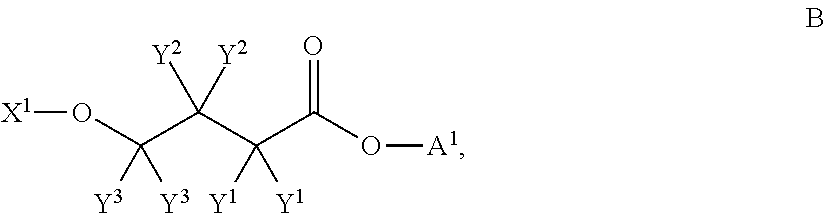
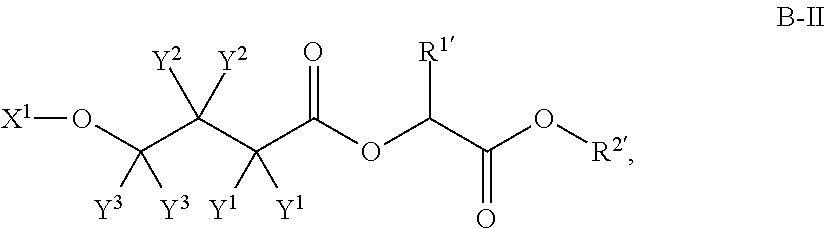
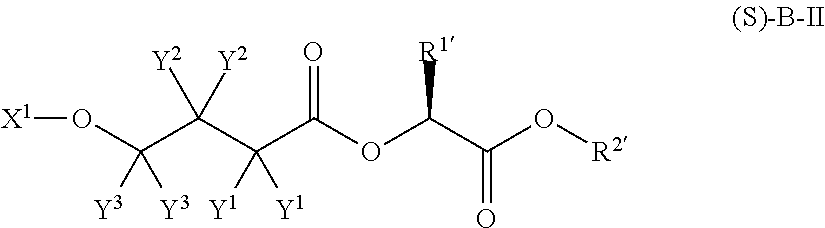
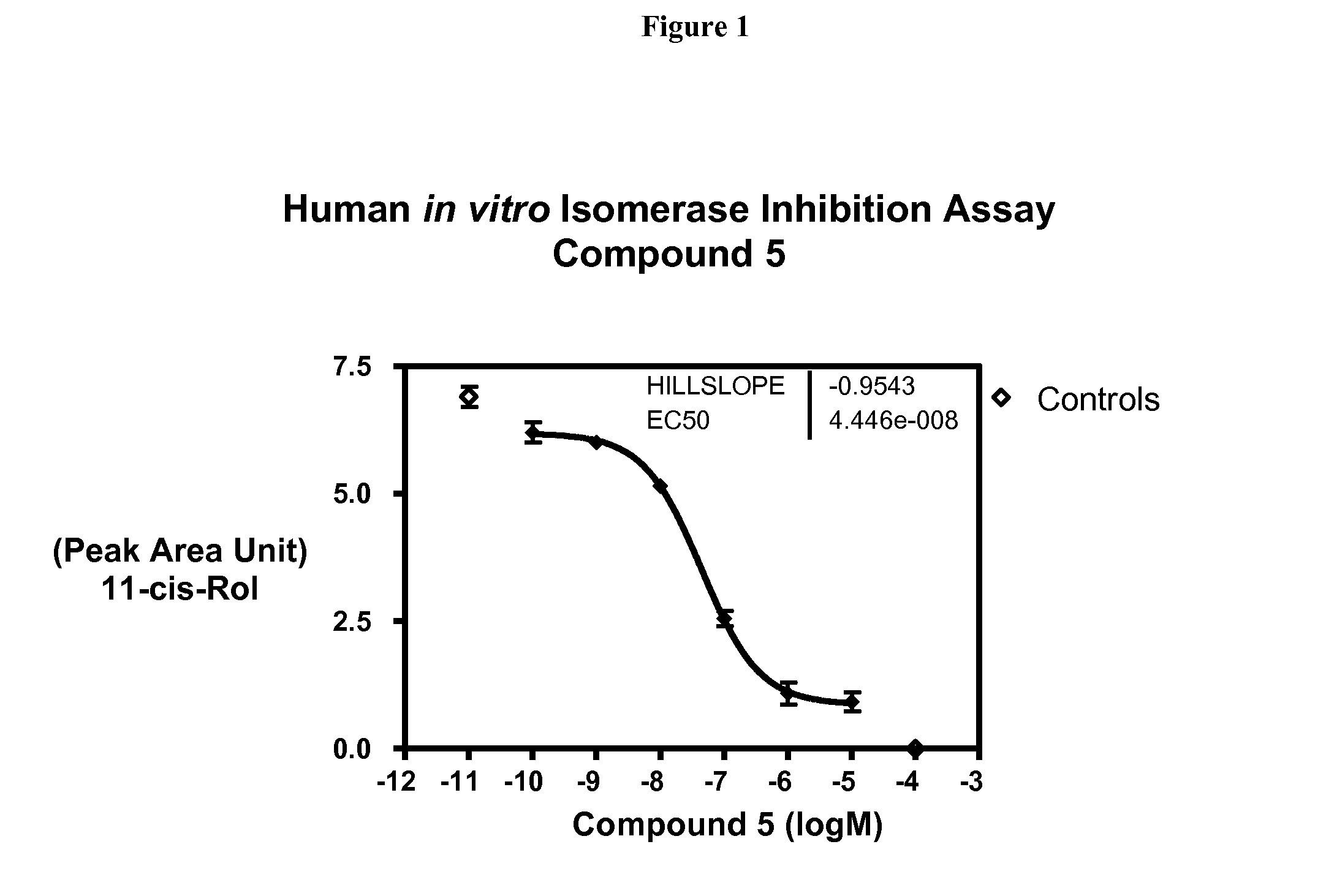
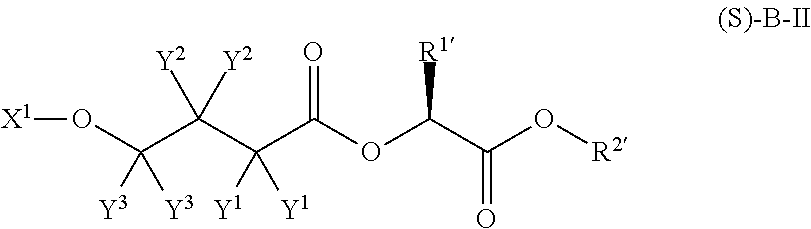
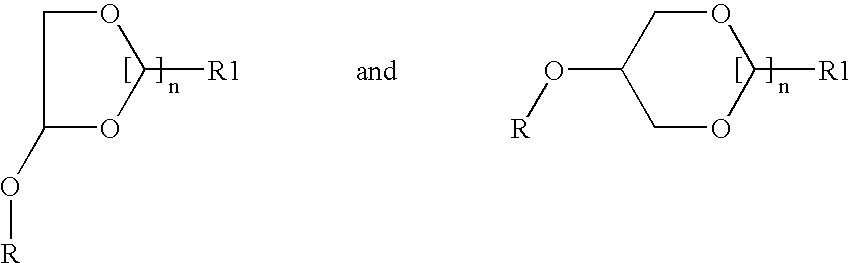
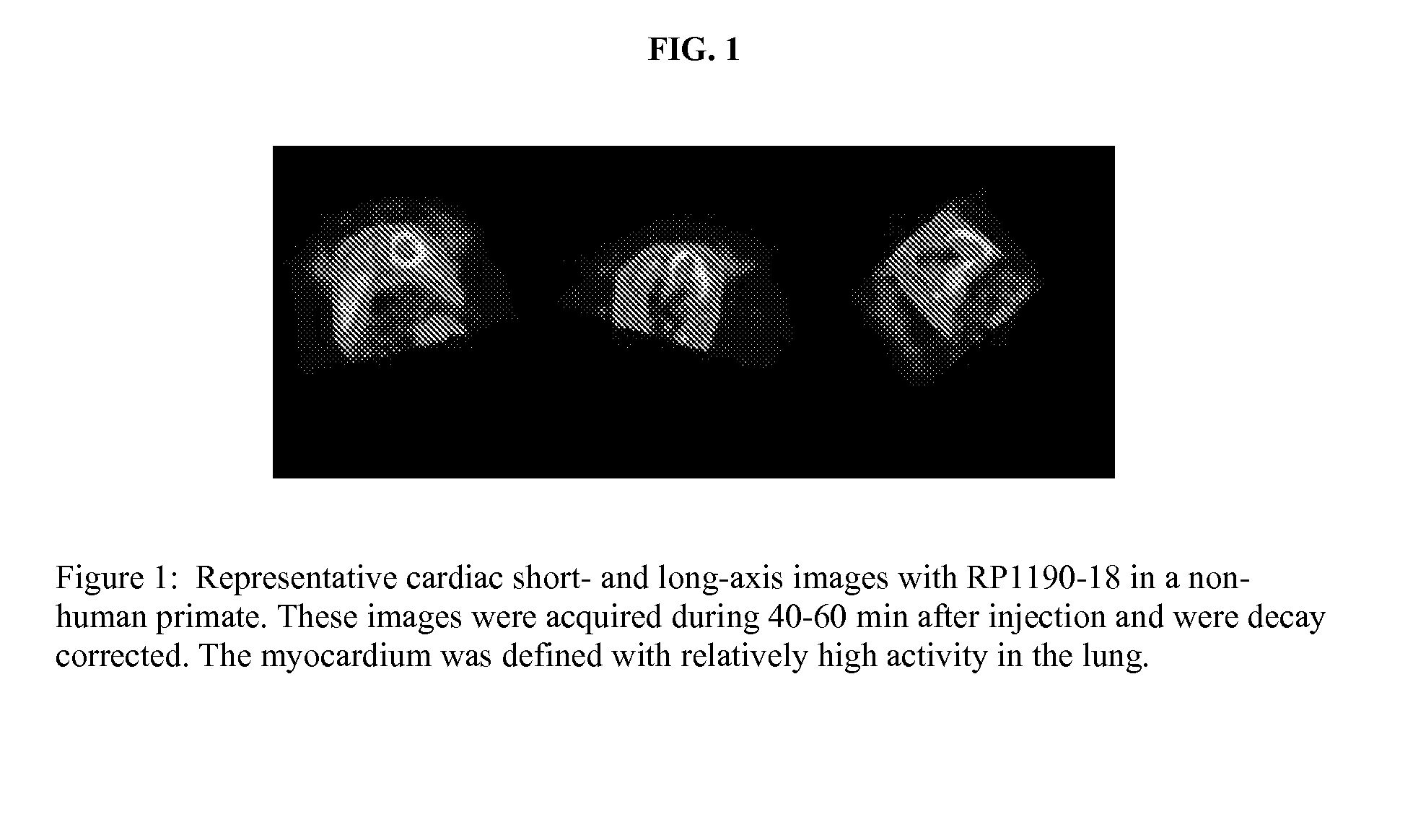
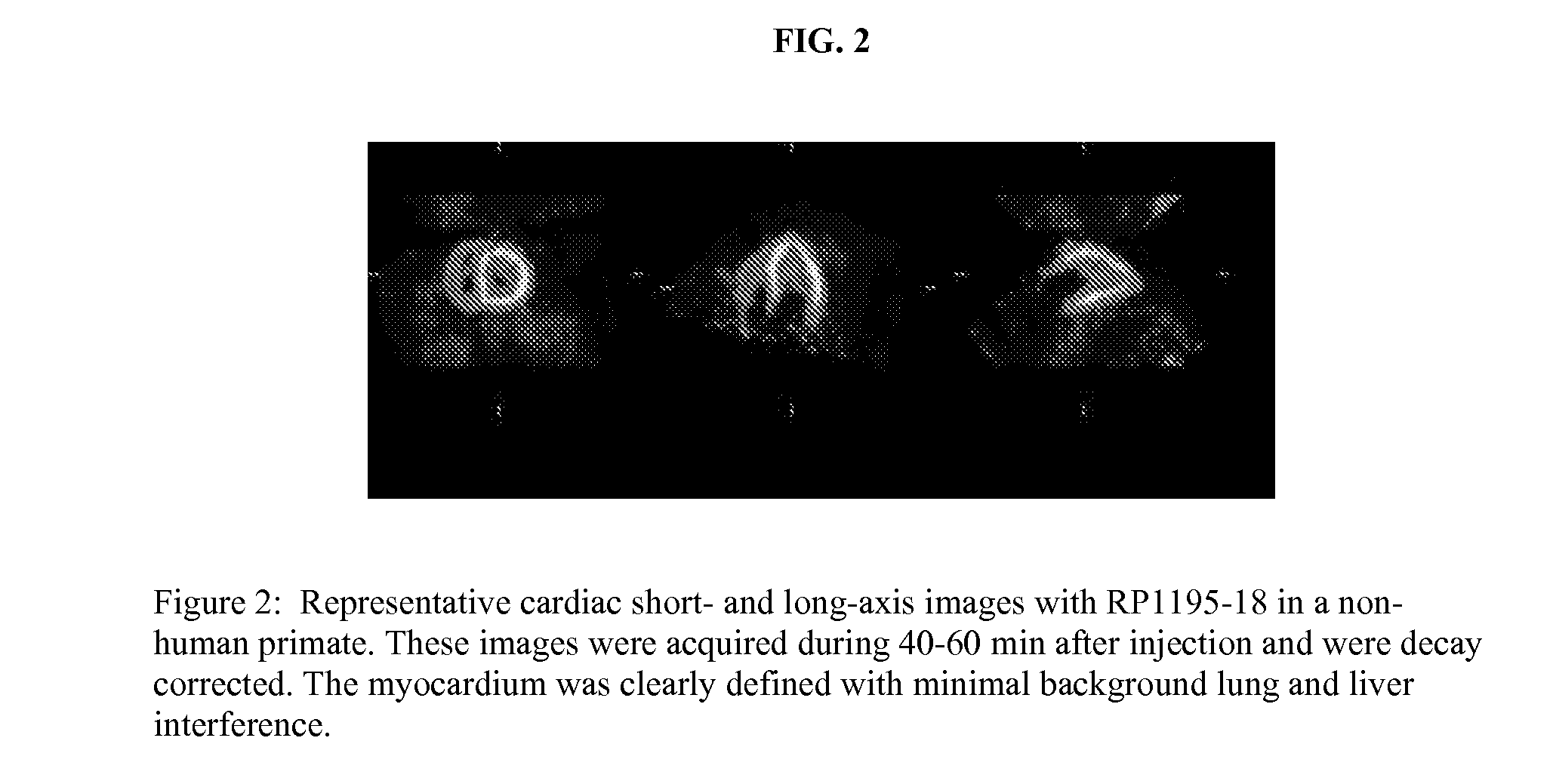
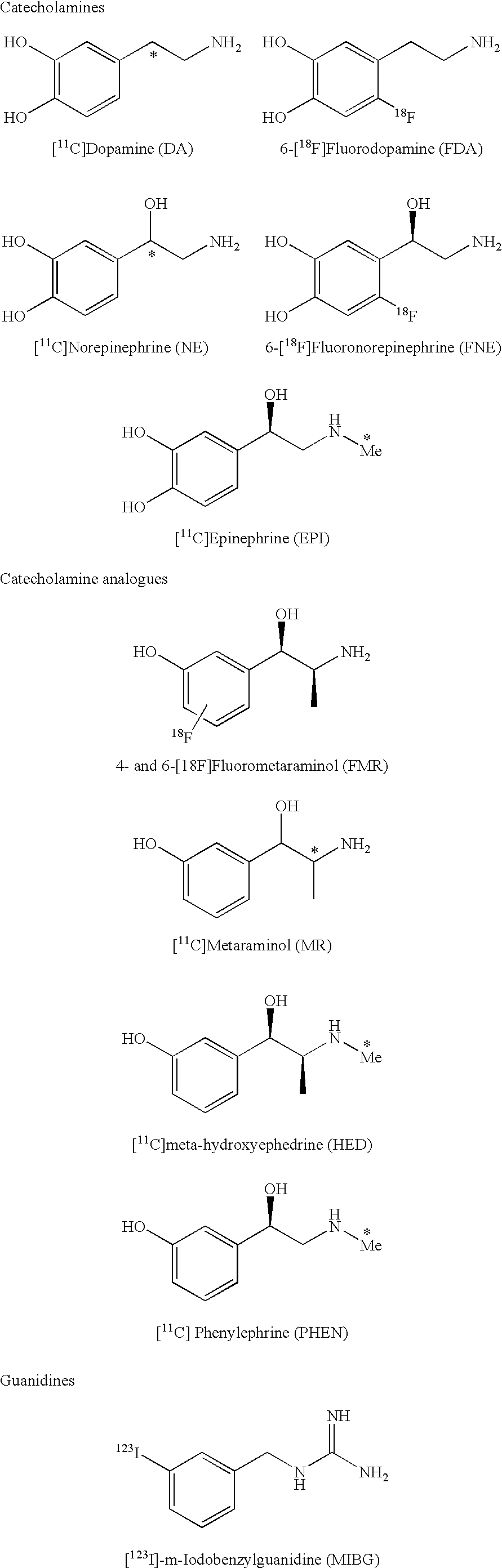
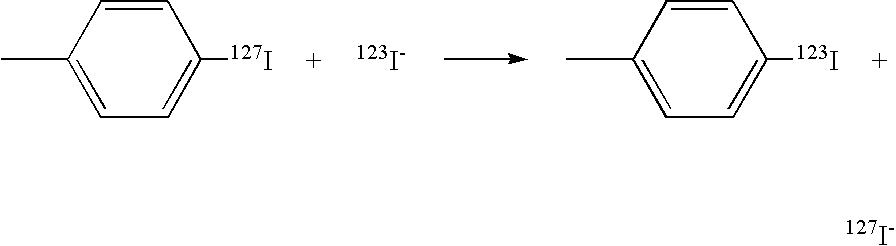
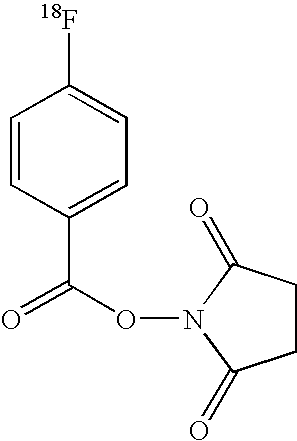
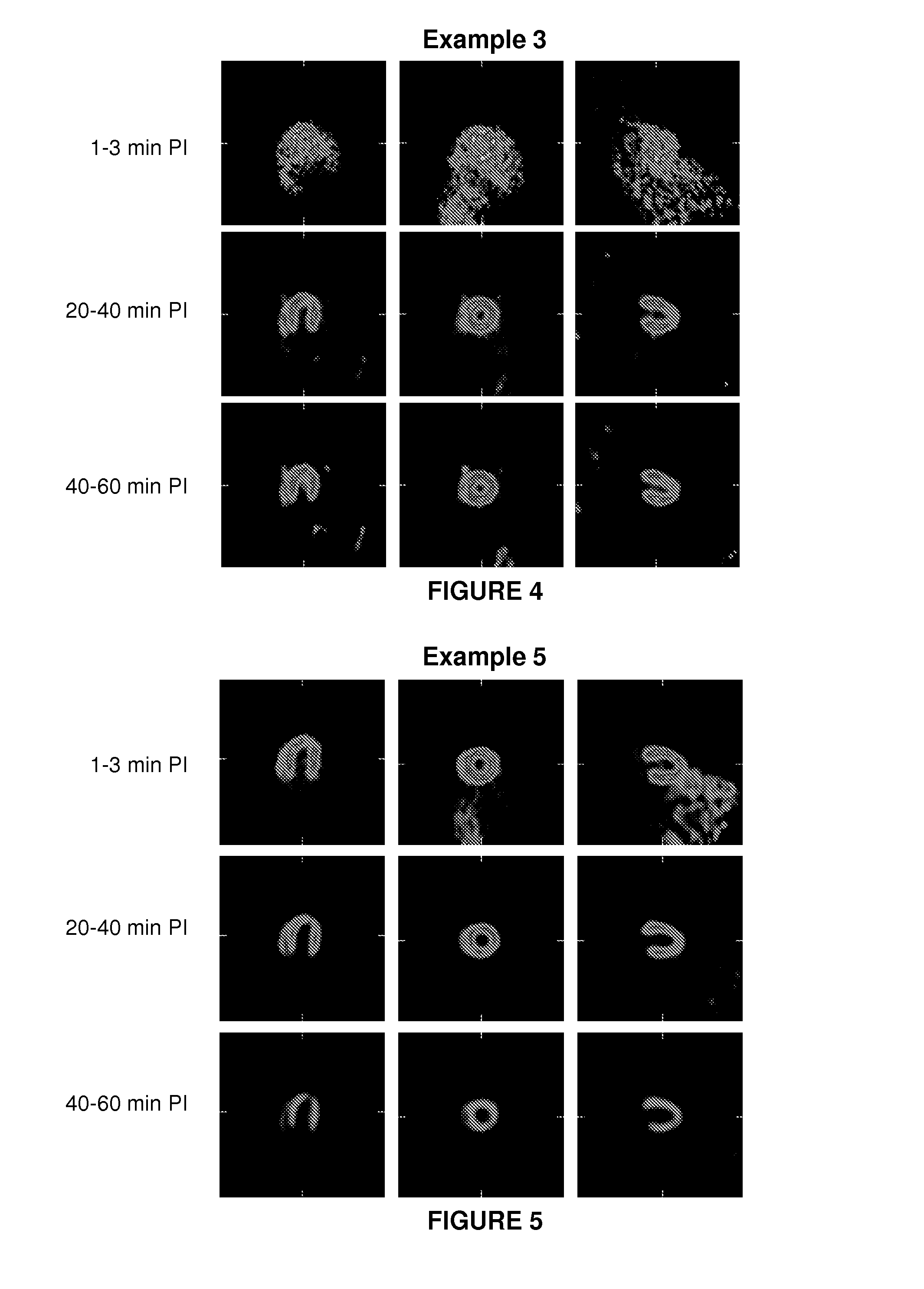
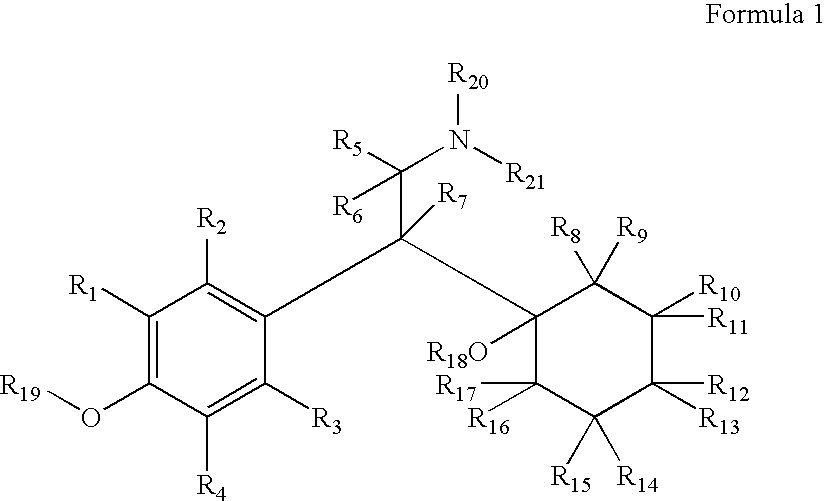
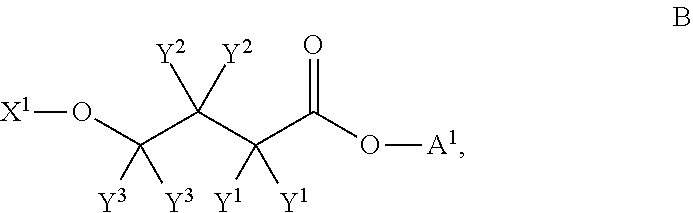
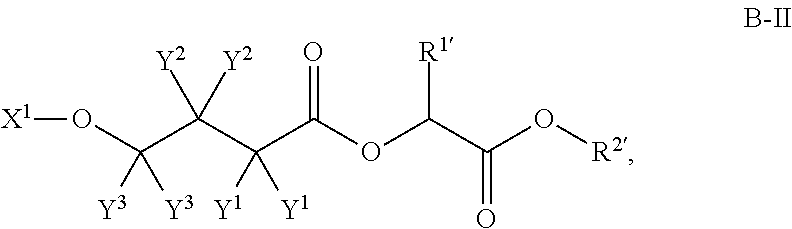
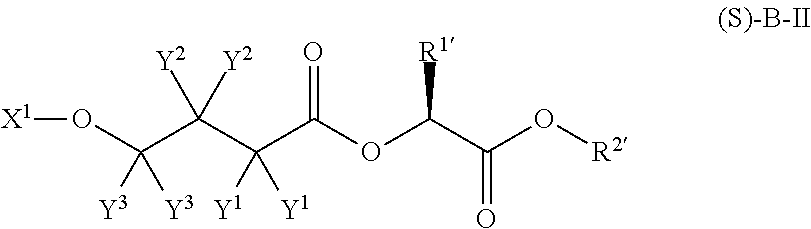
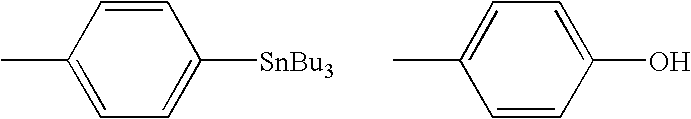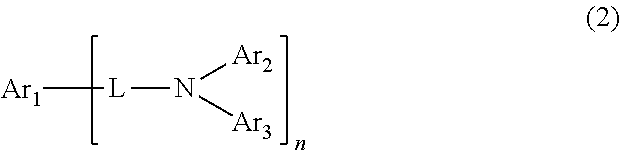Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
576results about "Isotope introduction to acyclic/carbocyclic compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for Deuteration of an Aromatic Ring
ActiveUS20070255076A1Improve working environmentAmino preparation from aminesOrganic compound preparationNickel catalystHydrogen
The present invention relates to a method for deuteration of a compound having an aromatic ring, using an activated catalyst, and the method comprises reacting a compound having an aromatic ring with heavy hydrogen source in the presence of an activated catalyst selected from a platinum catalyst, a rhodium catalyst, a ruthenium catalyst, a nickel catalyst and a cobalt catalyst.
