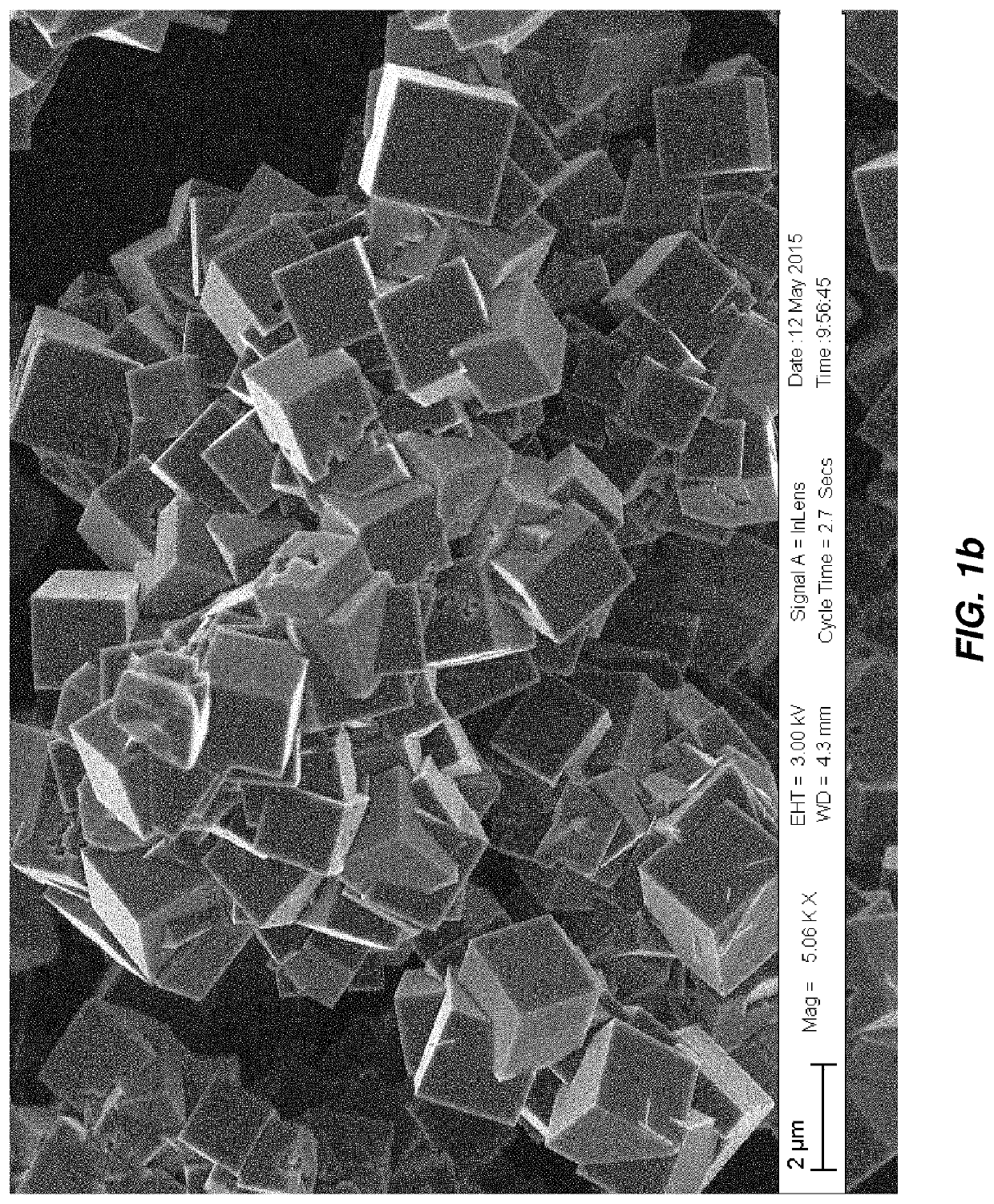
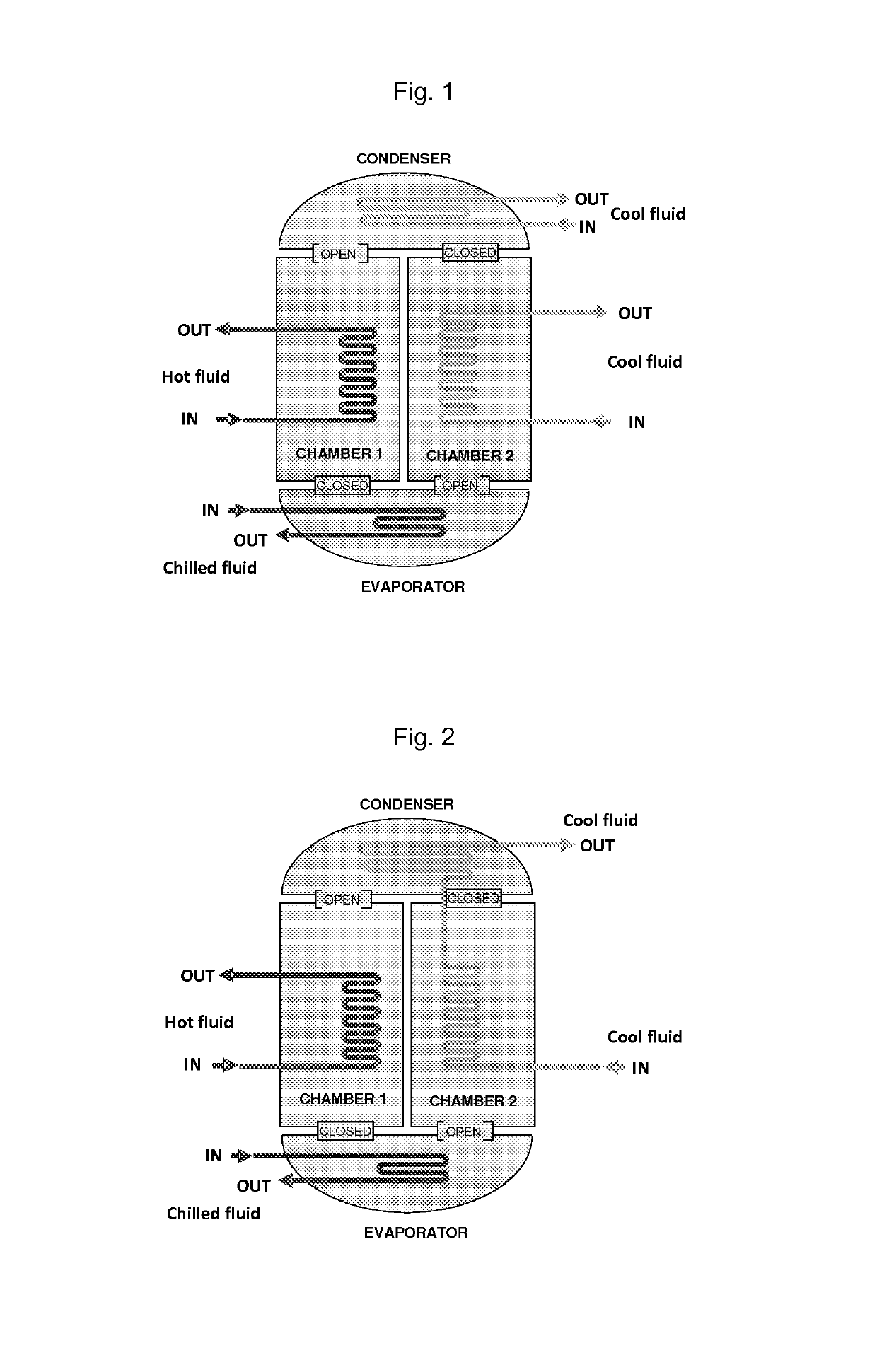
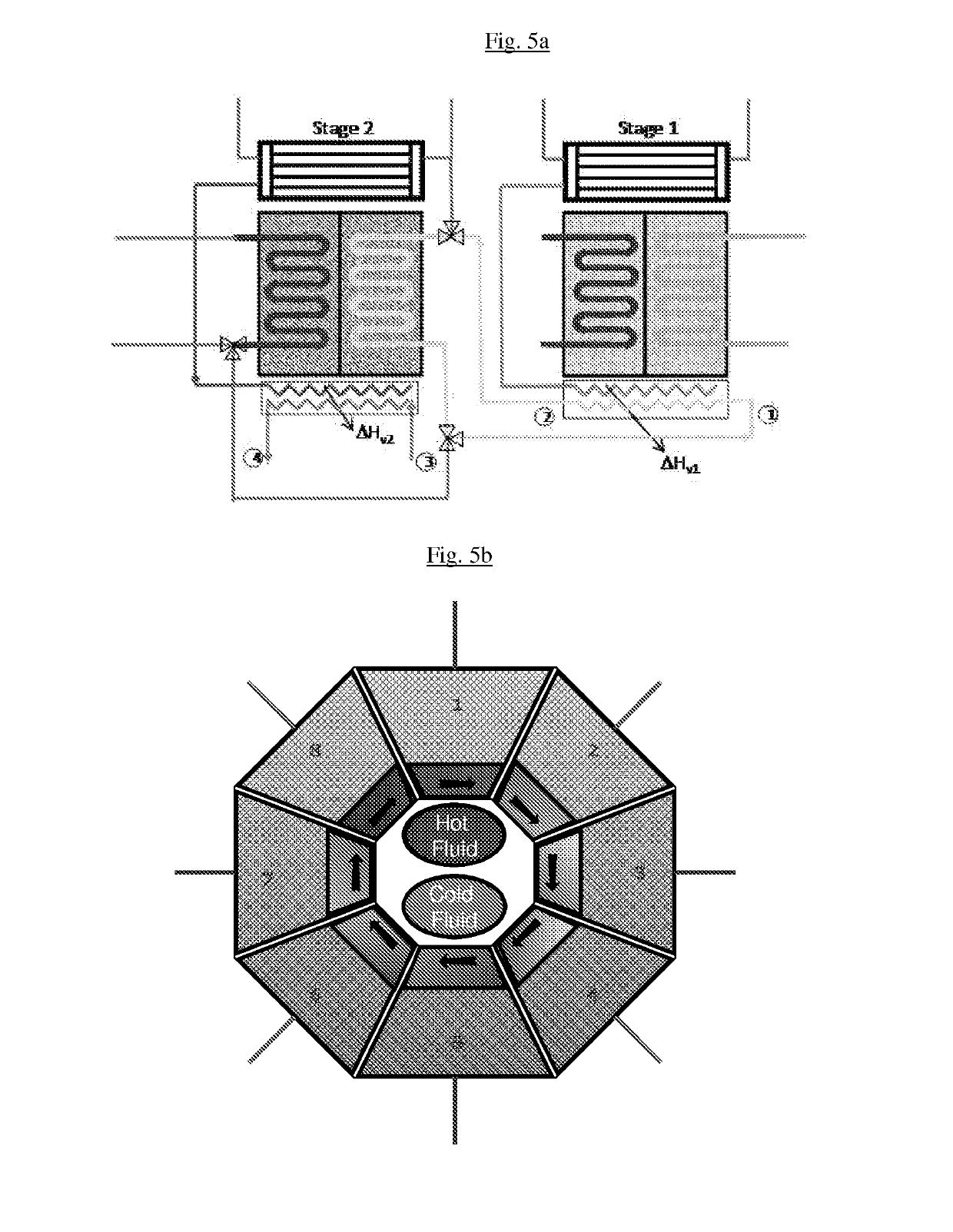
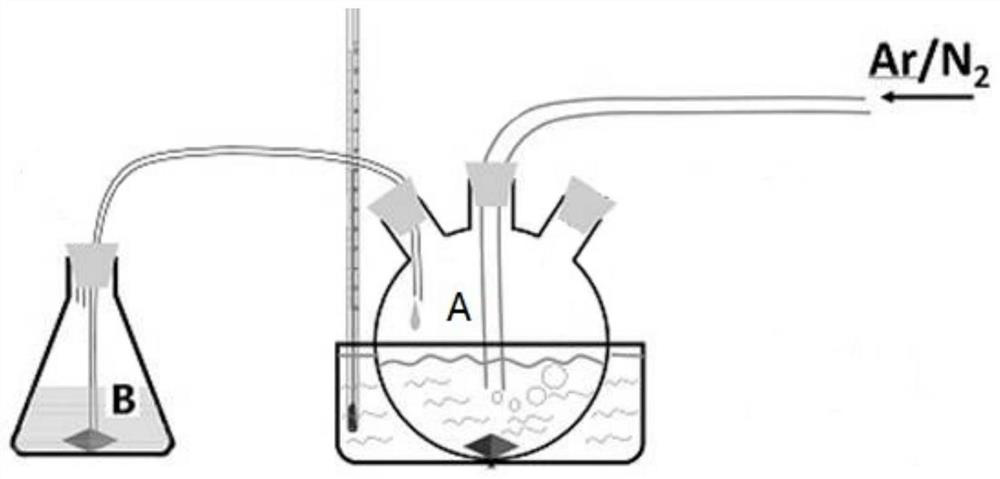
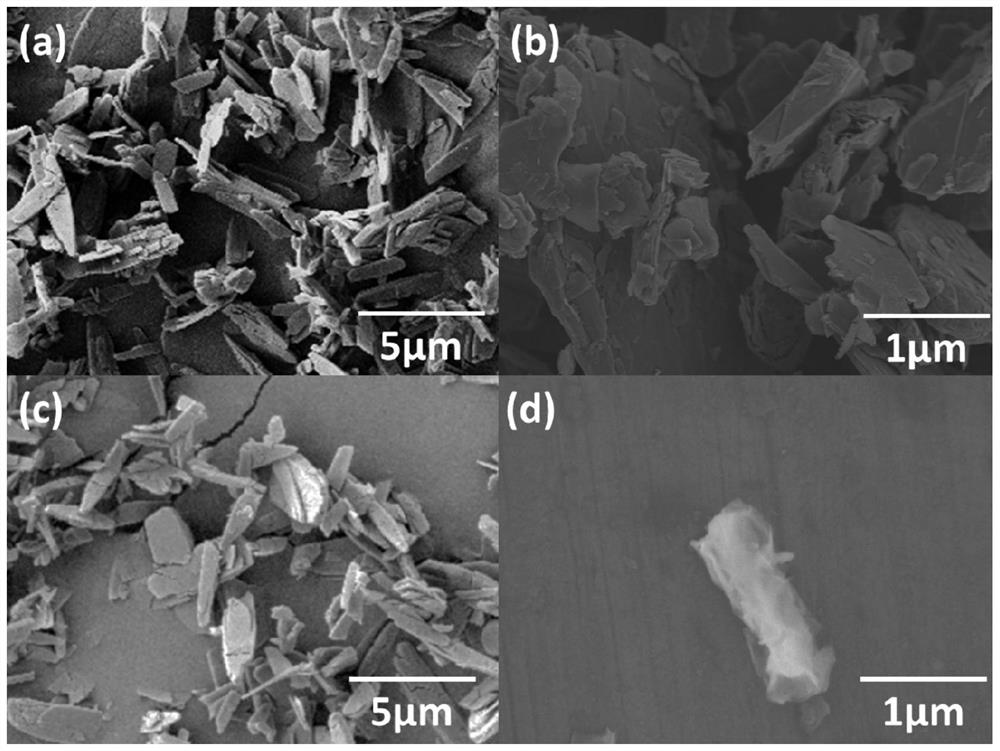
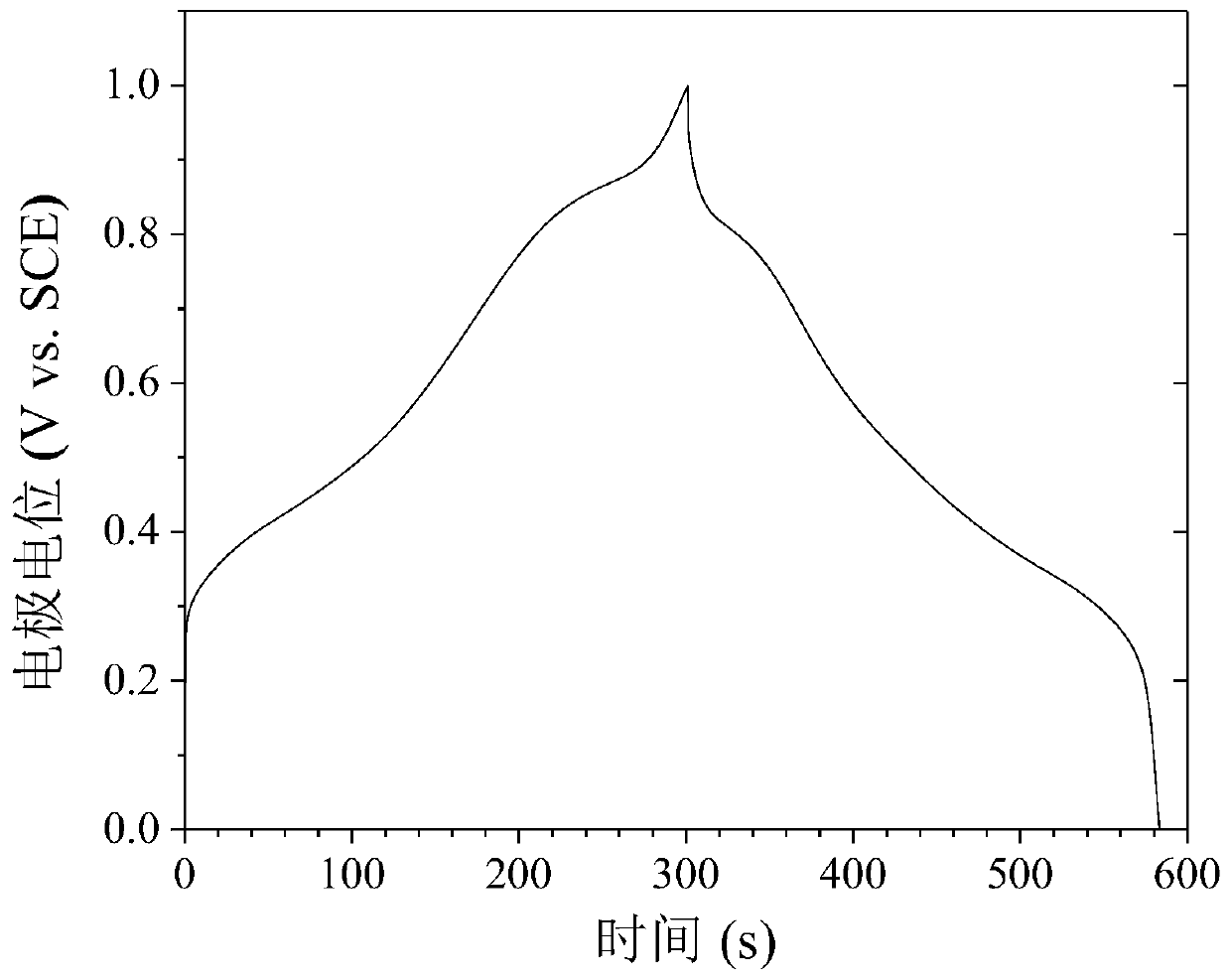
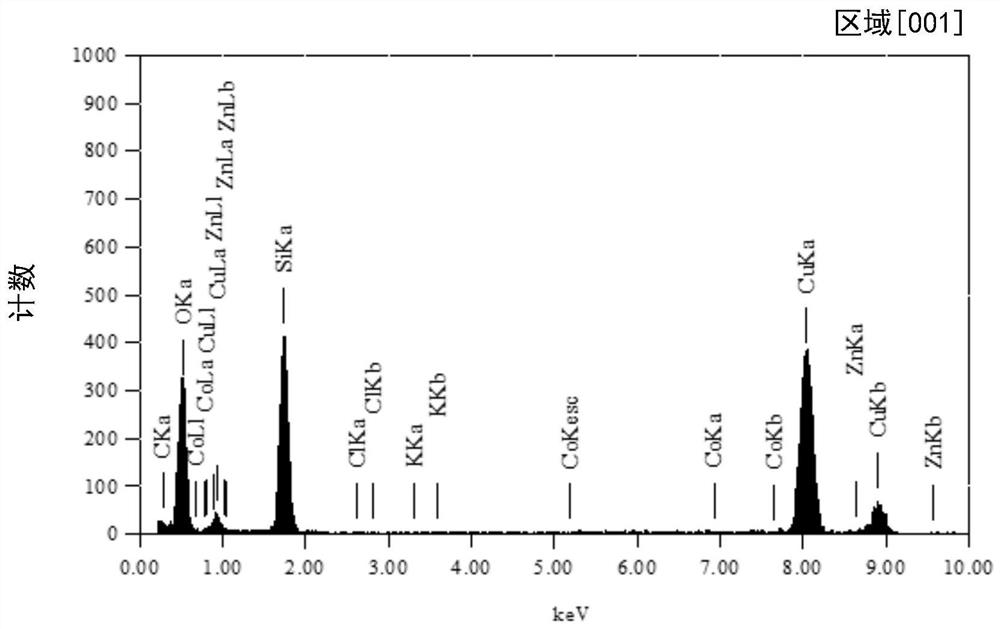
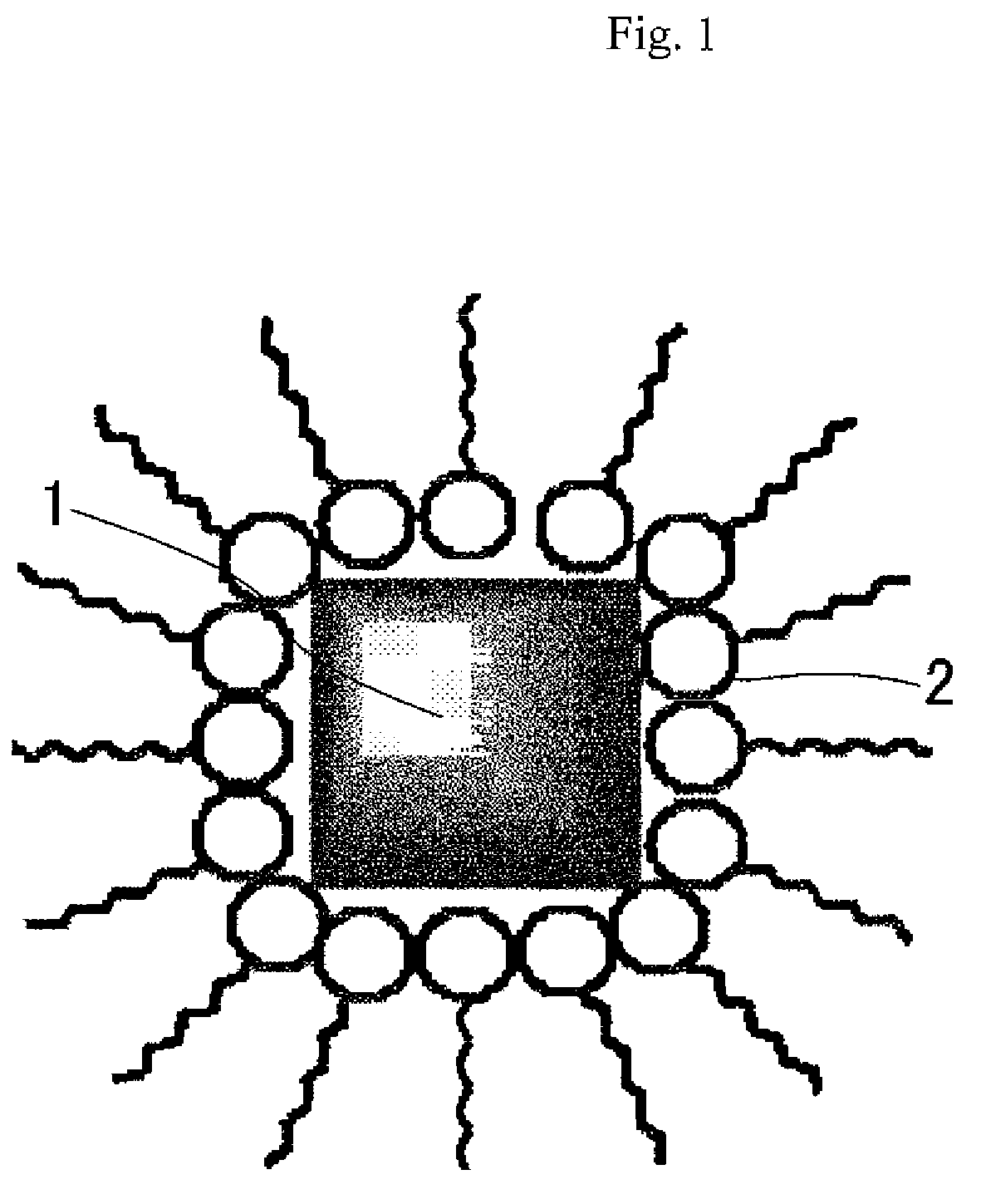
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
