered productsDivalent metalNitrogen
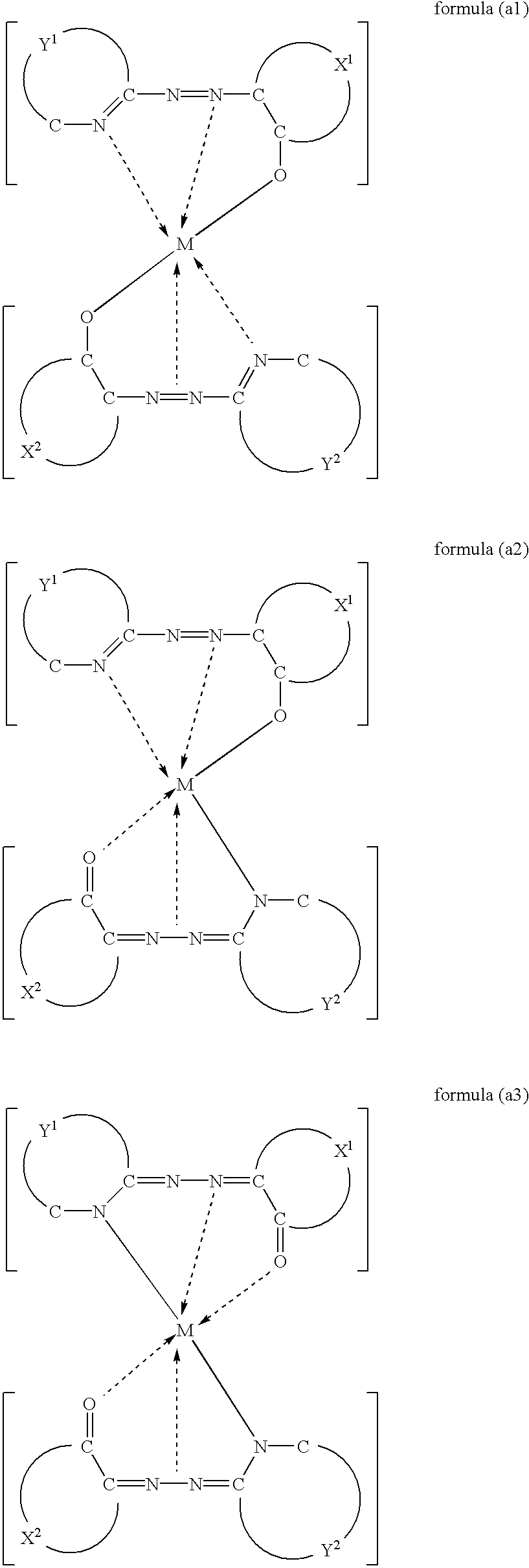
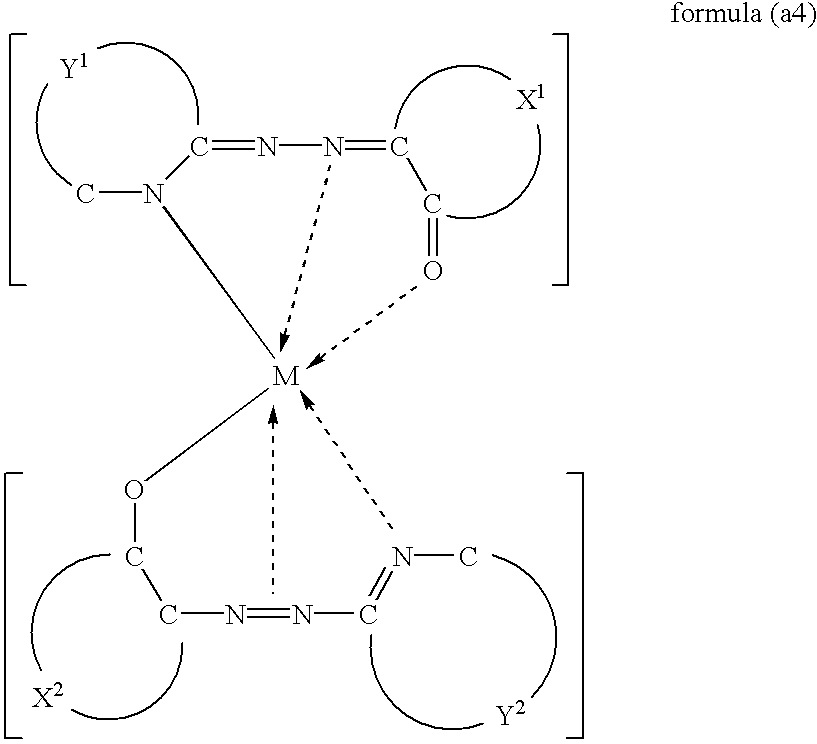
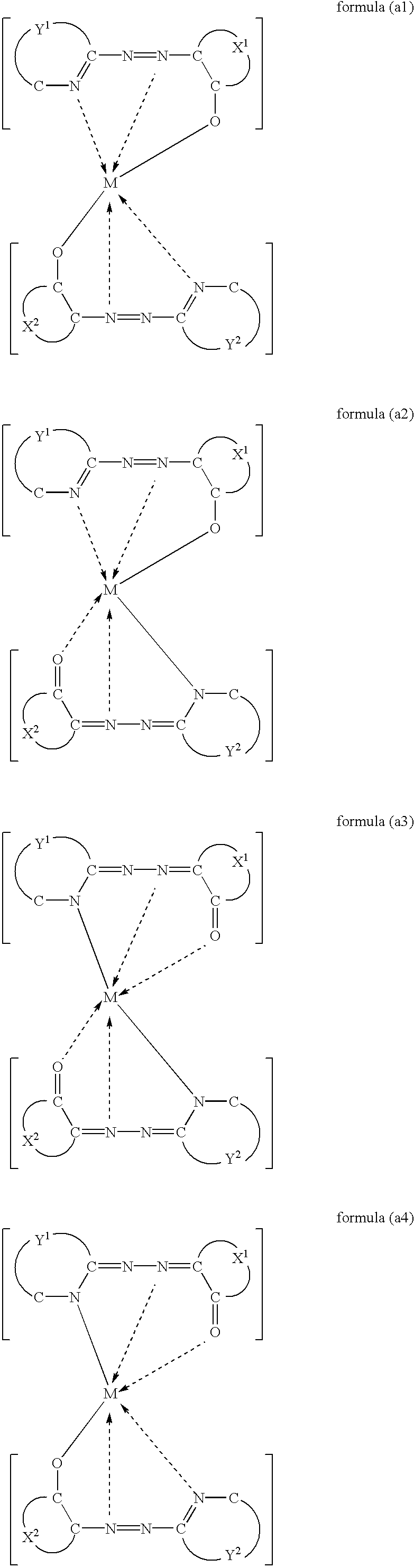
There is provided a metal-containing azo compound suitably used for an recording layer of an optical medium, which is represented by at least one of the following general formulae:wherein M(II) is a bivalent metal, X1 and X2 are each a residue that forms a monocyclic or polycyclic aromatic ring, Y1 and Y2 are each a residue that forms a nitrogen-containing aromatic heterocycle, and one residue is different from the other in at least one of a combination of X1 and X2, and a combination of Y1 and Y2; and there is also provided an optical recording medium of which recording layer comprises said metal-containing azo compound.