Owner:FUJIFILM WAKO PURE CHEM CORP
Hepatitis b antiviral agents
ActiveUS20170253609A1AntiviralsAmide active ingredientsHepatitis B immunizationPharmaceutical medicine
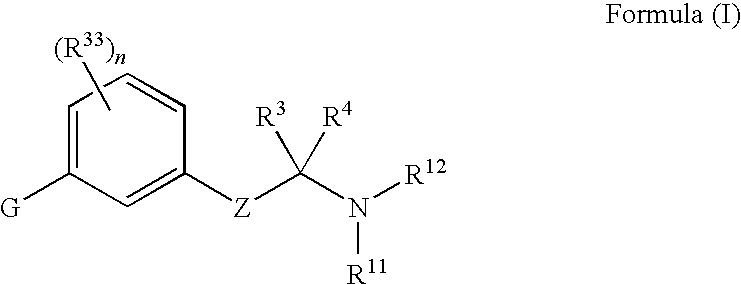
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:X-A-Y-L-R (I)which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
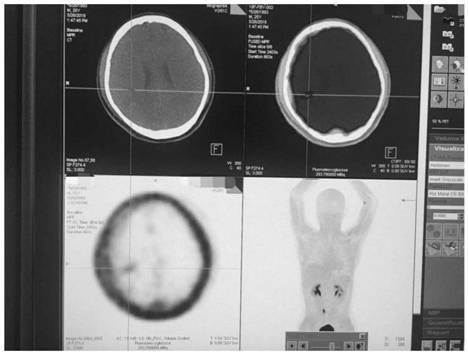
Imaging Agents
InactiveUS20070082879A1High yieldExcessive reactionBiocideIn-vivo radioactive preparationsRheniumAbnormal tissue growth
The present invention provides novel amino acid compounds useful in detecting and evaluating brain and body tumors. These compounds have the advantageous properties of rapid uptake and prolonged retention in tumors and can be labeled with halogen isotopes such as fluorine-18, iodine-123, iodine-124, iodine-125, iodine-131, bromine-75, bromine-76, bromine-77, bromine-82, astatine-210, astatine-211, and other astatine isotopes. These compounds can also be labeled with technetium and rhenium isotopes using known chelation complexes. The compounds disclosed herein bind tumor tissues in vivo with high specificity and selectivity when administered to a subject. Preferred compounds show a target to non-target ratio of at least 2:1, are stable in vivo and substantially localized to target within 1 hour after administration. Preferred compounds include 1-amino-2-[18F]fluorocyclobutyl-1-carboxylic acid (2-[18F]FACBC) and 1-amino-2-[18F]fluoromethylcyclobutyl-1-carboxylic acid (2-[18F]FMACBC). The labeled amino acid compounds of the invention are useful as imaging agents in detecting and / or monitoring tumors in a subject by PET or SPECT.
Owner:EMORY UNIVERSITY
4-hydroxybutyric acid analogs
This invention relates to novel derivatives of 4-hydroxybutyric acid and prodrugs thereof, and pharmaceutically acceptable salts of the foregoing. This invention also provides pharmaceutical compositions comprising a compound of this invention and the use of such compositions in methods of treating narcolepsy, fibromyalgia, other disorders or conditions that are beneficially treated by improving nocturnal sleep or by administering sodium oxybate.
Owner:SUN PHARMA IND INC
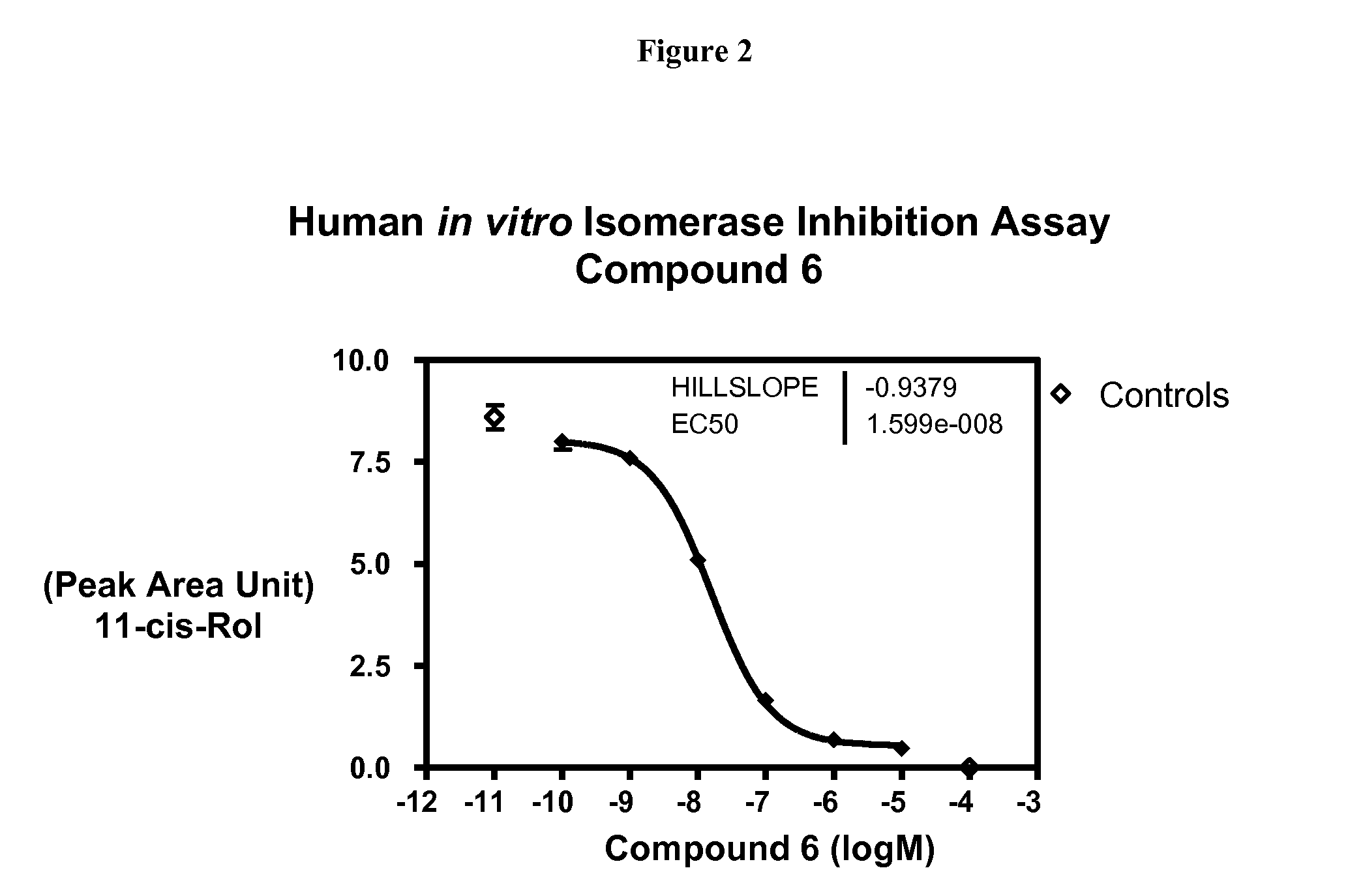
Compounds for treating ophthalmic diseases and disorders
Provided are compounds, pharmaceutical compositions thereof, and methods of treating ophthalmic diseases and disorders, such as age-related macular degeneration and Stargardt's Disease, using said compounds and compositions.
Owner:ACUACELA INC
4-hydroxybutyric acid analogs
This invention relates to novel derivatives of 4-hydroxybutyric acid and prodrugs thereof, and pharmaceutically acceptable salts of the foregoing. This invention also provides pharmaceutical compositions comprising a compound of this invention and the use of such compositions in methods of treating narcolepsy, fibromyalgia, other disorders or conditions that are beneficially treated by improving nocturnal sleep or by administering sodium oxybate.
Owner:SUN PHARMA IND INC
Nitrogen-containing compound, electronic element, and electronic device
ActiveCN111138298AImprove performanceSilicon organic compoundsSolid-state devicesPhysical chemistryElectronic component
The invention provides a nitrogen-containing compound shown as a chemical formula I, an electronic element and an electronic device, and belongs to the technical field of organic materials. The nitrogen-containing compound can improve the performance of an electronic component. The structure represented as the specification refers to a chemical bond, wherein R1 and R2 are respectively and independently selected from hydrogen and a group shown as a chemical formula 1-1, and only one of R1 and R2 is a group shown as a chemical formula 1-1; when R1 or R2 is selected from hydrogen, R1 or R2 selected from hydrogen can be substituted by R4.