85results about "Complex cyanides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
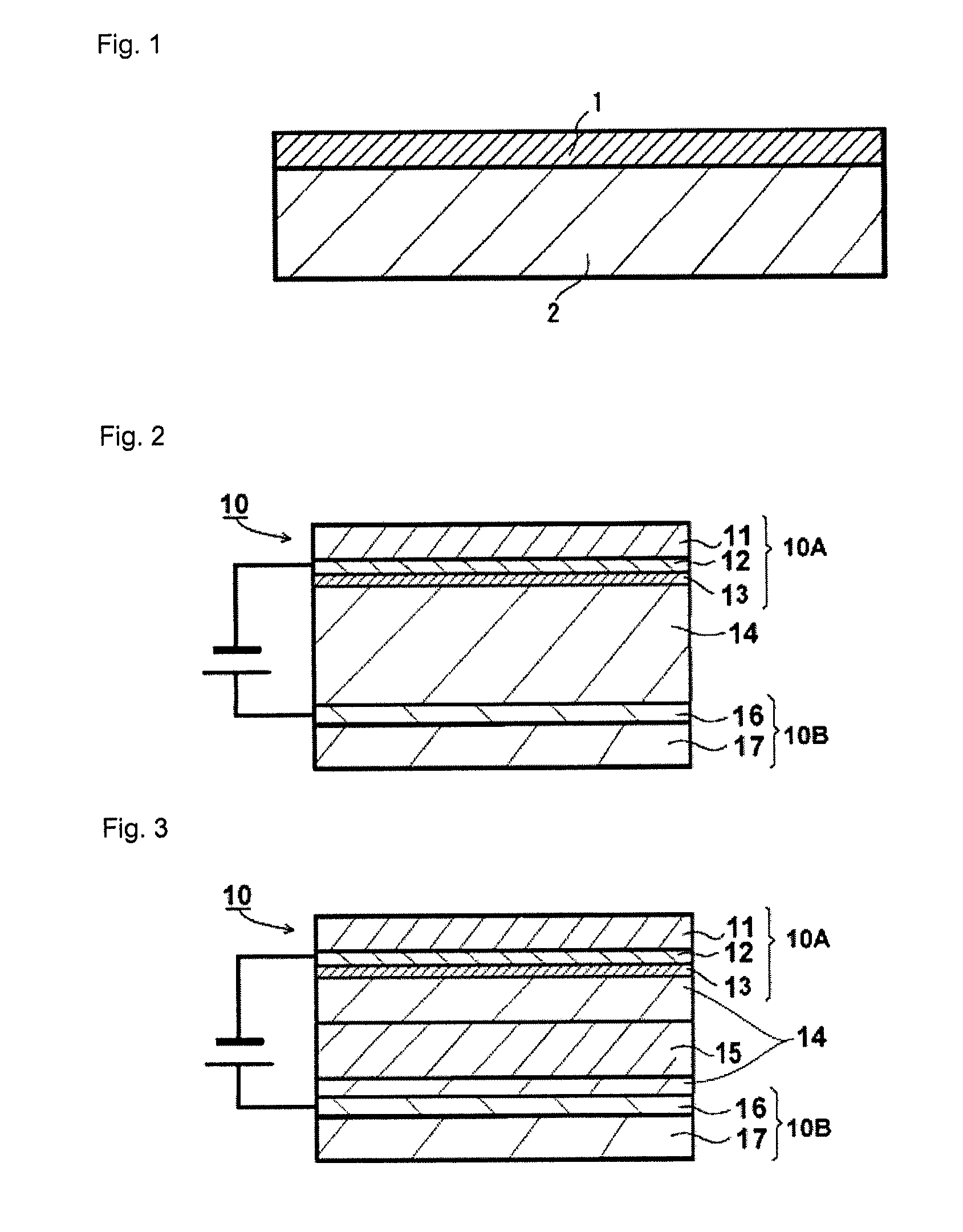
Prussian Blue Analogue Anodes for Aqueous Electrolyte Batteries
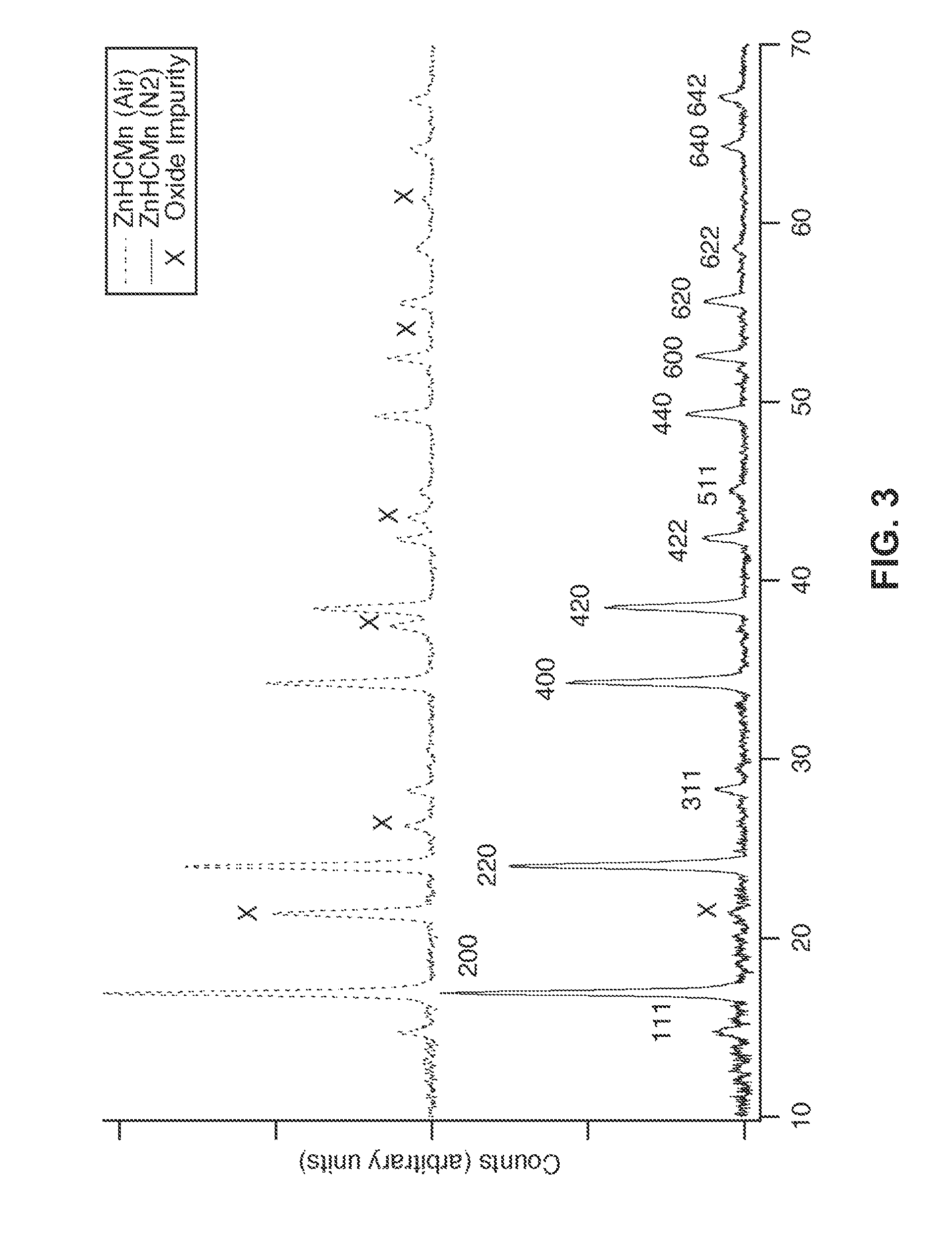
InactiveUS20140220392A1Maximizes energy storageReduces electrochemical decompositionIron cyanidesComplex cyanidesRedoxPrussian blue
A system and method producing electrodes in an aqueous electrolyte battery that maximizes energy storage, reduces electrochemical decomposition of the electrolyte, and uses Prussian Blue analogue materials for both electrodes, with an anode electrode including an electrochemically active hexacyanometalate group having two possible redox reactions of different potentials. These potentials may be tuned by substituting different electrochemically inactive components.
