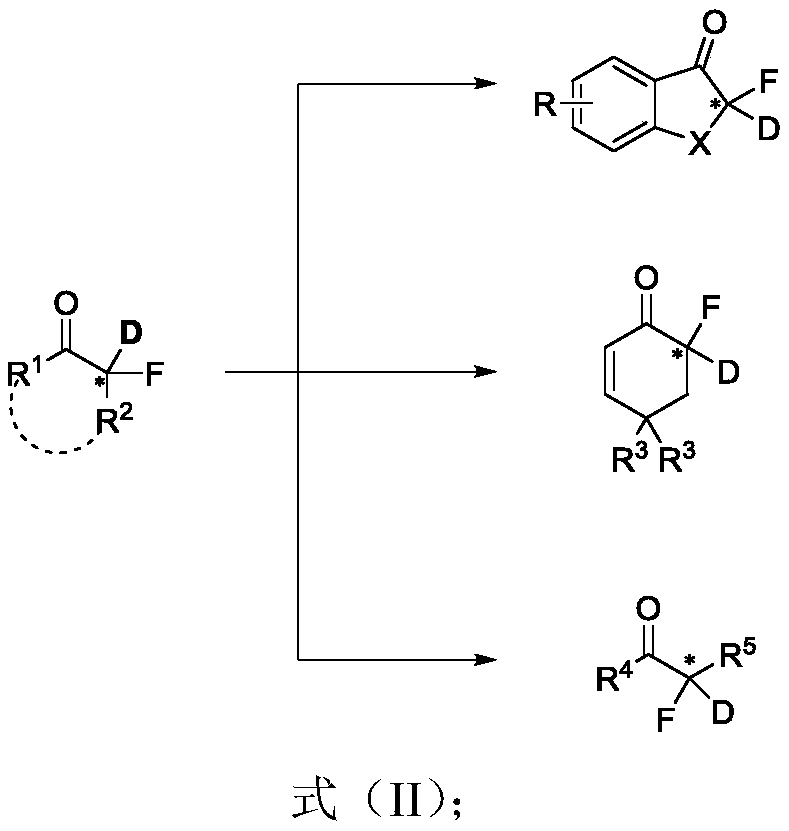
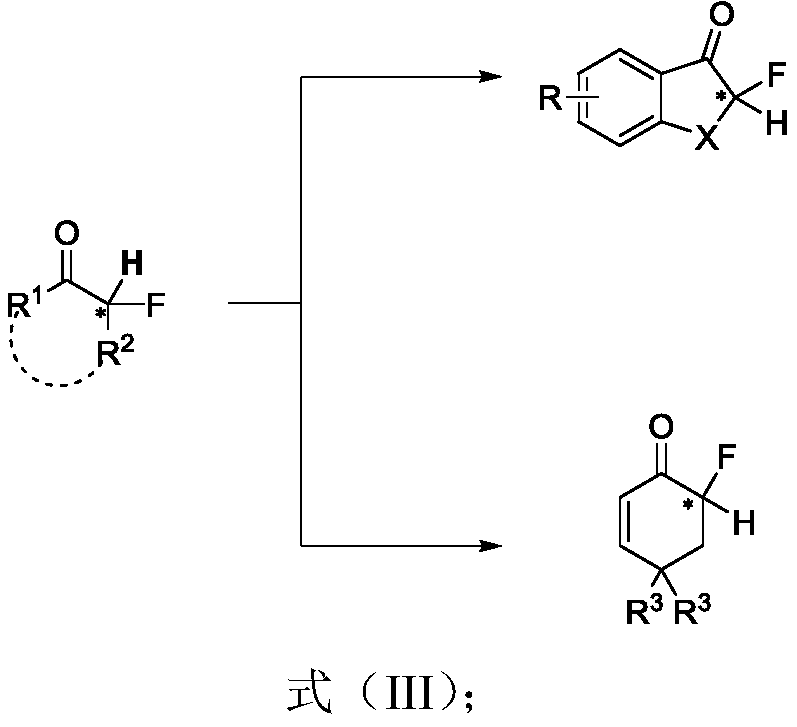
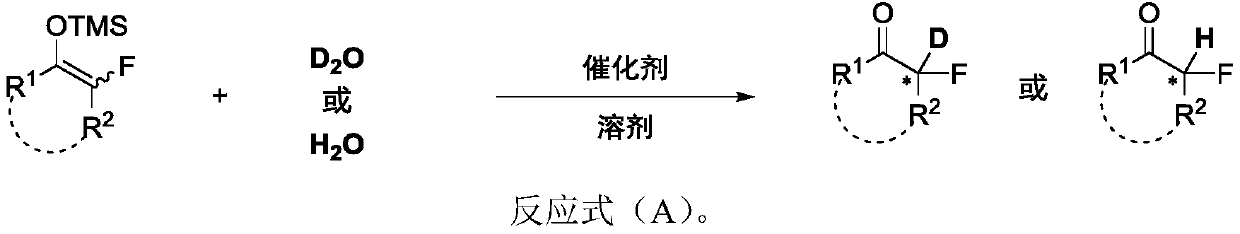
Chiral alpha-deuterium (hydrogen) alpha-fluoroketone compound and asymmetric catalytic synthesis method thereof
A synthetic method and technology of fluoroketones, applied in the field of asymmetric catalysis, can solve problems such as synthetic methods that have not been reported yet, and achieve the effect of enriching the library of chiral α-fluoroketones and promoting the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Synthesis of chiral α-deuterated α-fluoroketones II-1:
[0088]
[0089] Add chiral tetragonal acid catalyst A (9.0mg, 0.015mmol), deuterated methanol (3.0mL), and monofluoroenol silyl ether I-1 (65.4mg, 0.3mmol) to a 5.0mL reaction bottle in the glove box , deuterium water (5.5uL, 0.3mmol), and the reaction solution was stirred at 25°C for 2 days. TLC detects that the raw materials have basically reacted, and the reaction is stopped. Direct loading column chromatography obtained white solid product II-1 with 98% yield and 95% deuteration rate. HPLC analysis (Chiralpak AS-H, i PrOH / hexane=10 / 90, 1.0mL / min, 230nm; t r (minor) = 11.04min,t r (major)=13.41min) gave the isomeric composition of the product: 90%ee, [α] 20 D =-8.6 (c=0.5, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ): δ7.79(d, J=7.6Hz, 1H), 7.68-7.64(m, 1H), 7.47-7.41(m, 2H), 5.26(ddd, J=50.8Hz, J=7.6Hz, J= 4.4Hz, 0.06H), 3.62(dd, J=17.2Hz, J=7.2Hz, 1H), 3.22(dd, J=23.2Hz, J=17.2Hz, 1H); 13 C NMR (100MHz, ...
Embodiment 2
[0091] Synthesis of chiral α-deuterated α-fluoroketones II-2:
[0092]