Owner:SHAANXI LIGHTE OPTOELECTRONICS MATERIAL CO LTD
Ionizable isotopic labeling reagents for relative quantification by mass spectrometry
ActiveUS20080050833A1Improve ETD analysisConvenient charging statusOrganic compound preparationComponent separationMetaboliteIsotopic labeling
Relative quantification of metabolites by Electrospray Ionization Mass Spectrometry (ESI-MS) requiring a mechanism for simultaneous analysis of multiple analytes in two or more samples. Labeling reagents that are reactive to particular compound classes and differ only in their isotopic kit facilitating relative quantification and providing tangible evidence for the existence of specific functional groups. Heavy and light isotopic forms of methylacetimidate were synthesized and used as labeling reagents for quantification of amine-containing molecules, such as biological samples. Heavy and light isotopic forms of formaldehyde and cholamine were also synthesized and used independently as labeling reagents for quantification of amine-containing and carboxylic acid-containing molecules, such as found in biological samples. Advantageously, the labeled end-products are positively charged under normal acidic conditions involving conventional Liquid Chromatography Mass Spectrometry (LC / MS) applications. Labeled primary and secondary amine and carboxylic acid end-products also generated higher signals concerning mass-spectra than pre-cursor molecules and improved sensitivity. Improved accuracy concerning relative quantification was achieved by mixing heavy and light labeled Arabidopsis extracts in different ratios. Labeling strategy was further employed to ascertain differences in the amounts of amine-containing metabolites for two strains of Arabidopsis seeds.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
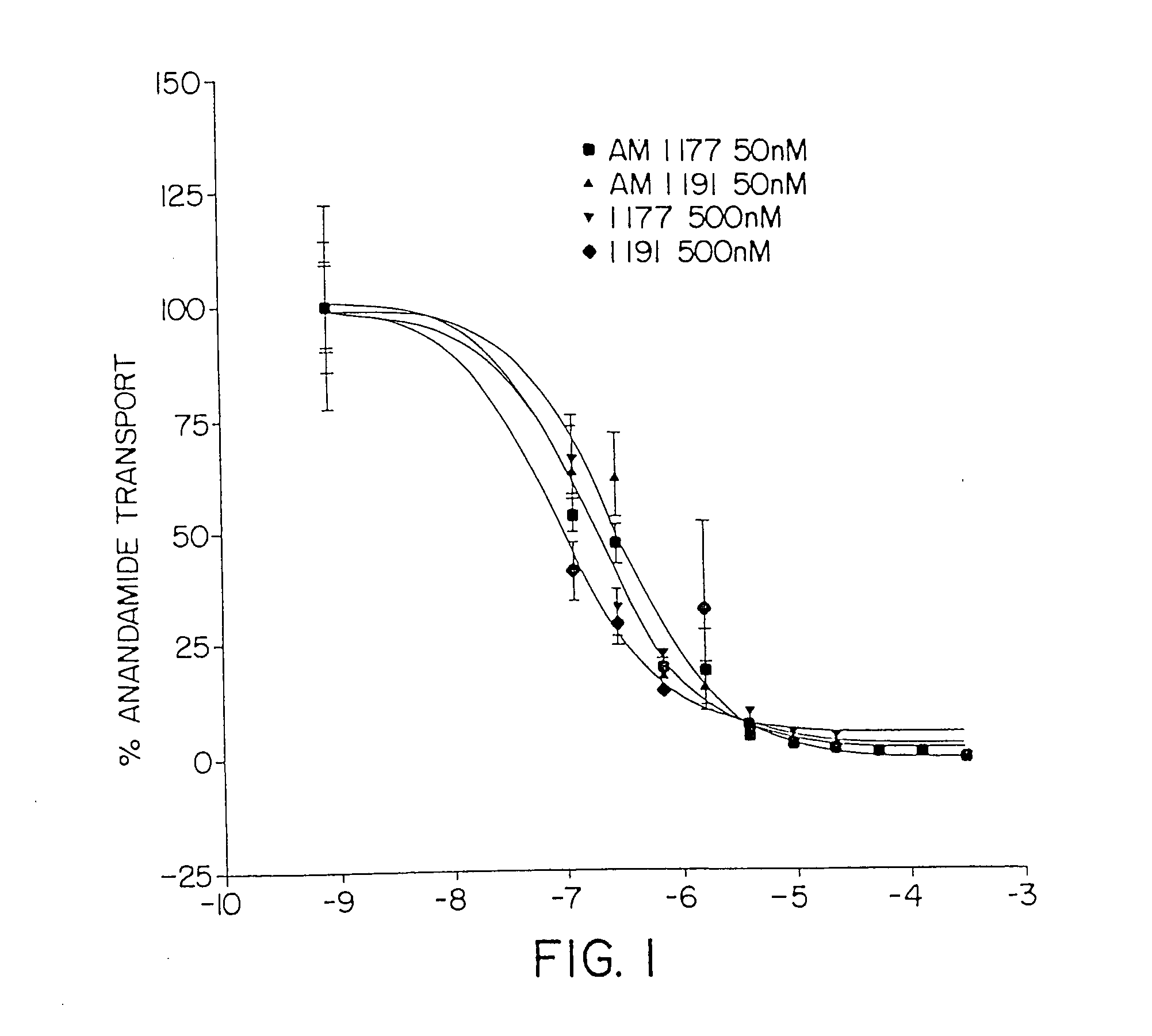
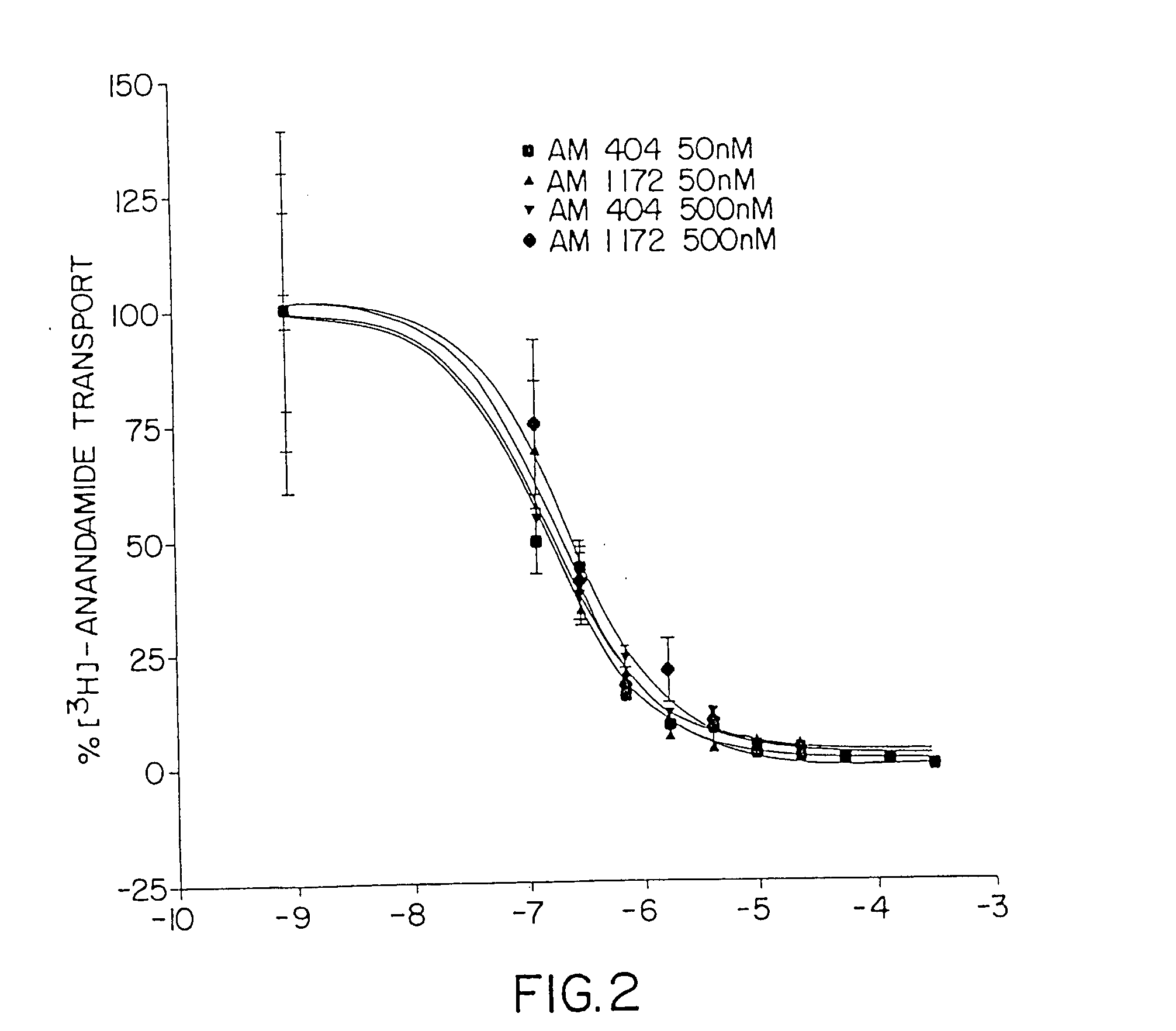
Inhibitors of the anandamide transporter
InactiveUS20050020679A1Raise the potentialIncrease appetiteBiocideSenses disorderTransport inhibitorAnandamide
Owner:UNIV OF CONNECTICUT
Ligands for imaging cardiac innervation
ActiveUS20100221182A1Improve stabilityDecreased NE releaseBiocideOrganic active ingredientsArylRadioactive tracer