Owner:NATRON ENERGY INC
Adsorption systems using metal-organic frameworks
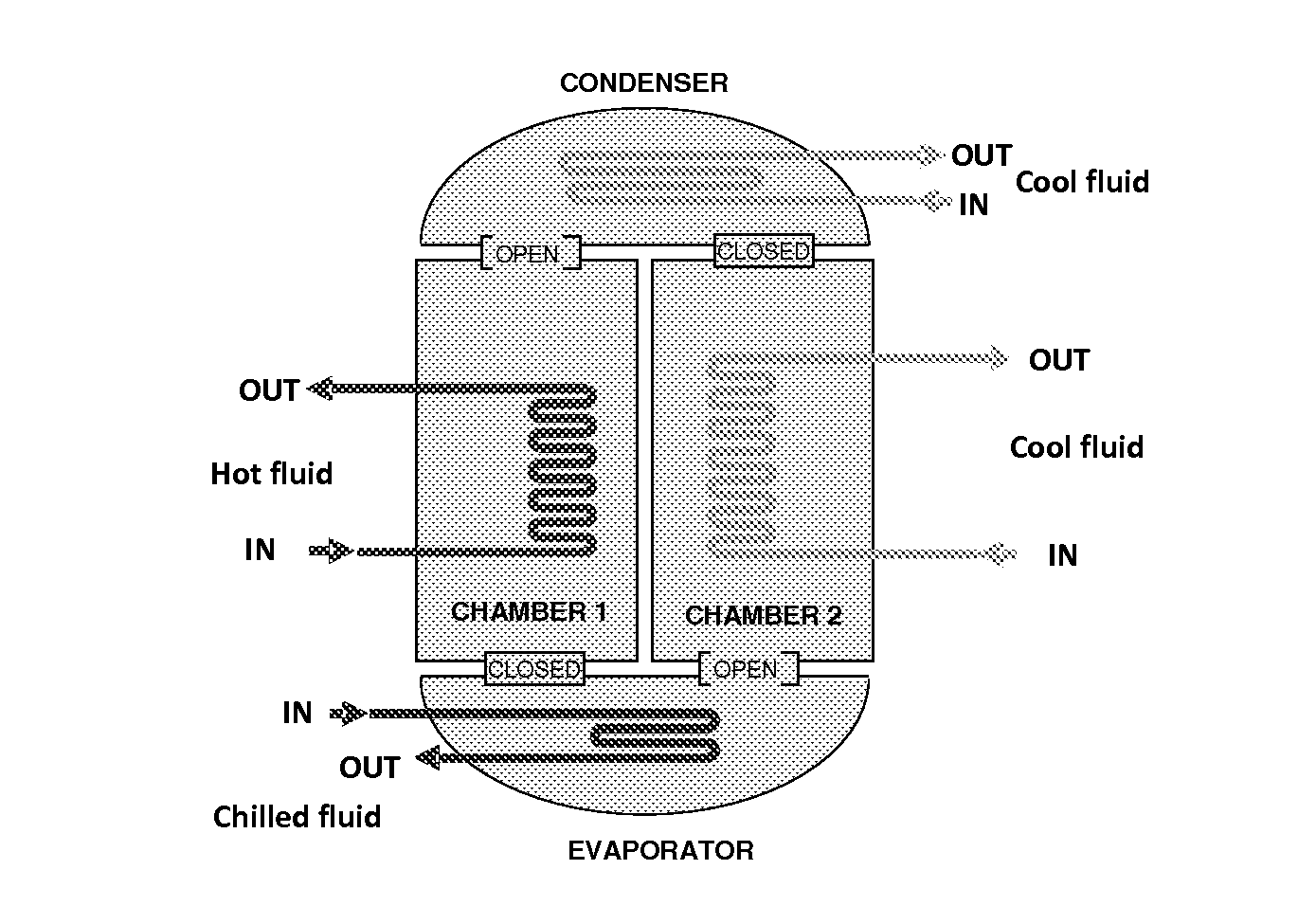
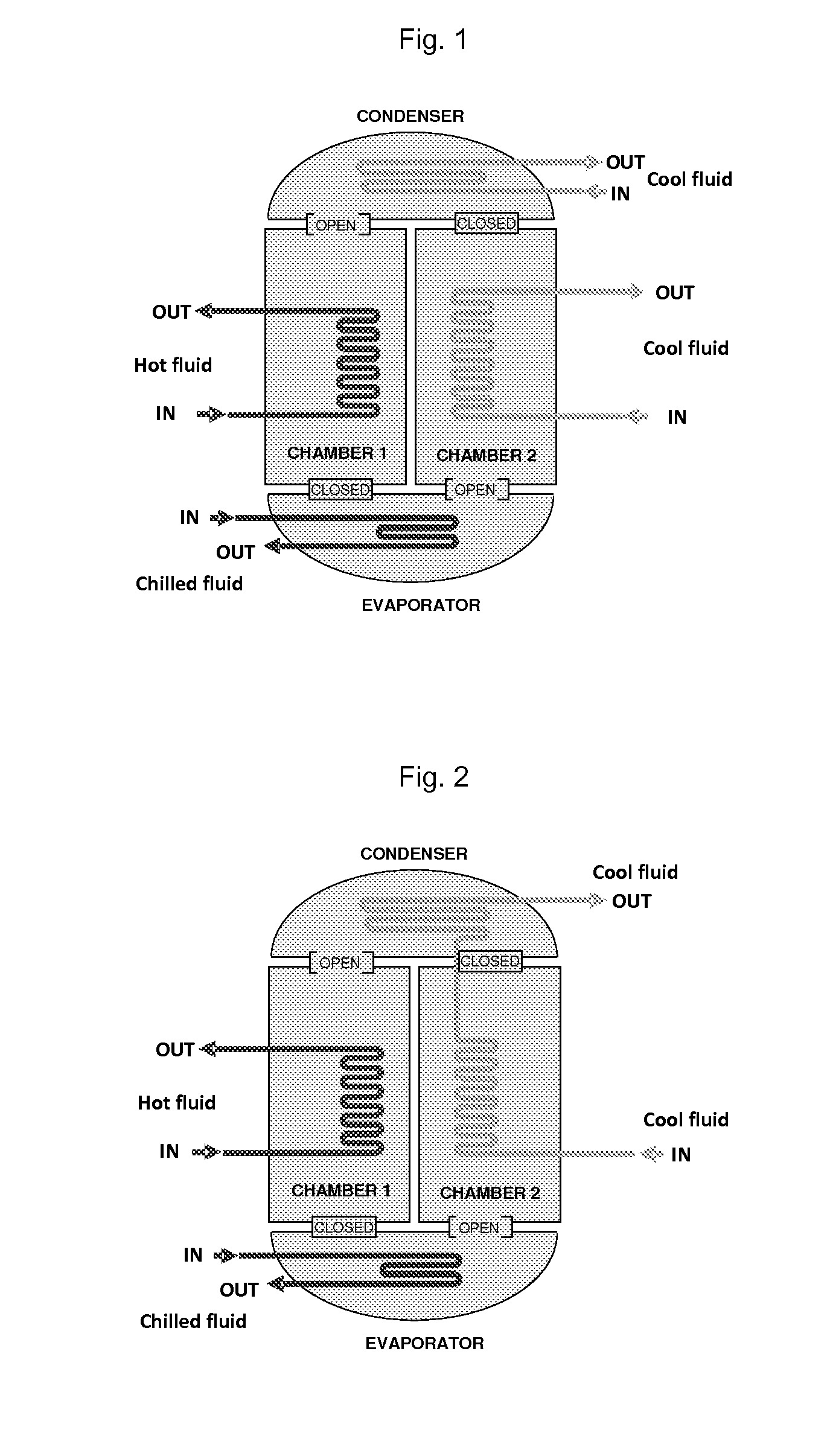
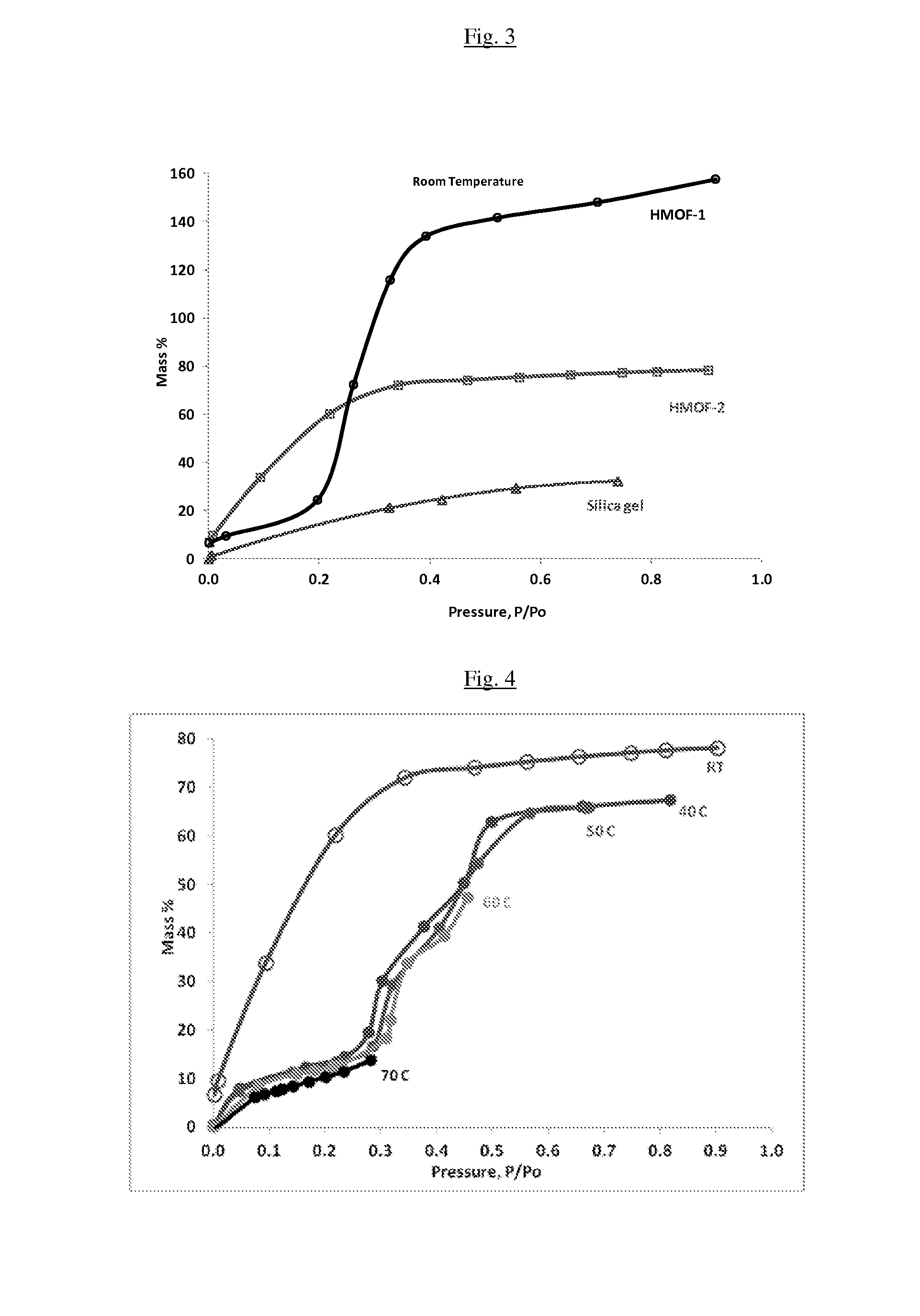
InactiveUS20150291870A1High mass loadingReduce heat of adsorptionGroup 1/11 organic compounds without C-metal linkagesComplex cyanidesAdsorption chillerSorbent
The present invention relates to sorbants such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs), porous aromatic frameworks (PAFs) or porous polymer networks (PPNs) for separations of gases or liquids, gas storage, cooling, and heating applications, including, but not limited to, adsorption chillers.