Owner:PANASONIC CORP +1
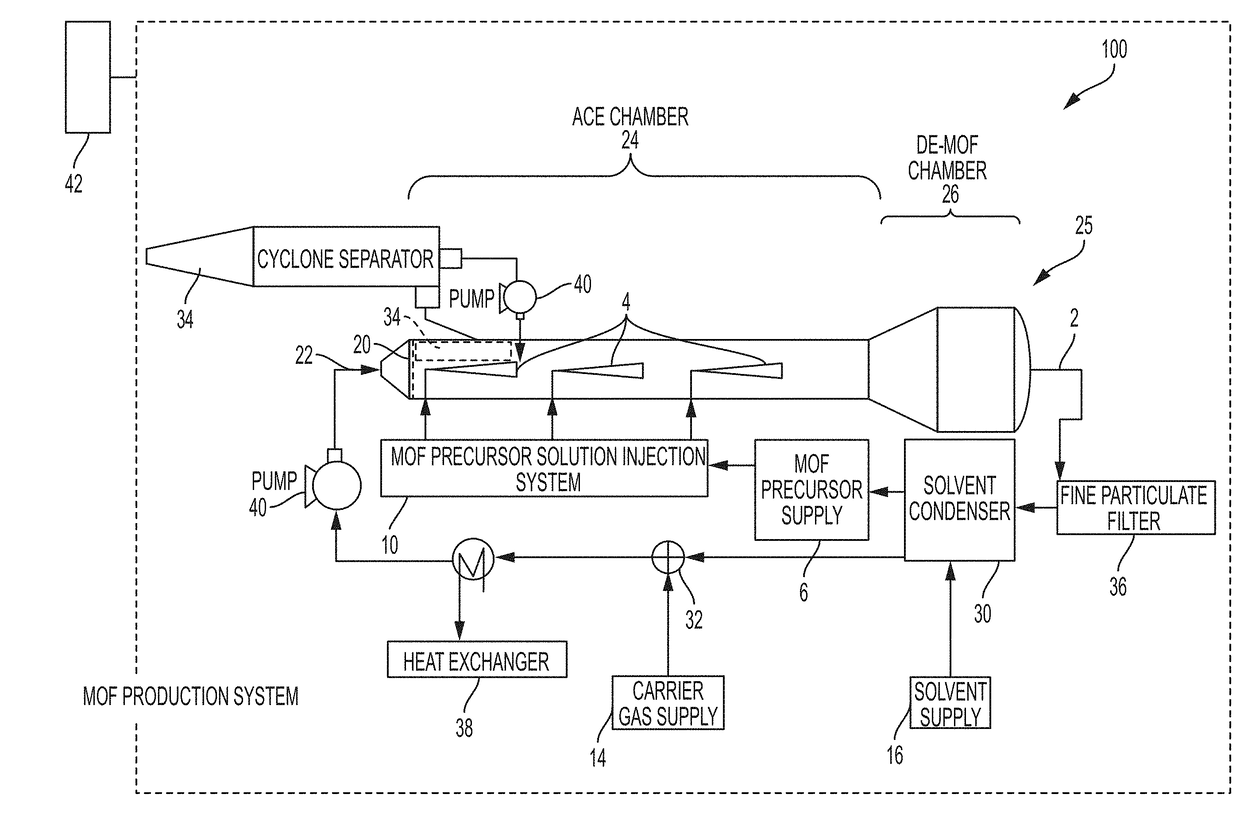
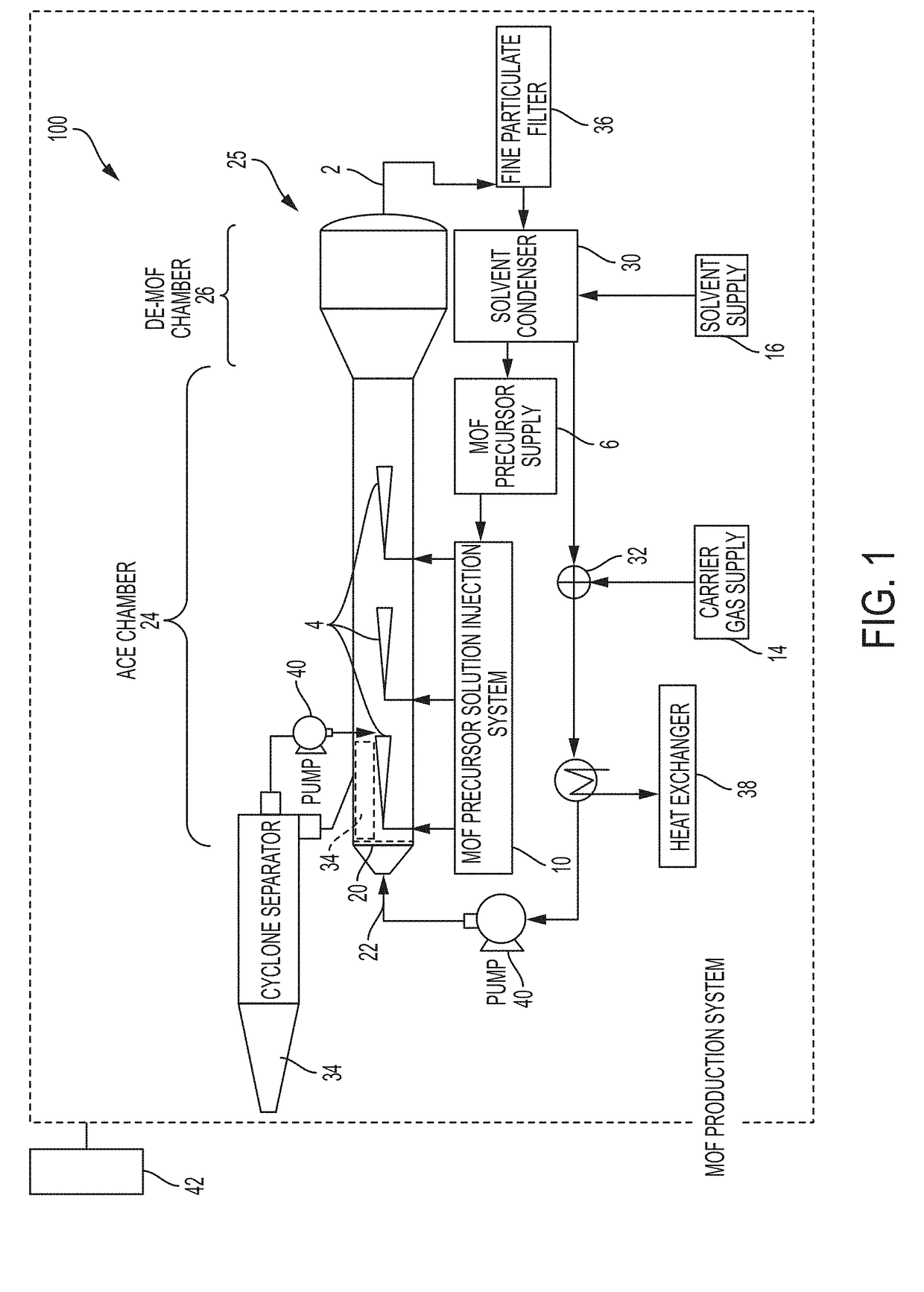
System and process for continuous and controlled production of metal-organic frameworks and metal-organic framework composites
ActiveUS20170361300A1Efficient scalable synthesisEffective and efficient MOF productCopper organic compoundsNickel organic compoundsMetal-organic frameworkMaterials science
Owner:BATTELLE MEMORIAL INST
Micro-channel chemical preparation method of porous metal-organic framework material
InactiveCN104892404AIncrease surface areaUniform particle sizeGroup 1/11 organic compounds without C-metal linkagesOrganic compound preparationLiquid mediumMixed materials
The purpose of the invention is providing a micro-channel chemical preparation method of porous metal-organic framework material. The method comprises the following steps: respectively injecting a liquid medium containing one or more organic compounds with at least one unidentate ligand, a liquid medium containing one or more metal ions, a liquid medium containing a deprotonation assistant and an inert gas into a micro-channel reactor through different inlets, carrying out a coordination reaction on the above obtained mixed material liquid in the micro-channel at a certain temperature under a certain pressure to form a coordination compound, crystallizing, filtering, washing, and drying to prepare the porous metal-organic framework material, wherein the micro-channel reactor has at least two inlets and one outlet; the addition amount of the deprotonation assistant is 0-50% of the mole number of all metal