Novel compounds that find use as imaging agents within nuclear medicine applications (PET imaging) for imaging of cardiac innervation are disclosed. These PET based radiotracers may exhibit increased stability, decreased NE release (thereby reducing side effects), improved quantitative data, and / or high affinity for VMAT over prior radiotracers. Methods of using the compounds to image cardiac innervation are also provided. In some instances the compounds are developed by derivatizing certain compounds with 18F in a variety of positions: aryl, alkyl, a keto, benzylic, beta-alkylethers, gamma-propylalkylethers and beta-proplylalkylethers. Alternatively or additionally, a methyl group a is added to the amine, and / or the catechol functionality is either eliminated or masked as a way of making these compounds more stable.
Owner:LANTHEUS MEDICAL IMAGING INC
Radiolabelling Method Using Polymers
InactiveUS20080305042A1Efficient responseDelayed recoveryRadioactive preparation carriersRadiation therapyImaging agentCombinatorial chemistry
The present invention provides a method for the preparation of radioisotopically-labelled imaging agent compositions. The method uses precursors which are bound to soluble polymers, so that the radiolabelling reaction is carried out in solution. Also described are radiopharmaceutical compositions, automated versions of the radiolabelling method and disposable cassettes for use in the automated method.
Owner:GACEK MICHEL +2
Anthracene derivatives and organic light emitting devices comprising the same
ActiveUS20140246657A1Group 4/14 element organic compoundsSolid-state devicesAnthraceneOrganic light emitting device
An anthracene derivative represented by Formula 1 is disclosed. An organic light-emitting device including an anode, a cathode, and an organic layer between the anode and the cathode, where the organic layer includes at least one anthracene derivative represented by Formula 1, is also disclosed. A method of manufacturing the organic light-emitting device is also disclosed.
Owner:SAMSUNG DISPLAY CO LTD +1
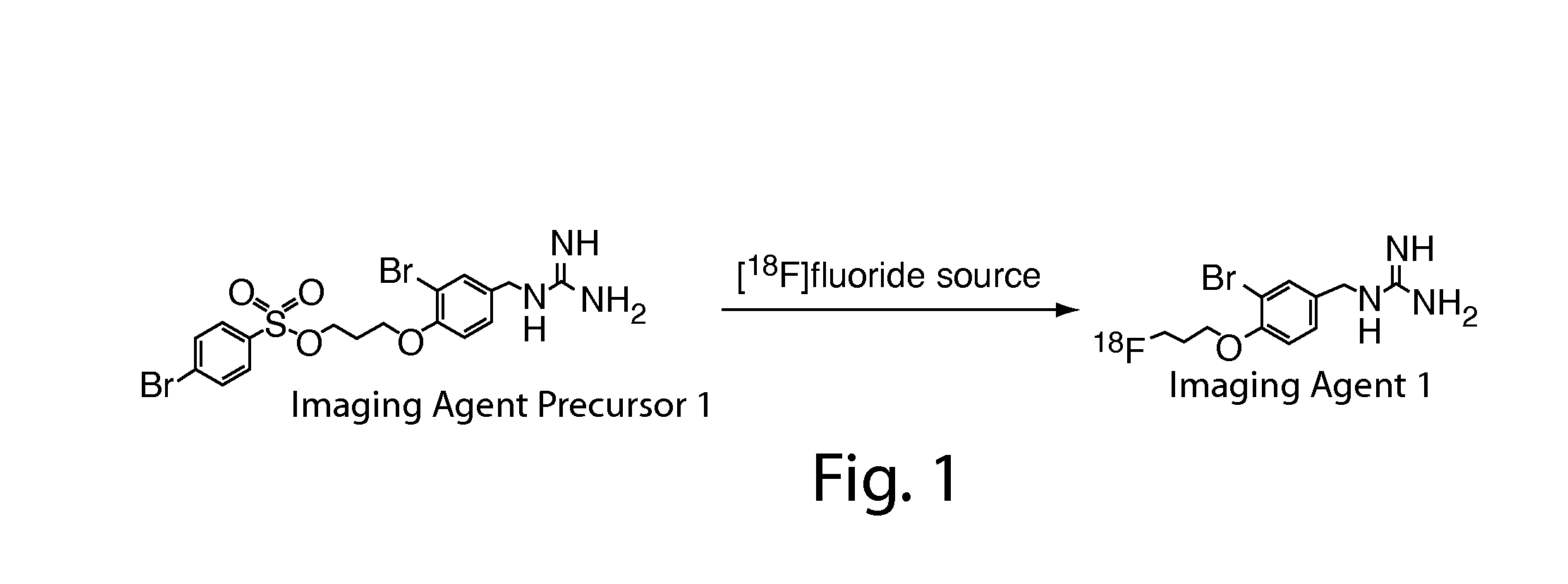
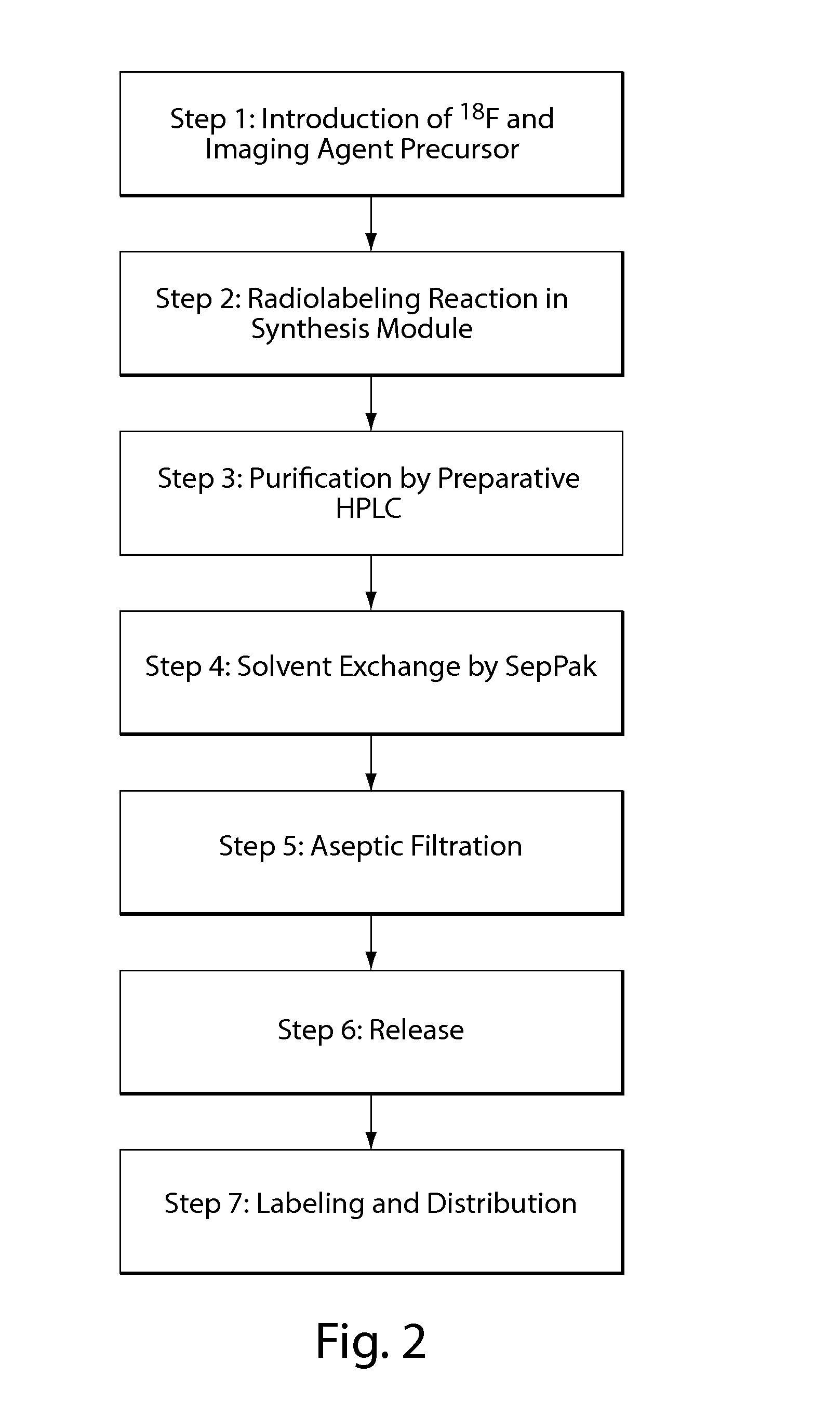
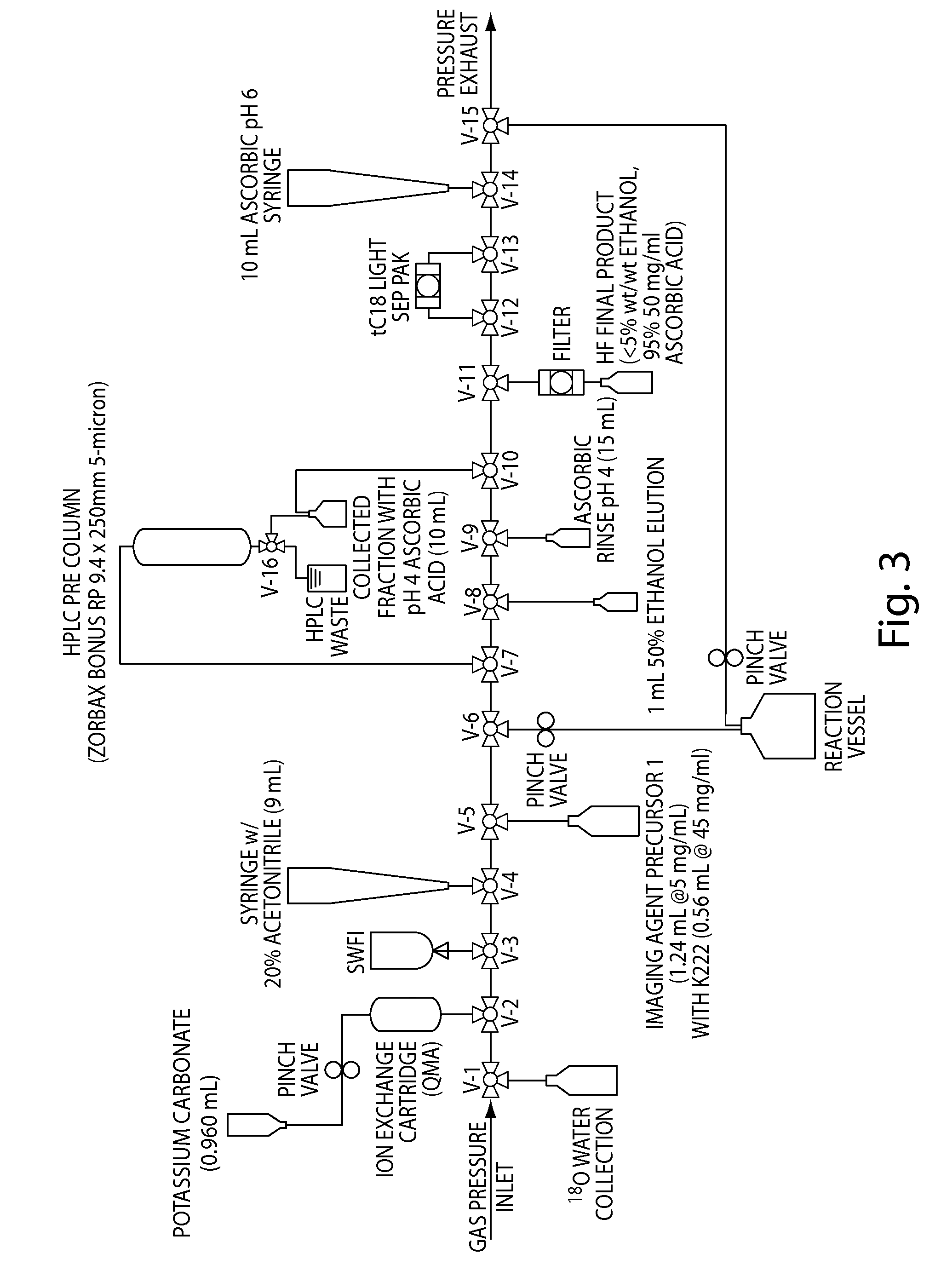
Compositions, methods, and systems for the synthesis and use of imaging agents