Owner:BATTELLE MEMORIAL INST +1
Prussian Blue Analogue Electrodes without Zeolitic Water Content
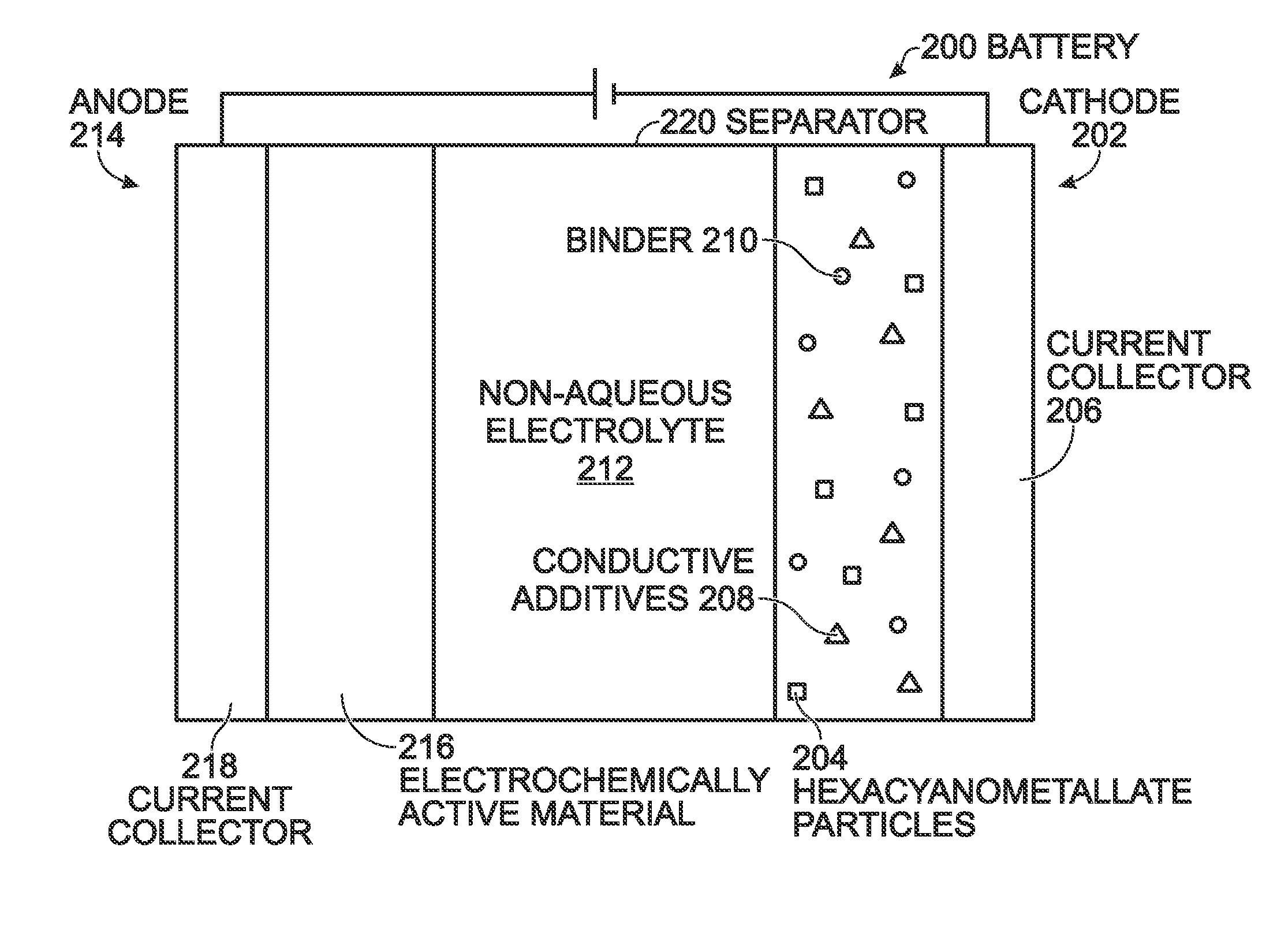
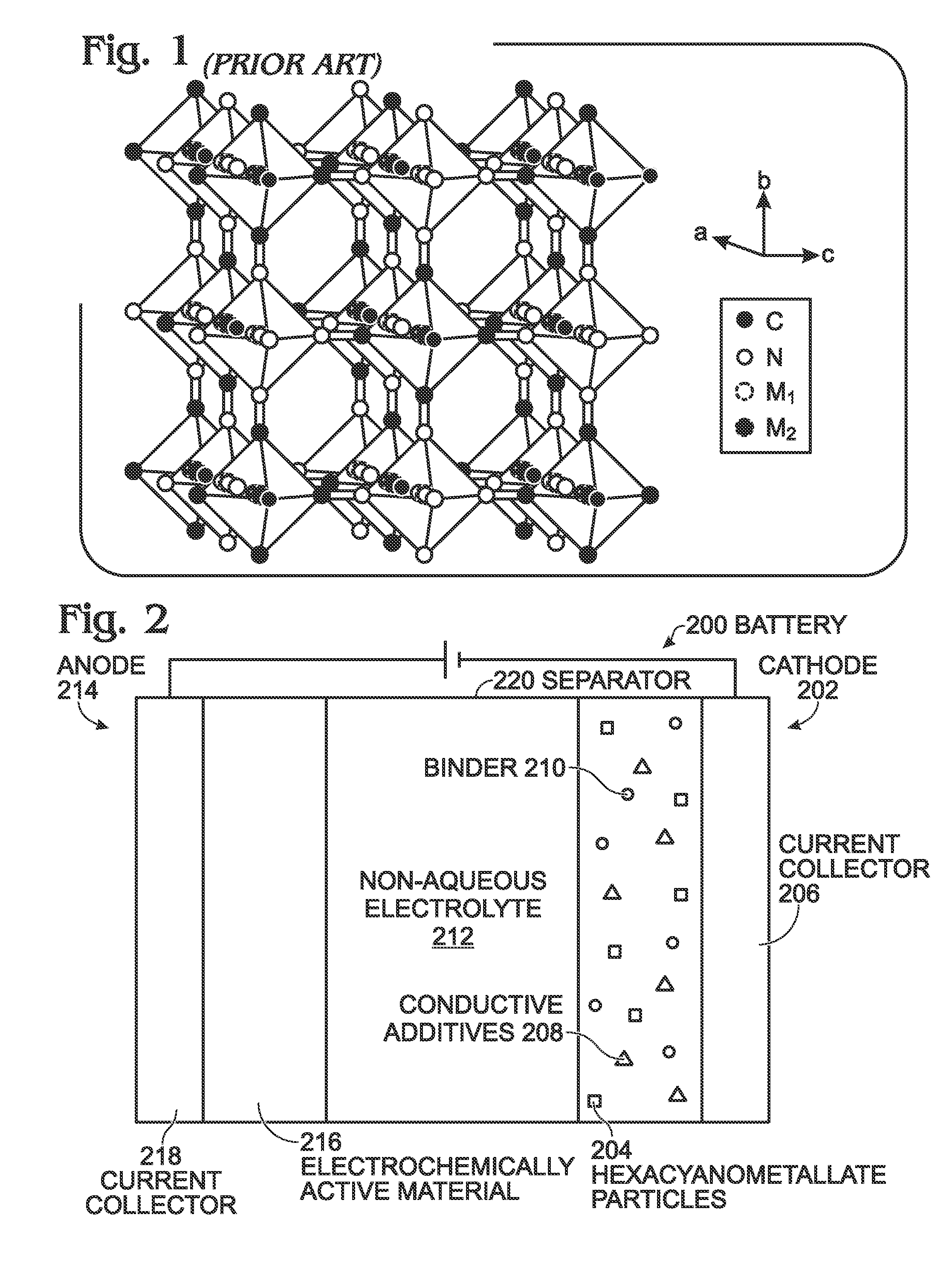
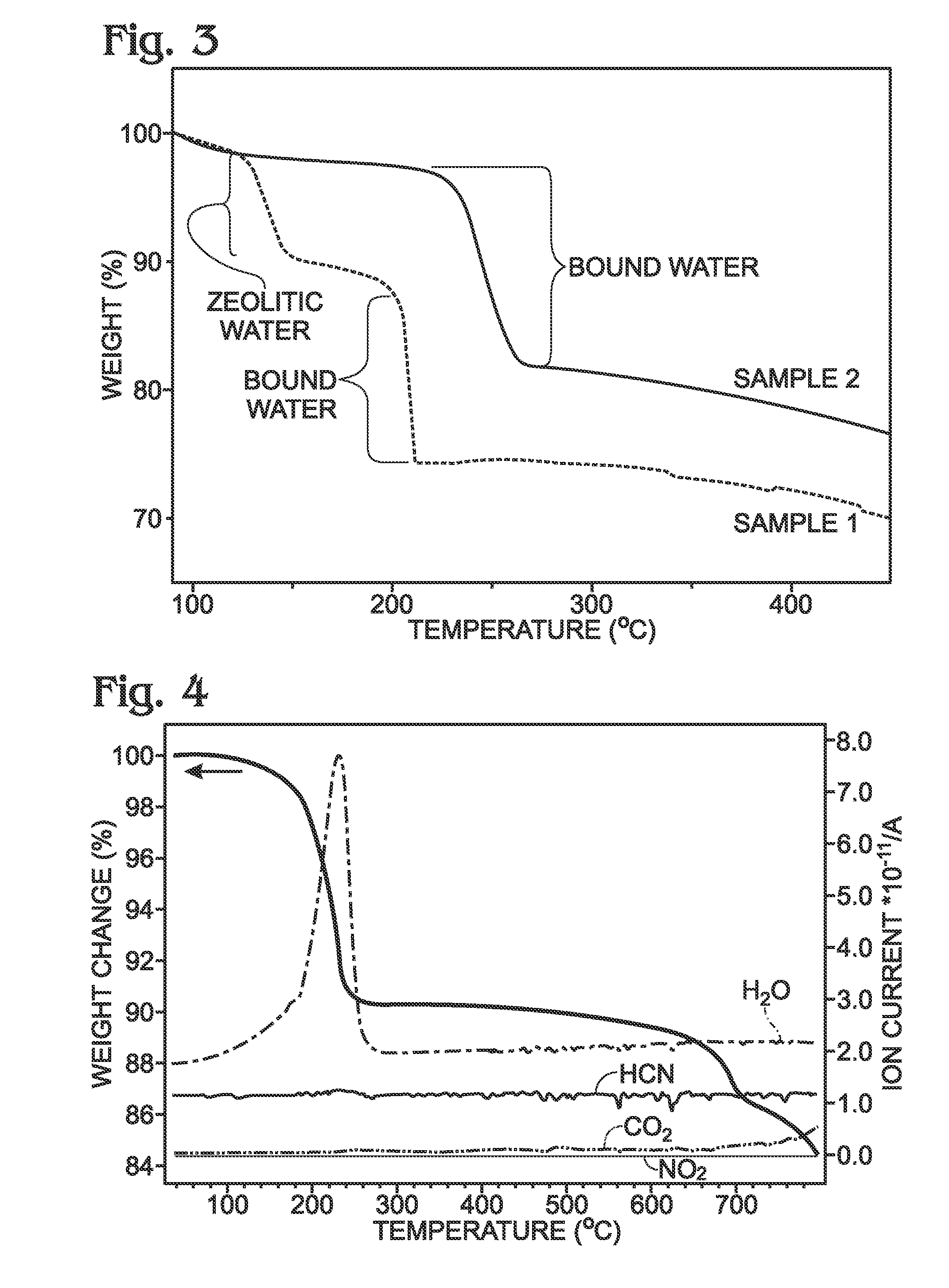
A battery is provided with a hexacyanometallate cathode. The battery cathode is made from hexacyanometallate particles overlying a current collector. The hexacyanometallate particles have the chemical formula AXM1MM2N(CN)Z.d[H2O]ZEO.e[H2O]BND. where A is a metal from Groups 1A, 2A, or 3A of the Periodic Table, where M1 and M2 are each a metal with 2+ or 3+ valance positions, where “ZEO” and “BND” indicate zeolitic and bound water, respectively, where d is 0, and e is greater than 0 and less than 8. The anode material may primarily be a material such as hard carbon, soft carbon, oxides, sulfides, nitrides, silicon, metals, or combinations thereof. The electrolyte is non-aqueous. A method is also provided for fabricating hexacyanometallate with no zeolitic water content in response to dehydration annealing at a temperature of greater than 120 degrees C. and less than 200 degrees C.