ions; a ratio of the volume velocity of inert gas added into the micro-channel reactor to the bulk volume velocity of the liquid phase is 0-100:1; and the crystallization process can be omitted. The method has the characteristics of simple and safe operation, high efficiency, large throughput and easy amplification.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Baicalin metal complex and preparation method and application thereof
InactiveCN102516341AReduce pollutionAvoid it happening againAntibacterial agentsGroup 1/11 organic compounds without C-metal linkagesDipotassium hydrogen phosphateSodium acetate
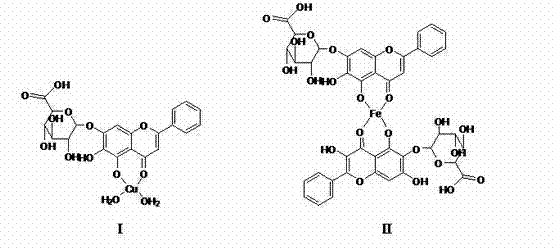
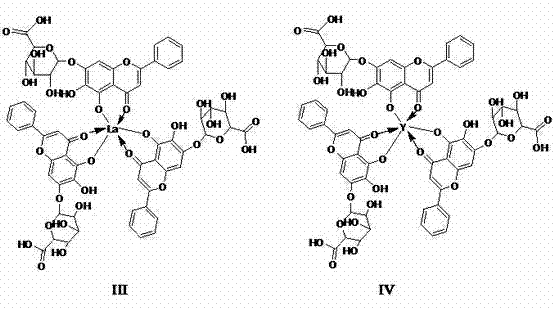
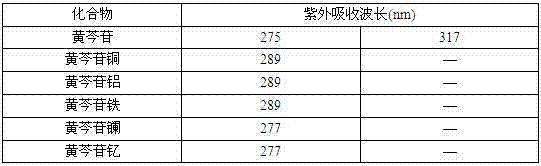
The invention discloses a baicalin metal complex of which the molecular formula is (C21H17O11) xM (H2O) y, wherein M in the formula is Cu (II), x is 1 and y is 2; or M is Fe (III), x is 2 and y is 0; or M is La (III) or Y (III), x is 3 and y is 0. The preparation method comprises the following steps: adding aqueous alkali into baicalin until the baicalin and alkali (sodium bicarbonate, potassium bicarbonate, sodium formate, potassium formate, sodium acetate, potassium acetate, disodium hydrogen phosphate, dipotassium hydrogen phosphate, sodium phosphate or potassium phosphate) are just completely reacted, then adding metal salt (copper salt, ferric salt, lanthanum salt or yttrium salt) for reaction to obtain the baicalin metal complex. The baicalin metal complex has obviously stronger antibacterial and antitumor activities than the baicalin, can be used for preparing