ActiveUS20140328756A1Eliminate needIsotope introduction to heterocyclic compoundsRadioactive preparation carriersImaging agentCardiac blood vessel
The present invention relates to systems, compositions, and methods for the synthesis and use of imaging agents, or precursors thereof. An imaging agent precursor may be converted to an imaging agent using the methods described herein. In some cases, the imaging agent is enriched in 18F. In some cases, an imaging agent may be used to image an area of interest in a subject, including, but not limited to, the heart, cardiovascular system, cardiac vessels, brain, and other organs. In some embodiments, methods and compositions for assessing perfusion and innervation mismatch in a portion of a subject are provided.
Owner:LANTHEUS MEDICAL IMAGING INC
Organic electroluminescent device comprising the organic electroluminescent compounds
InactiveUS20140346406A1High luminous efficiencyLong lastingGroup 4/14 element organic compoundsElectroluminescent light sourcesDopantBlue emission
The present invention relates to an organic electroluminescent device comprising a combination of specific host compounds and specific dopant compounds. The organic electroluminescent device according to the present invention shows a blue emission; and has a long operating lifespan, high efficiency, high brightness, good color purity, low driving voltage, and improved operational stability.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
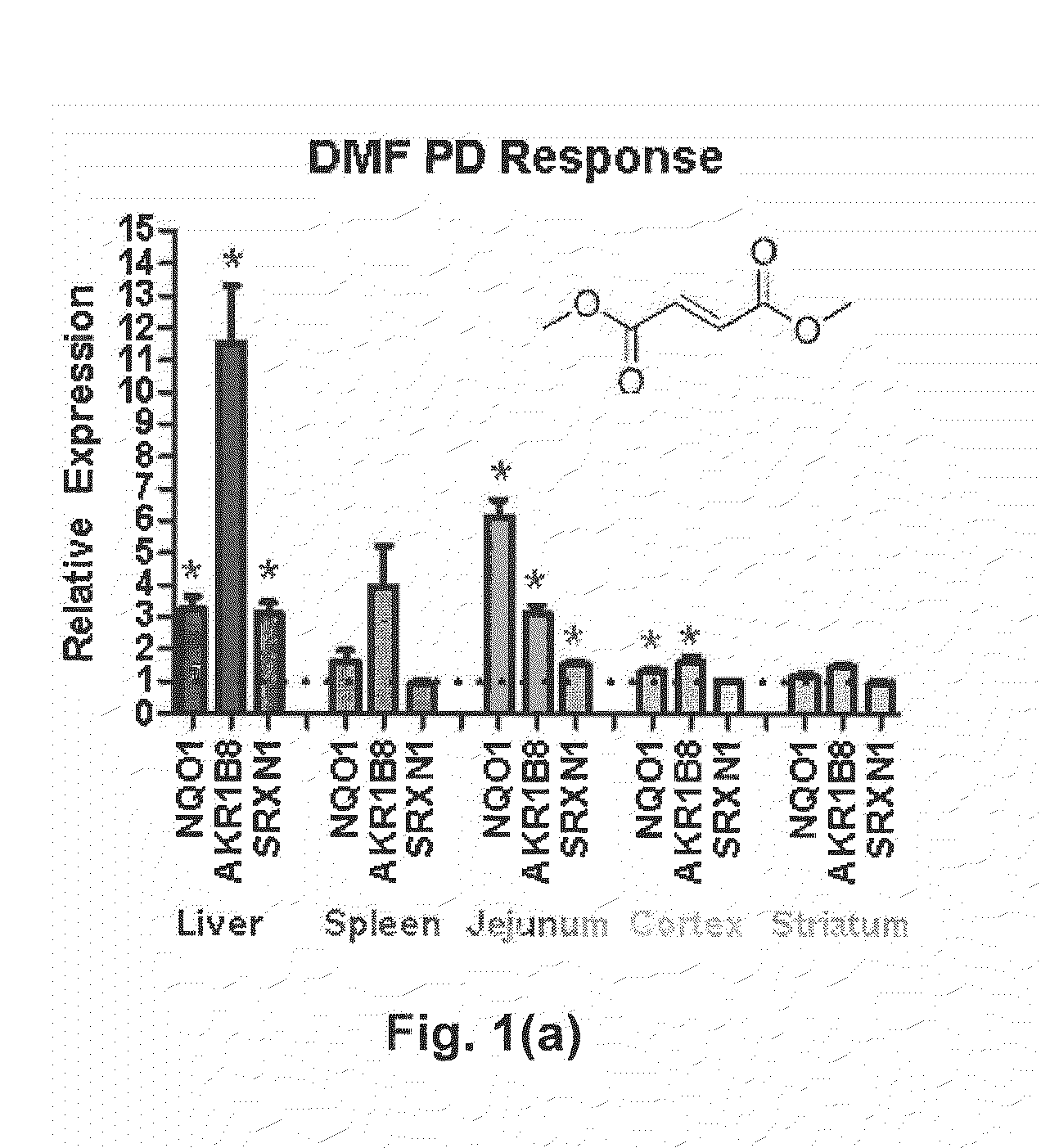
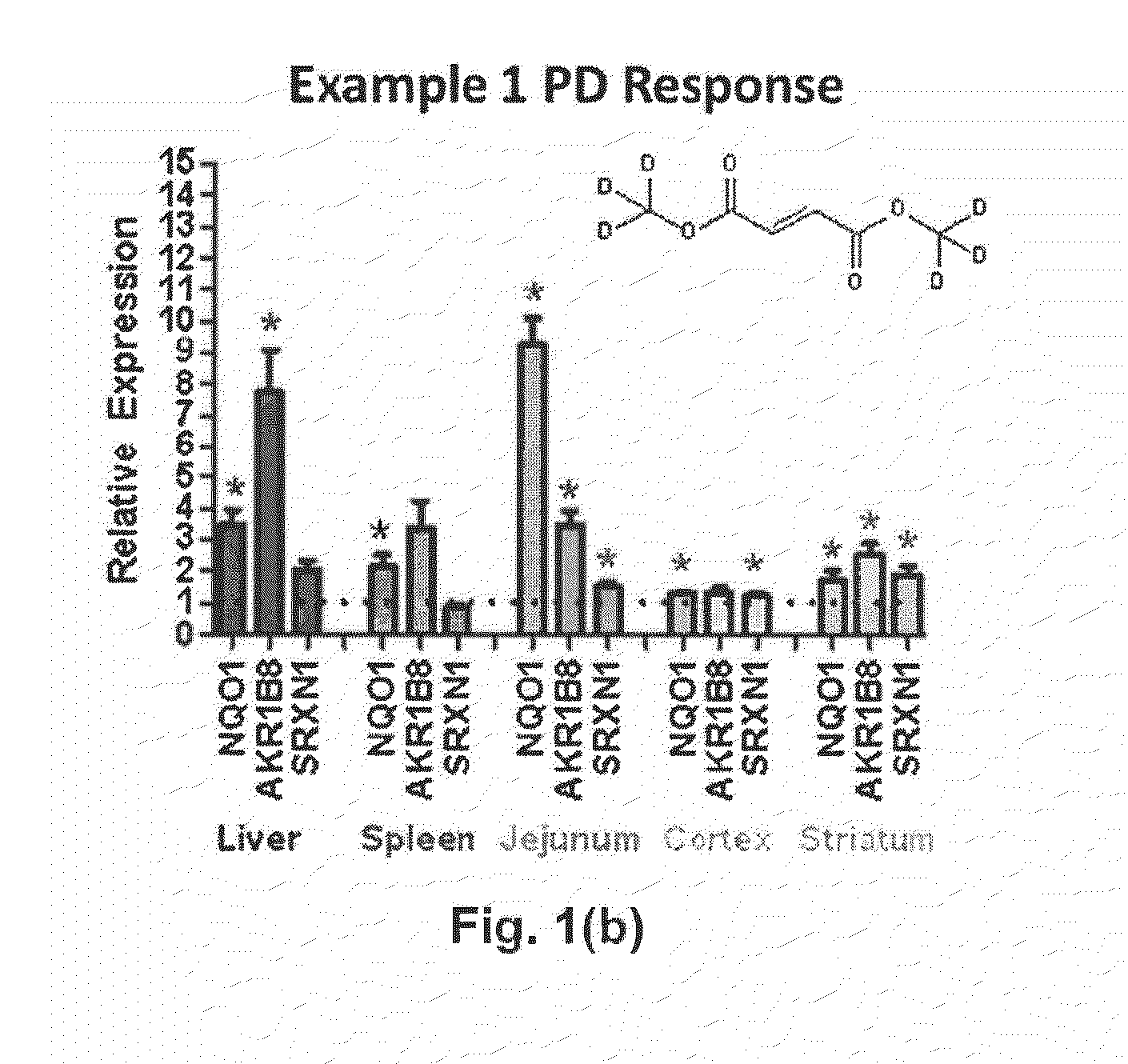
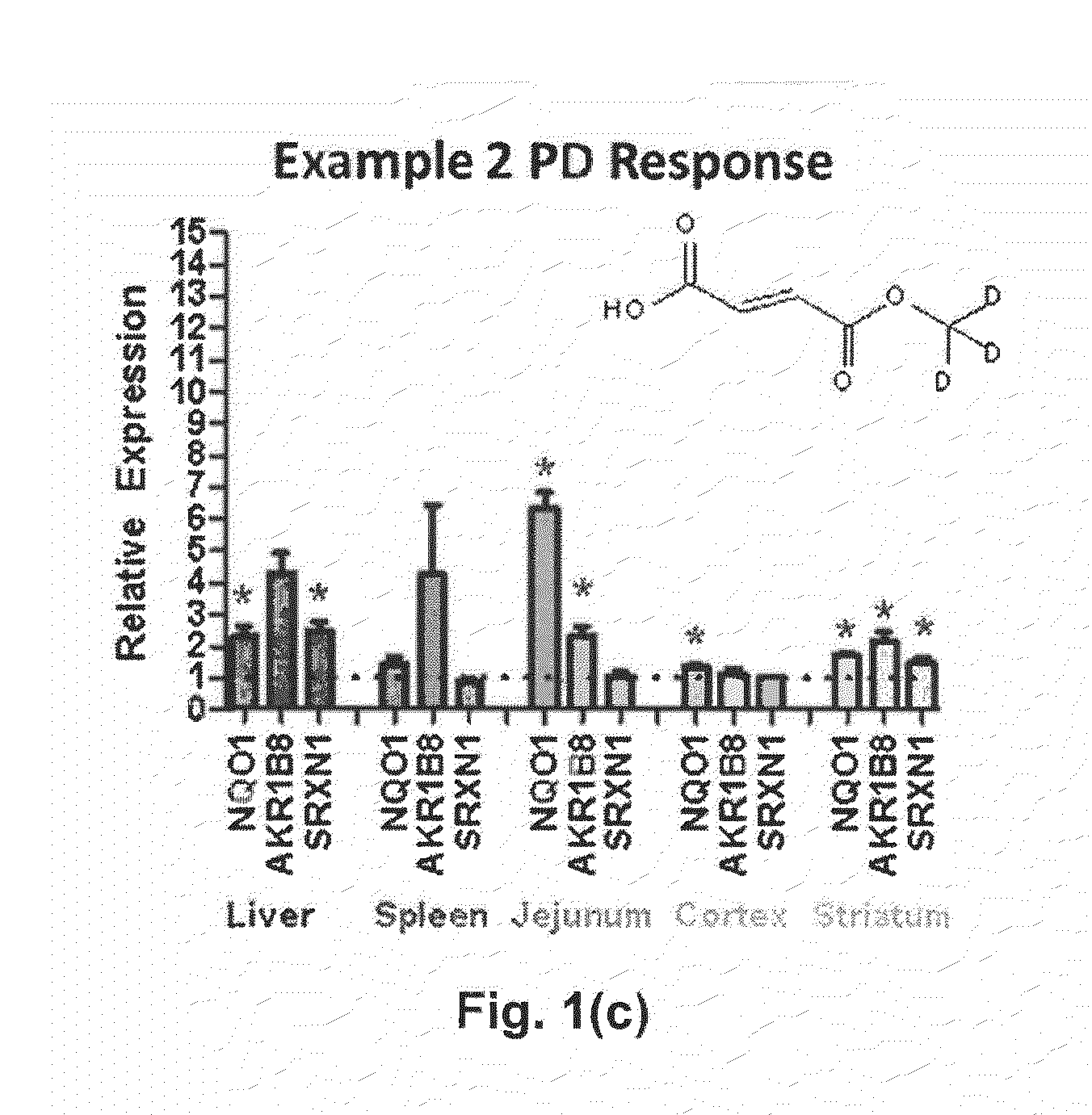
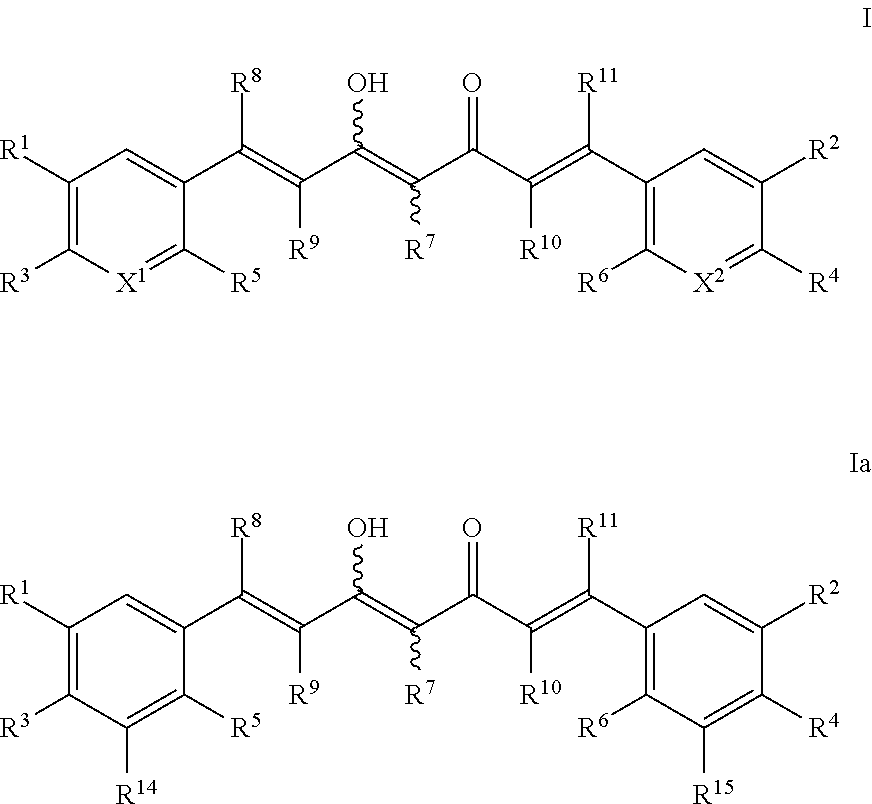
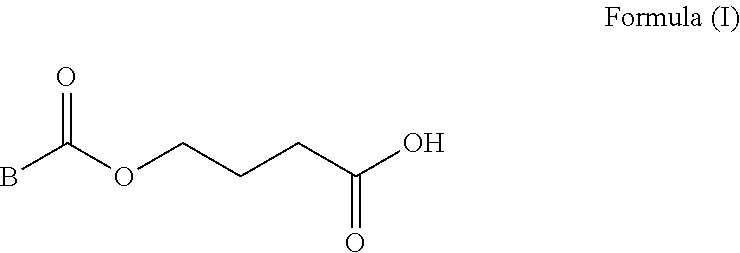
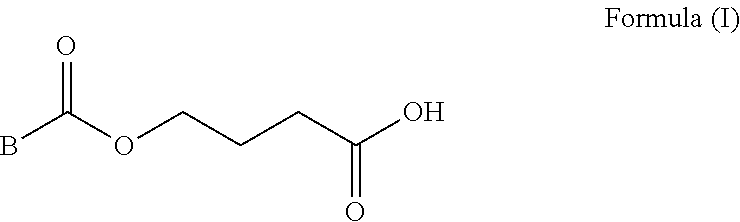
Deuterium Substituted Fumarate Derivatives
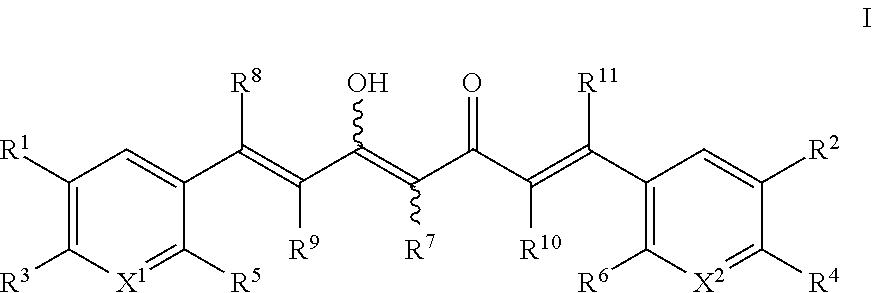
Provided is a compound of formula (I):or a pharmaceutically acceptable salt thereof. Also provided is a method of treating, prophylaxis, or amelioration of a disease, comprising administering to a subject in need of treatment for the disease an effective amount of a compound of formula (I) described herein. In one embodiment, the method is a neurodegenerative disease, such as multiple sclerosis, amyotrophic lateral sclerosis, Parkinson's disease, Huntington's disease, or Alzheimer's disease.