Owner:SHARP KK
Method of producing prussian blue-type metal complex nanoparticles, and prussian blue-type metal complex nanoparticles obtained by the method, dispersion of the nanoparticles, method of regulating the color of the nanoparticles, and electrode and transmitted light-regulator each using the nanoparticles
ActiveUS20100133487A1Suitable for mass productionExcessive amountMaterial nanotechnologyConductive materialNanometer sizeCoordination complex
To provide a method of producing Prussian blue-type metal complex nanoparticles without necessarily requiring complicated steps and an excessive amount of raw materials, but allowing one to obtain nanometer-size fine particles having desired fine particle properties, and Prussian blue-type metal complex nanoparticles obtained by the method, a dispersion of the nanoparticles, a method of regulating the color of the nanoparticles, and an electrode and a transmitted light-regulator each using the nanoparticles; Prussian blue-type metal complex nanoparticles are produced by: mixing an aqueous solution containing a metal cyano complex anion having predetermined metal atom MA as a central metal and an aqueous solution containing a cation of predetermined metal atom MB, thereby precipitating the crystal of a Prussian blue-type metal complex having the metal atom MA and the metal atom MB; and then mixing the Prussian blue-type metal complex with an aqueous solution containing a metal cyano complex anion having the metal atom MC as a central metal and / or an aqueous solution containing a cation of the metal atom MD. The thus-obtained Prussian blue-type metal complex nanoparticles have a preferable electrochemical responsiveness, and the particles can be used for forming a thin film including them to construct a light-transmitted regulator.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Prussian blue sodium ion battery positive electrode material and preparation method thereof
InactiveCN113488646ASlow crystallization rateHigh crystallinityIron cyanidesComplex cyanidesElectrical batteryPyrrolidinones
The invention relates to a Prussian blue sodium ion battery positive electrode material and a preparation method thereof. The method comprises the following steps: dissolving transition metal salt and tartaric acid in deionized water to form a solution A, dissolving sodium ferrocyanide decahydrate and ascorbic acid in deionized water to form a solution B, and dissolving polyvinylpyrrolidone and sodium chloride in deionized water to form a solution C; simultaneously adding the solution A and the solution B into the solution C through a peristaltic pump, and performing stirring while heating in an N2 atmosphere until the solution becomes a suspension liquid after dropwise adding is completed; keeping heating and stirring the suspension for 12 hours, finally aging at room temperature for 24 hours, then centrifugally washing with deionized water and absolute ethyl alcohol for three times respectively, and finally drying in vacuum at 120 DEG C for 24 hours to obtain the Prussian blue positive electrode material.
Owner:CHINA THREE GORGES UNIV
Process for the production of polyols using DMC catalysts having unsaturated tertiary alcohols as ligands
The present invention provides an active double metal cyanide (DMC) catalyst made of a non-hexanitrometallate containing double metal cyanide compound, one or more unsaturated tertiary alcohols and about 0 to about 80 wt. %, based on the amount of catalyst, of a functionalized polymer having a number average molecular weight greater than about 200. Also provided are methods of producing the inventive catalysts. The inventive catalysts may find use in the production of polyols.
Owner:COMBS GEORGE
Preparation method of potassium aurous cyanide
InactiveCN101624706AGood chemical stabilityPromote circulationElectrolysis componentsComplex cyanidesElectrolysisPotassium cyanide
The invention discloses a preparation method of potassium aurous cyanide, which adopts the following devices: an electrolysis bath, a diaphragm tank, an anode plate and a cathode plate. The anode plate is made of a coated titanium alloy plate and comprises a bottom plate, a lateral plate and an outer plate, wherein the outer plate is fixed with an anode bar. The diaphragm tank is arranged on the upper end face of the bottom plate of the anode plate. The cathode plate comprises a plumbing plate, a transverse plate, and an outer plate, wherein the outer plate is fixed with a cathode bar. The preparation method comprises the following steps: rolling gold into a plurality of flash gold sheets, and then winding and clustering, and then dispersedly placing on the bottom plate of the anode plate around the diaphragm tank; adding a potassium cyanide solution into an anode chamber, adding a potassium hydroxide solution into a cathode chamber, heating to 40-65 DEG C and then electrifying and electrolyzing until the current is broken down. A potassium aurous cyanide solution in the anode chamber after being electrolyzed is post treated to obtain the potassium aurous cyanide. The mass percent of gold in the product prepared by the invention is more than 68.3 percent; moreover, the device can be massively produced.
Owner:常州化工研究所有限公司
Prussian blue transition metal cyanide, preparation method thereof, and positive pole piece, secondary battery, battery pack and device related to prussian blue transition metal cyanide
ActiveCN112259730AIncrease compaction densityIncrease energy densityComplex cyanidesSecondary cellsCyanide compoundChemical physics
The invention belongs to the technical field of energy storage devices, and provides a prussian blue transition metal cyanide, a preparation method thereof, and a positive pole piece, a secondary battery, a battery pack and a device related to the prussian blue transition metal cyanide. The prussian blue transition metal cyanide comprises secondary particles, wherein the secondary particles comprise a plurality of primary particles; and the primary particles are in a spherical shape or a sphere-like shape. The prussian blue transition metal cyanide has the problem of relatively low gram volume, so that the energy density of the sodium ion battery is relatively poor, and the commercial application of the sodium ion battery is influenced. The secondary particles can increase the particle granularity and the powder compaction density, can further increase the compaction density of the positive pole piece, and improve the active ion transmission performance and the electronic conductivityof a positive pole piece, so that the energy density and the rate capability of the secondary battery adopting the secondary particles can be improved.
Owner:JIANGSU CONTEMPORARY AMPEREX TECHNOLOGY LIMITED
Surface-modified cyanide-based transition metal compounds
ActiveUS9299981B1Improve air stabilityImprovement of their fade capacity lossIron cyanidesNon-aqueous electrolyte accumulatorsCompound aCyanide
A system, method, and articles of manufacture for a surface-modified transition metal cyanide coordination compound (TMCCC) composition, an improved electrode including the composition, and a manufacturing method for the composition. The composition, compound, device, and uses thereof according to AxMn(y-k)Mjk[Mnm(CN)(6-p-q)(NC)p(Che)rq]z.(Che)rw(Vac)(1-z).nH2O (wherein Vac is a Mn(CN)(6-p-q)(NC)p(Che)rq vacancy); wherein Che is an acid chelating agent; wherein: A=Na, K, Li; and M=Mg, Al, Ca, Sc, Ti, V, Cr, Fe, Co, Ni, Cu, Zn, Ga, Pd, Ag, Cd, In, Sn, Pb; and wherein 0<j≦4; 0≦k≦0.1; 0≦(p+q)<6; 0<x≦4; 0≦y≦1; 0<z≦1; 0<w≦0.2; 0<n≦6; −3≦r≦3; and wherein: x+2(y−k)+jk+(m+(r+1)q−6)z+wr=0.