antibacterial drugs and antitumor drugs, and has good development and application prospects. In the preparation method, an organic solvent is not used, a strong basic condition is not adopted, and the method is simple and feasible, is green and environment-friendly and has the advantages of low cost, high product purity and high yield.
Owner:SOUTHWEST UNIVERSITY
Mesh-adjustable molecular sieve
InactiveUS20080184881A1Group 1/11 organic compounds without C-metal linkagesOther chemical processesMetal clustersMolecular sieve
Metal-organic framework-based molecular sieves comprising pores with a temperature-adjustable pore opening. The temperature-adjustable pore size molecular sieves comprise a plurality of metal clusters bound with a plurality of amphiphilic ligands, each ligand comprising a functionalized hydrophobic moiety and a functionalized hydrophilic moiety, and wherein the metal clusters and amphiphilic ligand hydrophilic moieties form a metal cluster layer, the metal cluster layer forming at least one hydrophilic pore. On each side of the metal cluster layer, a plurality of associated amphiphilic ligand hydrophobic moieties cooperate with the metal cluster layer to form a tri-layer and a plurality of tri-layers are held in proximity with each other to form at least one hydrophobic chamber. The hydrophobic moieties form temperature-adjustable pore size hydrophobic pores. When adjusted to a pre-selected temperature the temperature-adjustable pore openings allow for the passage of molecules having a size less than the size of the pre-selected temperature-adjustable pore opening.