Owner:BIOGEN MA INC
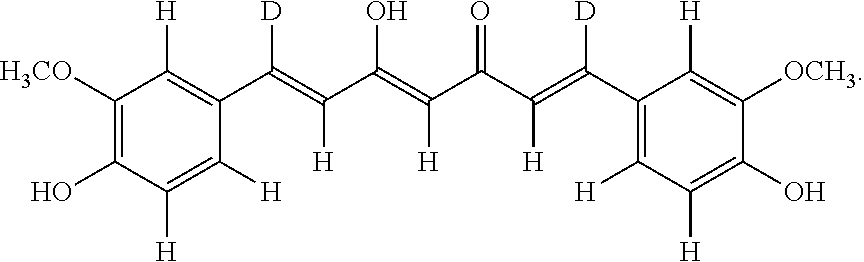
Derivatives of dimethylcurcumin
Owner:SUN PHARMA IND INC
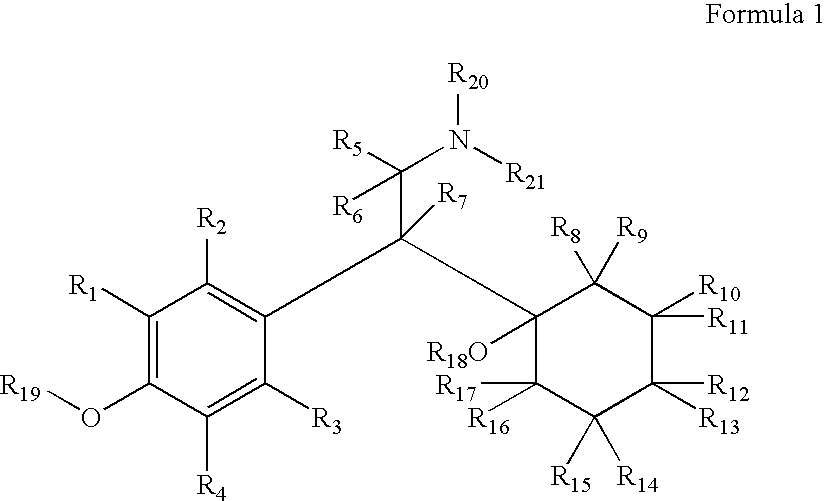
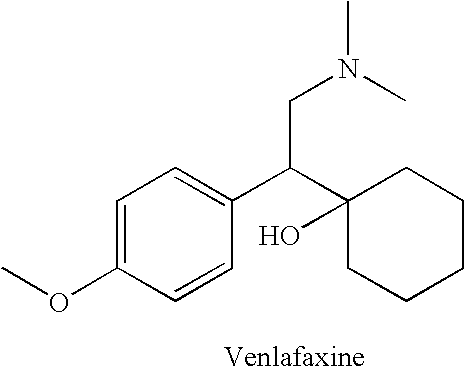
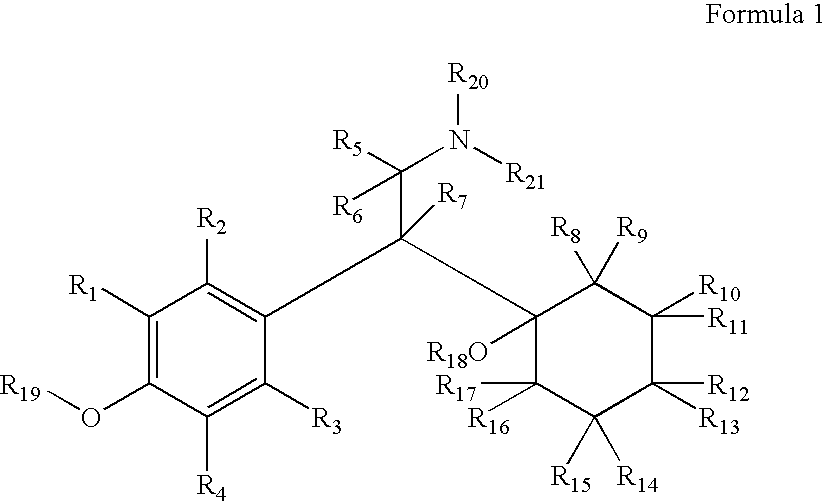
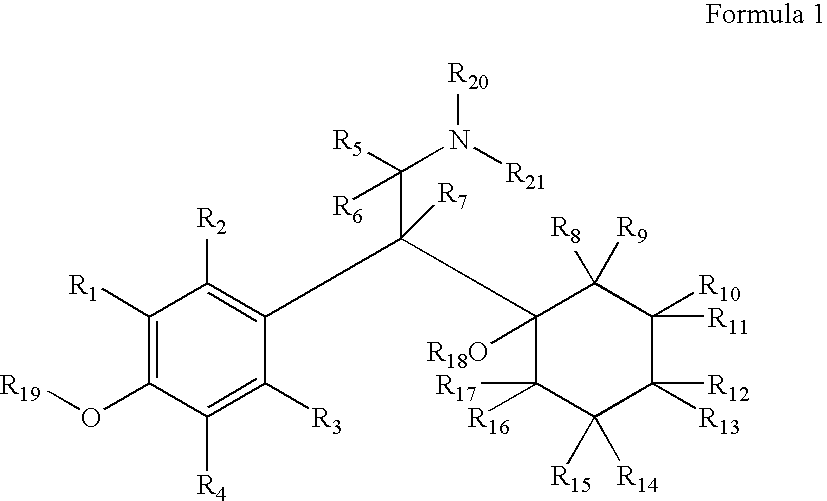
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Owner:ACADIA PHARMA INC
4-hydroxybutyric acid analogs
This invention relates to novel derivatives of 4-hydroxybutyric acid and prodrugs thereof, and pharmaceutically acceptable salts of the foregoing. This invention also provides pharmaceutical compositions comprising a compound of this invention and the use of such compositions in methods of treating narcolepsy, fibromyalgia, other disorders or conditions that are beneficially treated by improving nocturnal sleep or by administering sodium oxybate.
Owner:SUN PHARMA IND INC
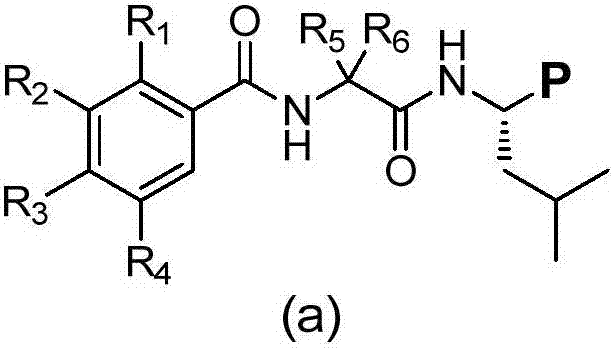
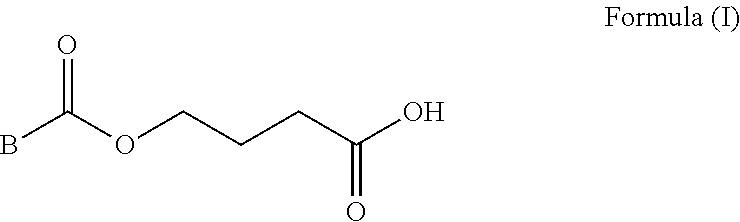
Deuterated dipeptide boric acids or esters thereof, and synthetic methods and uses of the compounds
Deuterated dipeptide boric acids or esters thereof, or crystal forms thereof, or pharmaceutically acceptable hydrates or solvates thereof are disclosed. The general structure of the compounds is shown as a formula (a) in the specification, wherein R1, R2, R3, R4, R5 and R6 are independently selected from hydrogen, deuterium or halogens, or C1-C4 alkyl one or a plurality of or all hydrogen atoms of which are deuterated; and at least one of the R1, the R2, the R3, the R4, the R5 and the R6 is deuterated or deuterium. The compounds can effectively inhibit proteasomes and effectively treat or prevent cancer, cardiovascular disease, inflammation, immune disease, nephropathy, vasculogenesis or prostate disease.
Owner:NANJING LINGRUI PHARM TECH CO LTD
Stilbene derivatives and their use for binding and imaging amyloid plaques
This invention relates to a method of imaging amyloid deposits and to labeled compounds, and methods of making labeled compounds useful in imaging amyloid deposits. This invention also relates to compounds, and methods of making compounds for inhibiting the aggregation of amyloid proteins to form amyloid deposits, and a method of delivering a therapeutic agent to amyloid deposits.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions, methods, and systems for the synthesis and use of imaging agents
ActiveUS9550000B2Eliminate needIsotope introduction to heterocyclic compoundsRadioactive preparation carriersImaging agentPerfusion
The present invention relates to systems, compositions, and methods for the synthesis and use of imaging agents, or precursors thereof. An imaging agent precursor may be converted to an imaging agent using the methods described herein. In some cases, the imaging agent is enriched in 18F. In some cases, an imaging agent may be used to image an area of interest in a subject, including, but not limited to, the heart, cardiovascular system, cardiac vessels, brain, and other organs. In some embodiments, methods and compositions for assessing perfusion and innervation mismatch in a portion of a subject are provided.
Owner:LANTHEUS MEDICAL IMAGING INC
Substituted phenethylamines with serotoninergic and/or norepinephrinergic activity
Chemical syntheses and medical uses of novel inhibitors of the uptake of monoamine neurotransmitters and pharmaceutically acceptable salts and prodrugs thereof, for the treatment and / or management of psychotropic disorders, anxiety disorder, generalized anxiety disorder, depression, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, hot flashes, senile dementia, migraine, hepatopulmonary syndrome, chronic pain, nociceptive pain, neuropathic pain, painful diabetic retinopathy, bipolar depression, obstructive sleep apnea, psychiatric disorders, premenstrual dysphoric disorder, social phobia, social anxiety disorder, urinary incontinence, anorexia, bulimia nervosa, obesity, ischemia, head injury, calcium overload in brain cells, drug dependence, and / or premature ejaculation are described.
Owner:ACADIA PHARMA INC
Deuterated aryl amine compound, preparation method thereof, and organic light emitting diode using the same
InactiveUS8026665B2Improve efficiencyImprove luminanceDischarge tube luminescnet screensLamp detailsArylHole injection layer
Disclosed are a novel deuterated aryl amine compound capable of enhancing thermal stability, hole transporting capability, luminescence efficiency, etc. of an organic light emitting diode at the time of being used as a hole-injecting layer, a preparation method thereof, and an organic light emitting diode using the same.
Owner:SOLUS ADVANCED MATERIALS CO LTD
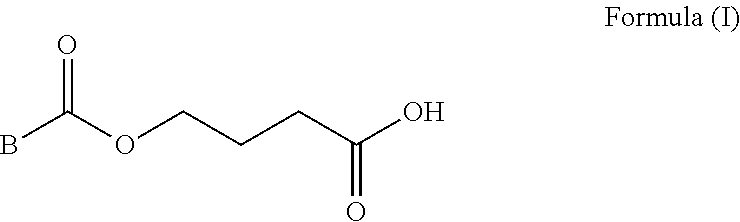
Boron carrying agent for integrating tumor diagnosis and treatment
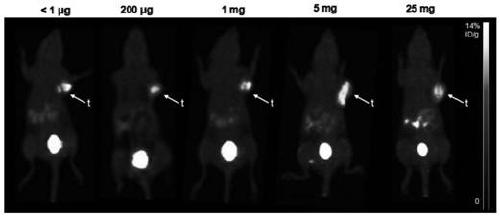
InactiveCN109053781AHigh enrichment efficiencyEasy to prepareEnergy modified materialsBoron compound active ingredientsImage diagnosisNeutron capture
The invention relates to a boron carrying agent for integrating tumor diagnosis and treatment. The boron carrying agent is tyrosine boron trifluoride (FBY) obtained by replacing a carboxyl group witha boron trifluoride group on the basis of tyrosine. FBY can be used for boron neutron capture therapy (BNCT) for treating malignant tumors; radioactive <18>F-labeled FBY can also be used for positronemission tomography (PET) imaging diagnosis. <18F>-FBY can simultaneously achieve imaging diagnosis and precise treatment of cancers.
Owner:PEKING UNIV
Prodrugs of gamma-hydroxybutyric acid, compositions and uses thereof
The present disclosure discloses prodrugs of gamma-hydroxybutyric acid as well as compositions and uses thereof.