[0093] Add chiral tetragonal acid catalyst B (9.0mg, 0.015mmol), deuterated methanol (3.0mL), and monofluoroenol silyl ether I-1 (65.4mg, 0.3mmol) to a 5.0mL reaction bottle in the glove box , deuterium water (5.5uL, 0.3mmol), and the reaction solution was stirred at 25°C for 2 days. TLC detects that the raw materials have basically reacted, and the reaction is stopped. Direct loading column chromatography obtained white solid product II-2 with 99% yield and 96% deuteration rate. HPLC analysis (Chiralpak AS-H, i PrOH / hexane=10 / 90, 1.0mL / min, 230nm; t r (minor) = 13.41min,t r(major)=11.04min) gave the isomeric composition of the product: 89%ee, [α] 20 D =+8.5 (c=0.5, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ): δ7.79(d, J=7.6Hz, 1H), 7.68-7.64(m, 1H), 7.47-7.41(m, 2H), 5.26(ddd, J=50.8Hz, J=7.6Hz, J= 4.4Hz, 0.04H), 3.62(dd, J=17.2Hz, J=7.2Hz, 1H), 3.22(dd, J=23.2Hz, J=17.2Hz, 1H); 13 C NMR (100MHz, C...
Embodiment 3
[0095] Synthesis of chiral α-deuterated α-fluoroketones II-3:
[0096]
[0097] Add chiral tetragonal acid catalyst A (9.0mg, 0.015mmol), deuterated methanol (3.0mL), and monofluoroenol silyl ether I-2 (67.2mg, 0.3mmol) to a 5.0mL reaction bottle in the glove box , deuterium water (5.5uL, 0.3mmol), and the reaction solution was stirred at 25°C for 2 days. TLC detects that the raw materials have basically reacted, and the reaction is stopped. The product II-3 was obtained as a yellow liquid with 86% yield and 96% deuteration rate by direct column chromatography. HPLC analysis (Chiralpak OJ-H, i PrOH / hexane=3 / 97, 1.0mL / min, 205nm; t r (minor) = 14.03min,t r (major)=16.71min) gave the isomeric composition of the product: 84%ee, [α] 27 D =-4.6 (c=0.5, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ): δ7.71-7.66(m, 2H), 7.20-7.15(m, 2H), 5.78(d, J=58.8Hz, 0.04H); 13 C NMR (100MHz, CDCl 3 ): δ192.97(d, J=14.0Hz, 1C), 171.40(d, J=3.0Hz, 1C), 139.48, 124.58(d, J=157.0Hz, 1C), 118.30,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com