Owner:NATRON ENERGY INC
Alkali-Ion Battery with Enhanced Transition Metal Cyanometallate Electrode Structure
ActiveUS20160056467A1Short assembly timeImprove throughputComplex cyanidesFinal product manufactureAlkali ionsAqueous solution
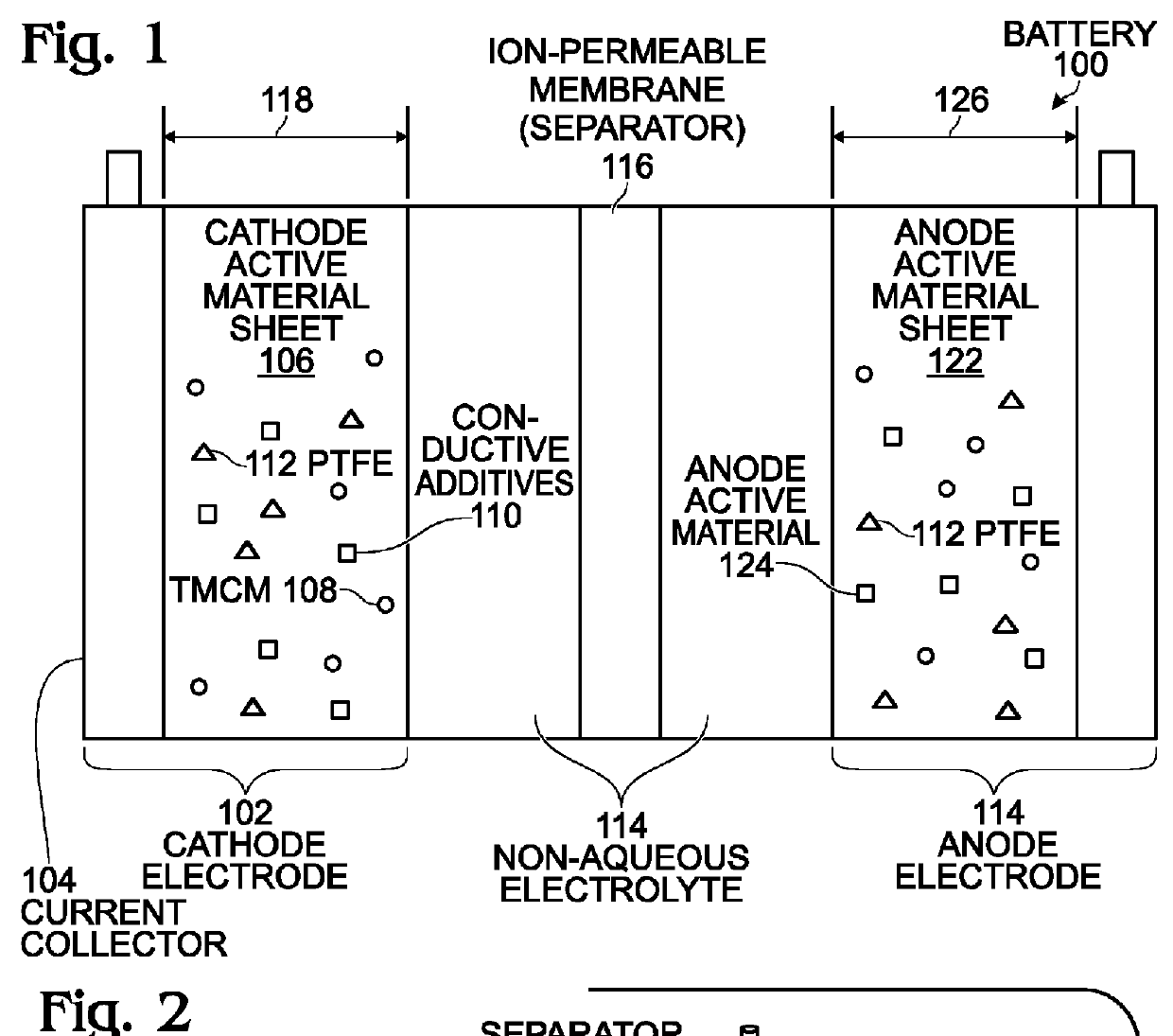
An alkali-ion battery is provided with a transition metal cyanometallate (TMCM) sheet cathode and a non-alkaline metal anode. The fabrication method mixes TMCM powders, conductive additives, and a polytetrafluoroethylene binder with a solution containing water, forming a wet paste. The wet paste is formed into a free-standing sheet of cathode active material, which is laminated to a cathode current collector, forming a cathode electrode. The free-standing sheet of cathode active material has a thickness typically in the range of 100 microns to 2 millimeters. The cathode electrode is assembled with a non-alkaline metal anode electrode and an ion-permeable membrane interposed between the cathode electrode and anode electrode, forming an assembly. The assembly is dried at a temperature of greater than 100 degrees C. The dried assembly is then inserted into a container (case) and electrolyte is added. Thick anodes made from free-standing sheets of active material can be similarly formed.
Owner:SHARP KK
Method of producing prussian blue-type metal complex nanoparticles, and prussian blue-type metal complex nanoparticles obtained by the method, dispersion of the nanoparticles, method of regulating the color of the nanoparticles, and electrode and transmitted light-regulator each using the nanoparticles
ActiveUS8349221B2Suitable for mass productionEfficiently obtainedMaterial nanotechnologyTin organic compoundsNanoparticlePrussian blue
A method of producing Prussian blue metal complex nanoparticles and Prussian blue metal complex nanoparticles obtained by the method, a dispersion of the nanoparticles, a method of regulating the color of the nanoparticles, and an electrode and a transmitted light-regulator each using the nanoparticles. Prussian blue metal complex nanoparticles are produced by: mixing an aqueous solution containing a metal cyano complex anion having metal atom MA as a central metal and an aqueous solution containing a cation of metal atom MB; thereby precipitating the crystal of a Prussian blue metal complex having the metal atom MA and the metal atom MB; and then mixing the Prussian blue metal complex with an aqueous solution containing a metal cyano complex anion having the metal atom MC as a central metal and / or an aqueous solution containing a cation of the metal atom MD.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Preparation method of manganese-based Prussian white positive electrode material and application of manganese-based Prussian white positive electrode material in sodium-ion battery electrode
ActiveCN111252784AHigh crystallinityImprove electrochemical performanceComplex cyanidesSecondary cellsManganeseSodium-ion battery
Owner:宇恒电池股份有限公司 +1
Method for the Synthesis of Metal Cyanometallates
Methods are presented for synthesizing metal cyanometallate (MCM). A first method provides a first solution of AXM2Y(CN)Z, to which a second solution including M1 is dropwise added. As a result, a precipitate is formed of ANM1PM2Q(CN)R·FH2O, where N is in the range of 1 to 4. A second method for synthesizing MCM provides a first solution of M2C(CN)B, which is dropwise added to a second solution including M1. As a result, a precipitate is formed of M1[M2S(CN)G]1 / T·DH2O, where S / T is greater than or equal to 0.8. Low vacancy MCM materials are also presented.
Owner:SHARP KK
Bimetallic complex catalyst and preparation method and application thereof
The invention discloses a bimetallic complex catalyst and a preparation method and application thereof. The preparation method of the bimetallic complex catalyst comprises the following steps: separately preparing a mixed microemulsion of water-soluble metal salt and calcium chloride and a mixed microemulsion of water-soluble metal complex and water-soluble sulfate on the premise of ensuring solution transparence; mixing and reacting the two mixed microemulsion at a certain temperature under stirring condition to obtain a mixed microemulsion after reaction; aging and standing for a while to form a layer and particle coexisting structure in the mixed microemulsion obtained after the reaction; washing and centrifuging the mixed solution of organic solvent and water; and drying the precipitate to obtain the bimetallic complex catalyst. The catalyst obtained by the invention has good catalysis effect.
Owner:CHINA BUILDING MATERIALS ACAD
Method of producing structural member having prussian blue-type metal complex nanoparticles, structural member obtained by the method, substrate, electrochromic device, rectifying device, and photo responding device, using the structural member
InactiveUS20110268963A1Good effectSuppressed and prevented from peelingMaterial nanotechnologyIron cyanidesWater dispersibleNanoparticle
A method of producing a structural member having Prussian blue-type metal complex nanoparticles, the method including: constructing the structural member stabilized by a particular process in producing the structural member by providing nanoparticles consisting of Prussian blue-type metal complex onto a substrate; and a structural member having Prussian blue-type metal complex nanoparticles, the structural member having water-dispersible nanoparticles consisting of Prussian blue-type metal complex provided on a substrate and the structural member being stabilized in water by a particular process.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Metal complex nanoparticles and method for producing the same
InactiveUS20120077037A1Improve productivityPreferable performanceMaterial nanotechnologyGlass/slag layered productsNanoparticleAqueous solution
A method for producing metal complex nanoparticles, the method having: providing an aqueous solution containing a metal cyano complex anion having a metal atom MA as a central metal, with an aqueous solution containing zinc cation, the pH of the aqueous solution containing zinc cation being adjusted; and mixing the solutions and thereby producing metal complex nanoparticles composed of the metal atom MA and zinc under controlling the properties of the obtained metal complex nanoparticles.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Method for directly synthesizing auro-potassium cyanide by controlling level
InactiveCN1141252CImprove efficiencyFast productionComplex cyanidesPotassium cyanideAqueous solution
Potassium cyanide and hydrogen peroxide are added into reaction container at certain speed and the gold potential is controlled in -400 mV to -800 mV during the reaction. The production of KAu(CN)2 in the method of the present invention has fast speed, high efficiency, high quality, less investment and low cost.
Owner:张东山
Surface-modified cyanide-based transition metal compounds
ActiveUS9359219B1Improve air stabilityReduced responseNon-aqueous electrolyte accumulatorsIron cyanidesCompound aCyanide
A system, method, and articles of manufacture for a surface-modified transition metal cyanide coordination compound (TMCCC) composition, an improved electrode including the composition, and a manufacturing method for the composition. The composition, compound, device, and uses thereof according to AxMn(y-k)Mjk[Mnm(CN)(6-p-q)(NC)p(Che)rq]z. (Che)rw(Vac)(1-z).nH2O (wherein Vac is a Mn(CN)(6-p-q)(NC)p(Che)rq vacancy); wherein Che is an acid chelating agent; wherein: A=Na, K, Li; and M=Mg, Al, Ca, Sc, Ti, V, Cr, Fe, Co, Ni, Cu, Zn, Ga, Pd, Ag, Cd, In, Sn, Pb; and wherein 0<j≦4; 0≦k≦0.1; 0≦(p+q)<6; 0<x≦4; 0≦y≦1; 0<z≦1; 0<w≦0.2; 0<n≦6; −3≦r≦3; and wherein: x+2(y−k)+jk+(m+(r+1)q−6)z+wr=0.
Owner:NATRON ENERGY INC
Method and apparatus for directly synthesizing auro-potassium cyanide by controlling electric potential
The invention utilizes the reaction container with built-in stirrer, temperature sensor, gold electrode, reference electrode and baffle-plate and the equipments of temperature display, potentiometer and potassium cyanide solution and hydrogen peroxide solution storage tanks with control valves, and uses the gold powder, potassium cyanide and hydrogen peroxide as raw materials in the reaction container, and controls the speed of adding solutions in the course of reaction and adopts proper gold potential and temperature to directly synthesize potassium aurocyanide. Said invention is reasonable in structure design, small in volume and stable in performance, safe and reliable.
Owner:张东山
Method for synthesizing metal cyanides by use of Fenton reagent
ActiveCN104556150ANo pollutionNot involved in the productionComplex cyanidesMetal/metal-oxides/metal-hydroxide catalystsPlatinumFenton reagent
Provided is a preparation method of metal cyanide, comprising the following steps: 1) mixing supported metal nanoparticles, a Fenton reagent and nitrile to form suspension, and then stirring, the supported metal nanoparticles being a complex wherein the supporter supports nano metal supports nano metal, nano metal oxide or nano metal salt; and 2) centrifugalizing and drying a product after stirring to obtain the metal cyanide. Also provided are the prepared metal cyanide and the use of same in the fields of sensing, batteries, medicine, electroplating and catalysis. The preparation method does not relate to highly toxic substance CN -, does not require ultraviolet light, is an entirely green method for synthesizing metal cyanide, and applies to industrial production of a large scale.
Owner:ZHEJIANG UNIV
Fluorine free tungsten ald/cvd process
A tungsten precursor useful for forming tungsten-containing material on a substrate, e.g., in the manufacture of microelectronic devices. The tungsten precursor is devoid of fluorine content, and may be utilized in a solid delivery process or other vapor deposition technique, to form films such as elemental tungsten for metallization of integrated circuits, or tungsten nitride films or other tungsten compound films that are useful as base layers for subsequent elemental tungsten metallization.
Owner:ENTEGRIS INC
Method for producing silver potassium cyanide
InactiveCN102874844AHigh purityReduce moisture contentComplex cyanidesPotassium cyanideVacuum drying
The invention discloses a method for producing silver potassium cyanide. The silver potassium cyanide with high purity can be obtained by steps of dissolving, detecting, neutralizing, precipitating, cleaning, detecting, dissolving again, cooling for crystallization, leaching, washing and the like. Compared with conventional production methods, the method for producing silver potassium cyanide is added in multi-time detection and filtering and improved in purity of final product, and vacuum drying is simultaneously performed, so that water content of the obtained silver potassium cyanide is low, and product quality is improved.