Owner:XWPHARMA LTD
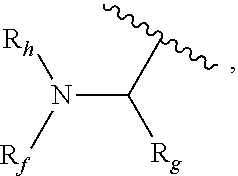
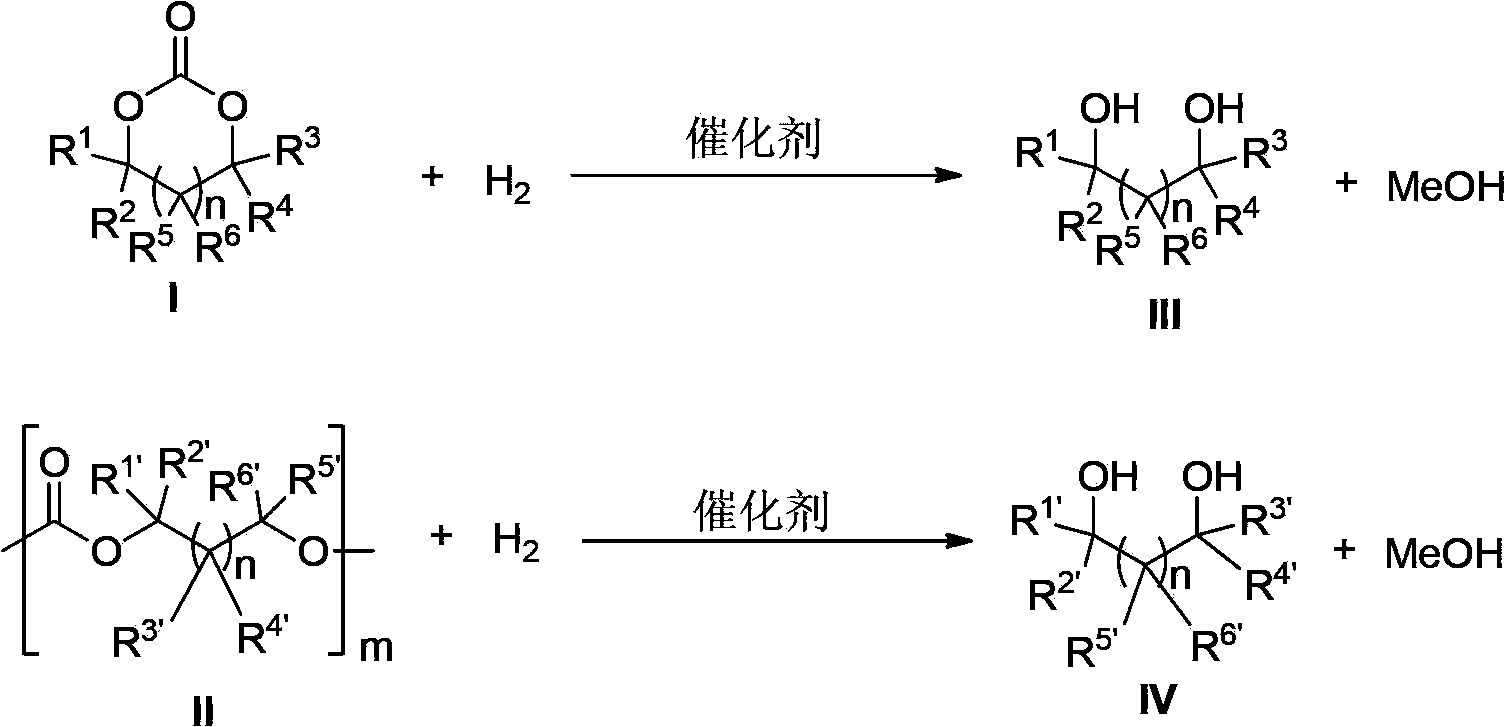
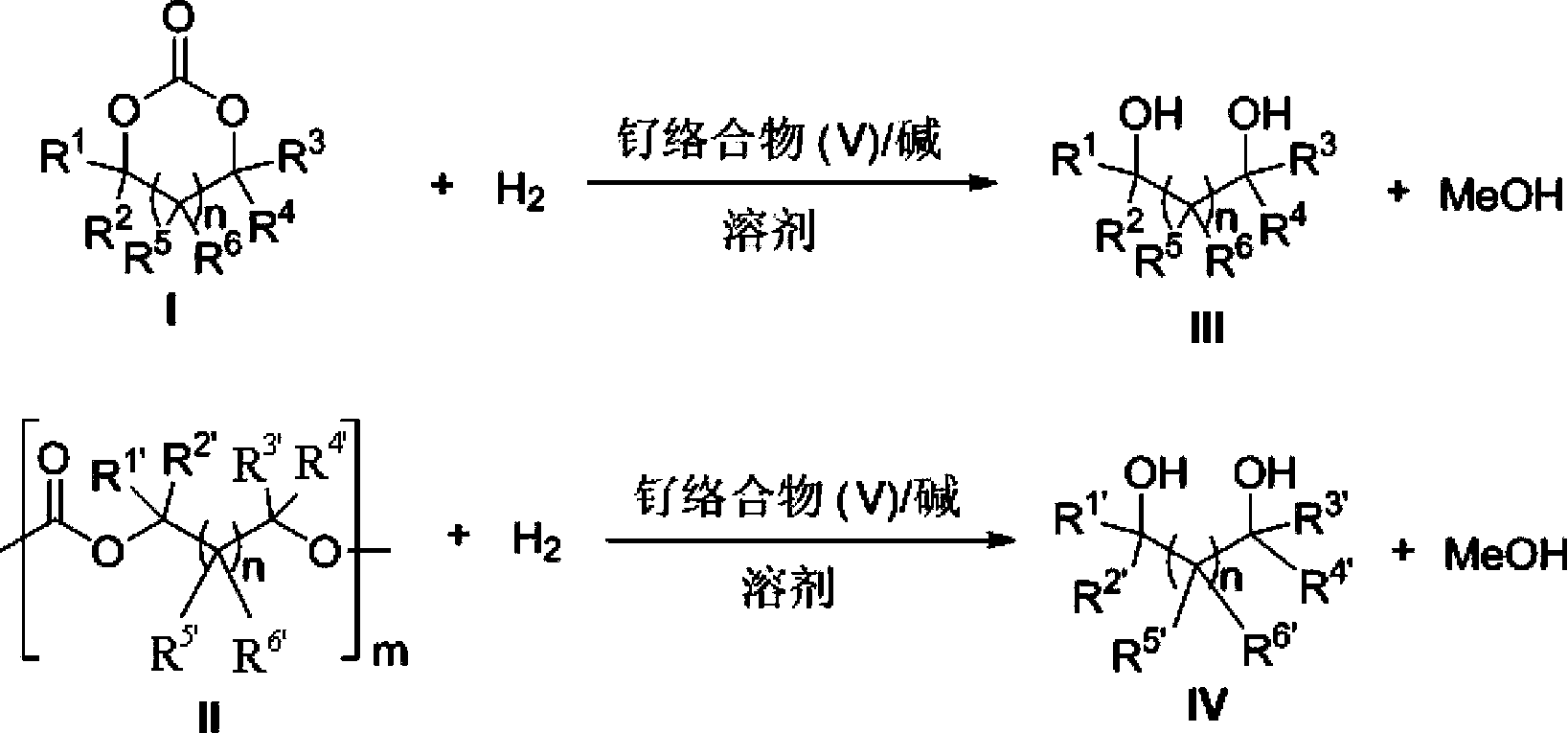
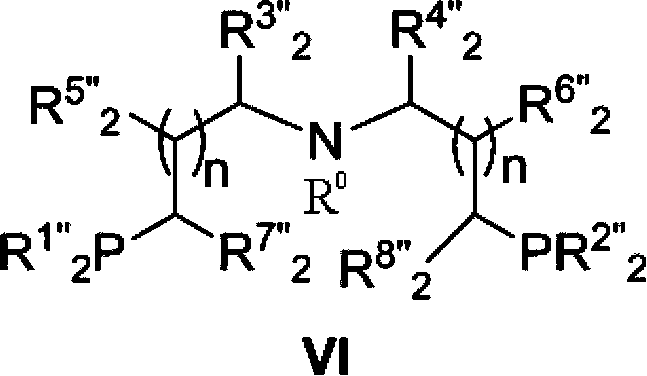
Novel ruthenium complex and method for preparing methanol and diol
ActiveCN103772142AFacilitate large-scale industrial productionImprove efficiencyRuthenium organic compoundsOrganic compound preparationOrganic solventHydrogen atmosphere
The invention provides a method for preparing methanol and diol from cyclic carbonates and polycarbonates. The method comprises: in hydrogen atmosphere, in an organic solvent, and in the presence of a ruthenium complex (Ru(L)XYY') and an alkali, performing hydrogenation reduction reaction on a cyclic carbonate or a polycarbonate, so as to obtain methanol and diol, wherein all groups in the formula are defined in the specification. The invention also provides the ruthenium complex formed by ruthenium and a tridentate amino diphosphine ligand. The invention also provides a method for preparing deuterated methanol and deuterated diol by employing the above preparation method. The method provided by the invention is high in efficiency, high in selectivity, economic, environment-friendly, and simple for operation, can be performed under mild conditions, and has complete atom economy.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Compositions, methods, and systems for the synthesis and use of imaging agents
ActiveUS20130149244A1Improve purification effectImprove solubilityNervous disorderOrganic compound preparationImaging agentCardiac blood vessel
The present invention generally relates to novel synthetic methods, systems, kits, salts, and precursors useful in medical imaging. In some embodiments, the present invention provides compositions comprising an imaging agent precursor, which may be formed using the synthetic methods described herein. An imaging agent may be converted to an imaging agent using the methods described herein. In some cases, the imaging agent is enriched in 18F. In some cases, an imaging agent including salt forms (e.g., ascorbate salt) may be used to image an area of interest in a subject, including, but not limited to, the heart, cardiovascular system, cardiac vessels, brain, and other organs.
Owner:LANTHEUS MEDICAL IMAGING INC
Prodrugs of gamma-hydroxybutyric acid, compositions and uses thereof
The present disclosure discloses prodrugs of gamma-hydroxybutyric acid as well as compositions and uses thereof.
Owner:XWPHARMA LTD
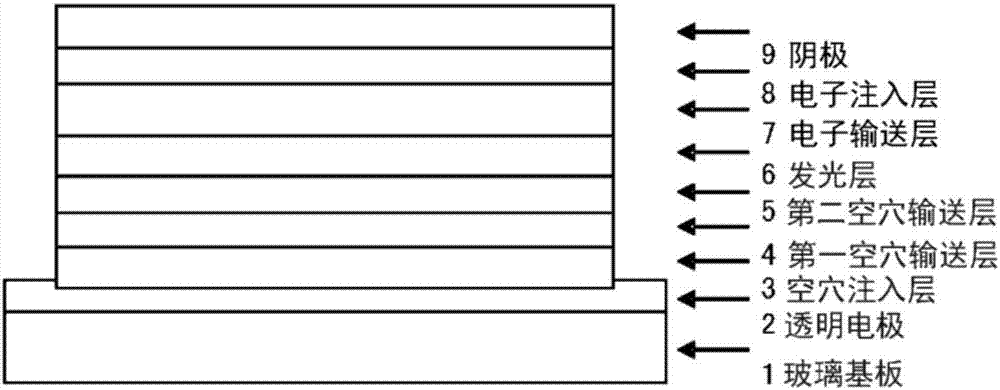
Organic electroluminescent element
ActiveCN107112427AImprove efficiencySolution to short lifeSolid-state devicesSemiconductor/solid-state device manufacturingHole injection layerOrganic electroluminescence
Owner:HODOGOYA CHEMICAL CO LTD
Photocatalytic halohydrocarbon dehalogenation conversion method
InactiveCN109438156AHigh economic valueAvoid it happening againPhysical/chemical process catalystsHydrocarbon from halogen organic compoundsHalohydrocarbonAlkyne
The invention provides a photocatalytic halohydrocarbon dehalogenation conversion method which comprises the following steps: adding a photocatalyst quantum dot / rod into a solvent to obtain a solutionA; adding halohydrocarbon and an electronic sacrificial body into the solution A to obtain a solution B; utilizing a light source to irradiate the solution B and catalyzing the solution B to performhalohydrocarbon dehalogenation conversion. According to the photocatalytic halohydrocarbon dehalogenation conversion method disclosed by the invention, a nano quantum dot and a nano quantum rod are applied to dehalogenation conversion reaction of alkyl halide, alkenyl halide and alkyne halide for the first time; the reaction conditions are moderate, visible light is utilized as driving energy, a product is hydrocarbon compound, and the whole process has the advantages of environmental protection, conciseness and high efficiency. In addition, higher hydrocarbon of carbon chain growth can be generated after dehalogenation reaction, so that the method has potential application in preparation of higher hydrocarbon. According to the method disclosed by the invention, halohydrocarbon dehalogenation conversion and deuteration marking processes are jointly performed; hydrocarbon deuteration marking can be finished when a halohydrocarbon dehalogenation process is finished. The invention furtherprovides a method for performing deuteration marking on hydrocarbon.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Popular searches
Hydrocarbon by hydrogenation Hydrocarbons Carboxylic compound preparation Isotope introduction to acyclic/carbocyclic compounds Nitrile/isonitrile active ingredients Heterocyclic compound active ingredients X-ray constrast preparations Drug compositions Animal repellants Group 7/17 organic compounds without C-metal linkages
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com