Owner:MIAMI UNIVERSITY
Coordination Polymer Crystal With Porous Metal-Organic Frameworks and Preparation Method Thereof
ActiveUS20100174047A1Low efficiencyInhibition releaseLithium organic compoundsReversible hydrogen uptakeSimple Organic CompoundsMetal clusters
Disclosed is a coordination polymer crystal with porous metal-organic frameworks (MOFs), in which, while a crystal state of the coordination polymer crystal is maintained, an additional material selected from the group consisting of an organic compound, a metal cluster, and an organometallic compound is chemically bonded to the coordination polymer crystal. Therefore it is possible easily adsorb and store more guest molecules regardless of a change in an ambient temperature or pressure due to the chemically bonded additional material.
Owner:INSILICO CO LTD
Preparation of metal alkoxides
InactiveUS6355821B1Heat dissipationReduce thermal decompositionPlatinum group organic compoundsCopper oxides/halidesSolventEthanol
Methods of forming metal alkoxides and methods of forming precursor solutions of metal alkoxides suitable for the coating of glass in the manufacture of electrochromic devices are disclosed. The method of forming metal alkoxides involves dissolving the metal halide in an anhydrous solvent and reacting it with an alcohol and (together with the addition of the alcohol or subsequently) adding an epoxide, and then evaporating-off the volatile components of the reaction product to leave a solid metal alkoxide that is substantially free of halide. The alkoxide may then be dissolved in a solvent including an alcohol (preferably ethanol) containing a small proportion of water to produce a precursor solution suitable for coating glass, the coating then being hydrolyzed to form a sol-gel and then baked to remove volatile components and to yield a thin layer of metal oxide.
Owner:DYESOL PTY LTD
Method for the preparation of metal organic frameworks
ActiveUS20140284829A1Delayed reaction timePrevent steppingGroup 1/11 organic compounds without C-metal linkagesOrganic compound preparationMetal-organic frameworkSolvent
A process for the preparation of a dry crystalline metal organic framework which comprises: a) spray drying at least one metal ion and at least one organic ligand which is at least bidentate into a spray dryer in the presence of a solvent, wherein the reaction of the at least one metal ion with the at least one organic ligand to yield the metal organic framework and the drying of the obtained metal organic framework take place simultaneously inside the spray dryer, and b) collecting the formed dry crystalline metal organic framework.
Owner:FUNDACIO PRIVADA INST CATALA DE NANOTECNOLOGIA
Sol solution and method for film formation
InactiveUS6149967AImprove stabilityImprove responseGas-filled discharge tubesOther chemical processesDisplay deviceAlternating current
A method for forming a protective layer for a dielectric material in an alternating current type plasma display is provided. In this connection, a coating sol solution is provided which can form a protective layer on a large area substrate without the need to introduce any expensive equipment, the protective layer thus formed being excellent in properties such as strength, adhesion, transparency, and protective properties and capable of being formed by a sol-gel process without use of the conventional vacuum process. The sol solution comprises a dispersion of a precursor to magnesium oxide in a specific form. A method for film formation, using this solution is also provided.
Owner:DAI NIPPON PRINTING CO LTD
Metal organic framework (MOF) yellow phosphors and their applications in white light emitting devices
Owner:RUTGERS THE STATE UNIV
Doped metal organic frameworks for reversible H2 storage at ambient temperature
Metal-organic frameworks (MOFs) are provided. An exemplary MOF includes a plurality of metal clusters, at least one linking ligand, and at least one dopant. Doped MOFs according to embodiments of the present invention have significantly increased H2 uptake capacity, and some embodiments meet the 2010 DOE H2 storage target of 6 wt % at a temperature ranging from −30 to 80° C. and a pressure less than or equal to 100 bar.
Owner:GODDARD WILLIAM A +1
Water stable zinc-based metal organic framework and method of use
ActiveUS20200190114A1Organic-compounds/hydrides/coordination-complexes catalystsEnergy inputMetal-organic frameworkEngineering
A zinc-based metal organic framework and method of making is described. The zinc-based metal organic framework is in the form of an interpenetrating diamondoid framework where each Zn2+ ion center is linked with four other Zn2+ ion centers in a distorted tetrahedral geometry. The linking occurs through diamine and dicarboxylic acid linkers. The zinc-based metal organic framework may be deposited on a transparent conducting film and used as a photoelectrode for photoelectrochemical water splitting.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com