Owner:河南银城科技股份有限公司
Transition metal cyanide coordination compounds having multiple reactions
ActiveUS20200071175A1Improve air stabilityImprovement of their fade capacity lossNon-aqueous electrolyte accumulatorsIron cyanidesCyanide compoundElectrochemical cell
A system, method, and articles of manufacture for a surface-modified transition metal cyanide coordination compound (TMCCC) composition, an improved electrode including the composition, and a manufacturing method for the composition according to Formula III—An electrochemical cell including a system having an anode, a cathode, and an electrolyte wherein the anode includes a material, including the material including at least one composition represented by Formula III: AxMny[Mn(CN)(6)]z(Vac)(1-z).n(H2O)m(Che) wherein, in Formula III, A includes one or more alkali metals including Na; and wherein 0<j≤4, 0≤k≤0.1, 1.2<x≤4, 0<y≤1, 0.8<z≤1, 0<n≤4; 0≤m≤0.2 and wherein x+2y−4z=0.
Owner:NATRON ENERGY INC
Adsorption systems using metal-organic frameworks
InactiveUS10266737B2Group 1/11 organic compounds without C-metal linkagesOther chemical processesAdsorption chillerMetal-organic framework
The present invention relates to sorbants such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs), porous aromatic frameworks (PAFs) or porous polymer networks (PPNs) for separations of gases or liquids, gas storage, cooling, and heating applications, including, but not limited to, adsorption chillers.
Owner:BATTELLE MEMORIAL INST +1
Prussian blue sodium ion battery positive electrode material and preparation method thereof
ActiveCN111943225AImproved magnification performanceOpen Stereoscopic Frame StabilizationComplex cyanidesCell electrodesElectrical batteryPhysical chemistry
The invention provides a preparation method of a Prussian blue sodium ion battery cathode material, which comprises the following steps: adding a chelating agent into a precursor solution B and addingan auxiliary solvent to enhance the solubility of the chelating agent; reacting a transition metal salt with the chelating agent, so that transition metal ions can be slowly released when the precursor solution A reacts with the precursor solution B, thereby obtaining the Prussian blue sodium ion battery cathode material, and achieving control on the reaction rate. Therefore, the morphology of the generated Prussian blue sodium ion battery positive electrode material is controlled, the flaky high-crystallinity Prussian blue material is obtained, the diffusion and transmission paths of ions and electrons can be shortened, and the conductivity of the positive electrode material is improved. As a result, the rate performance of the sodium ion battery is improved, the open three-dimensional frame of the Prussian blue material is stable due to the high crystallinity, and the transition metal is not easily separated out during the charge-discharge process so as to improve the cycle stability of the sodium ion battery.
Owner:GLOBAL ENERGY INTERCONNECTION RES INST CO LTD +2
Anode for Sodium-ion and Potassium-ion Batteries
ActiveUS20160028086A1Improve Coulombic efficiencyIncrease irreversible capacityElectrode thermal treatmentComplex cyanidesCarbon compositesMetallic materials
A first method for fabricating an anode for use in sodium-ion and potassium-ion batteries includes mixing a conductive carbon material having a low surface area, a hard carbon material, and a binder material. A carbon-composite material is thus formed and coated on a conductive substrate. A second method for fabricating an anode for use in sodium-ion and potassium-ion batteries mixes a metal-containing material, a hard carbon material, and binder material. A carbon-composite material is thus formed and coated on a conductive substrate. A third method for fabricating an anode for use in sodium-ion and potassium-ion batteries provides a hard carbon material having a pyrolyzed polymer coating that is mixed with a binder material to form a carbon-composite material, which is coated on a conductive substrate. Descriptions of the anodes and batteries formed by the above-described methods are also provided.
Owner:SHARP KK
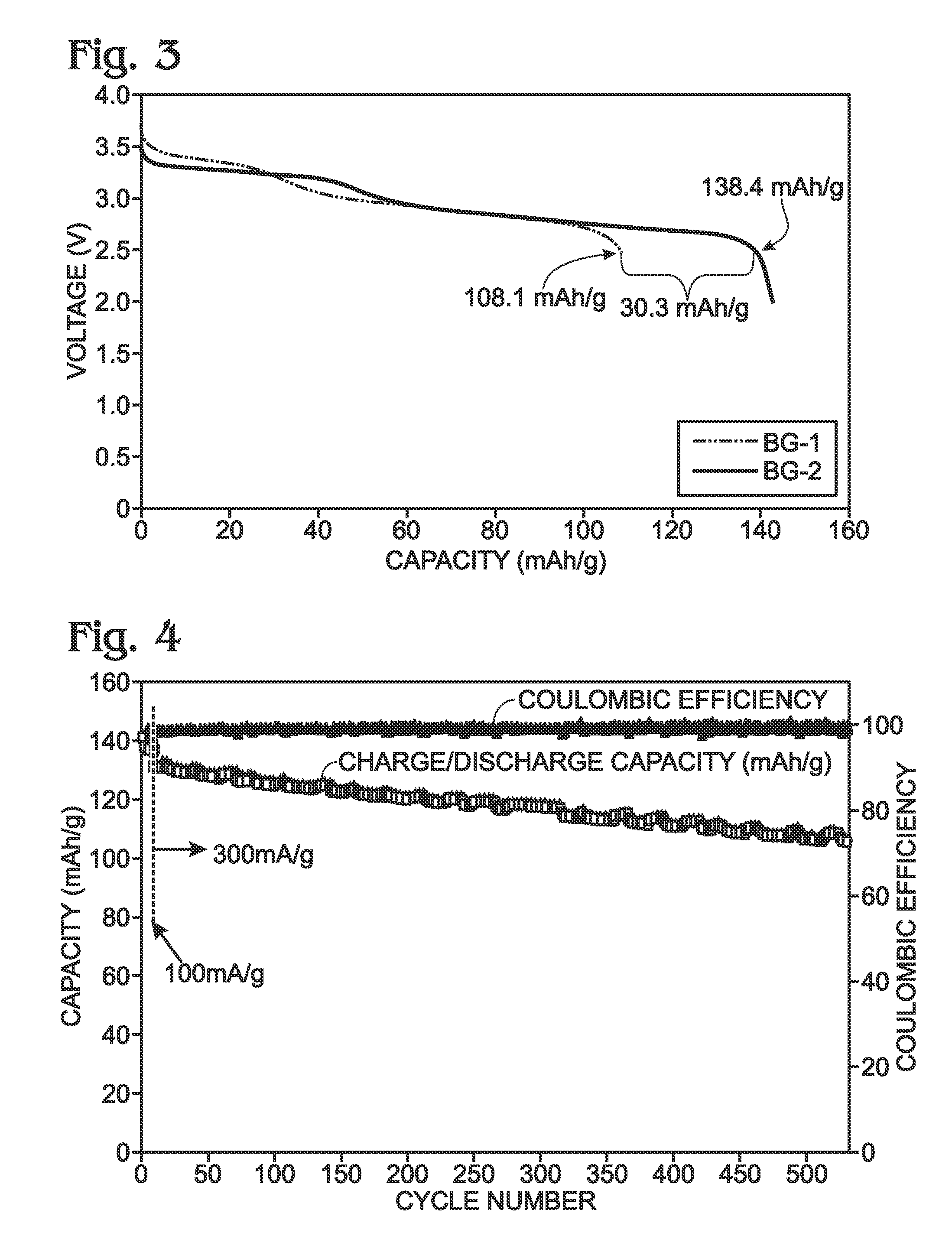
Prussian blue analogue and preparation method, negative electrode material and application thereof
ActiveCN112694104ALow priceSimple processIron cyanidesComplex cyanidesHigh current densityElectrical battery
The invention discloses a prussian blue analogue and a preparation method, a negative electrode material and application thereof, which belong to the field of new energy batteries. The chemical formula of the Prussian blue analogue is KxMn[R(CN)6]1-y<vacancy>y.nH2O, x is more than or equal to 0 and less than or equal to 2, y is more than 0.3 and less than 1, and vacancy is a [R(CN)6] vacancy. The negative electrode material composed of the Prussian blue analogue is obtained at a rapid crystallization rate, and has relatively high crystal water content (24wt%) and relatively long Mn-N bond length (2.214 angstrom). The material has the advantages of being low in cost, environmentally friendly and high in Li storage capacity, transfer of 5 mol electrons can be achieved under the low potential, breakage and recombination of Mn-N bonds are involved, and stable circulation can be conducted for 1000 cycles or above with the high reversible capacity of 480 mAh g<-1> under the high current density of 1A g<-1>.
Owner:HUAZHONG UNIV OF SCI & TECH
Carbon quantum dot-CoFe type Prussian blue nanocomposite material as well as preparation method and application thereof
The invention relates to a carbon quantum dot-CoFe Prussian blue nanocomposite material as well as a preparation method and application thereof, and belongs to the technical field of electrode material preparation. The preparation method is a two-step synthesis method and comprises the steps: firstly, taking potassium citrate as a carbon source of carbon quantum dots, controlling the dosage of potassium citrate, hydrothermal synthesis temperature, hydrothermal synthesis time and other reaction conditions, and preparing a carbon quantum dot solution through a hydrothermal synthesis method; thentaking carbon quantum dots, inorganic cobalt salt and potassium ferricyanide as reaction raw materials, preparing the carbon quantum dot-CoFe Prussian blue composite material with good dispersity byadopting a liquid-phase coprecipitation reaction method, and enabling the size of nanospheres to be about 200-400 nm. When the carbon quantum dot-CoFe Prussian blue composite material prepared by themethod is used as an electrode material, the capacity of a supercapacitor is improved, the rapid charging and discharging capacity of the supercapacitor is greatly improved, and the supercapacitor hasexcellent rate capability and long cycle service life.
Owner:DALIAN UNIV OF TECH
Highly active double metal cyanide compounds
PendingCN112368317AGroup 8/9/10/18 element organic compoundsGroup 5/15 element organic compoundsCyanide compoundPolymer science
The present invention is directed to supported catalyst having utility in the polymerization and co-polymerization of epoxide monomers, the supported catalyst having the general Formula (I): [DMCC] *b Supp, wherein: [DMCC] denotes a double metal cyanide complex which comprises a double metal cyanide (DMC) compound, at least one organic complexing agent and a metal salt; Supp denotes a hydrophobicsupport material; and b represents the average proportion by weight of the support material, based on the total weight of [DMCC] and Supp, and is preferably in the range 1 wt.% <= b <= 99 wt.%.
Owner:HENKEL KGAA
Ultrafine particles of Prussian blue-type metal complex, dispersion liquid thereof and their production methods
InactiveUS7678188B2Improve stabilityEasily and massivelyMaterial nanotechnologyPigmenting treatmentPrussian blueSolvent
Owner:NAT INST OF ADVANCED IND SCI & TECH
Popular searches
Secondary cells charging/discharging Non-aqueous electrolyte accumulator electrodes Climate change adaptation Iron group organic compounds without C-metal linkages Isotope separation Heat-exchange elements Group 2/12 organic compounds without C-metal linkages Sorption machines Group 6/16 organic compounds without C-metal linkages Negative electrodes
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com