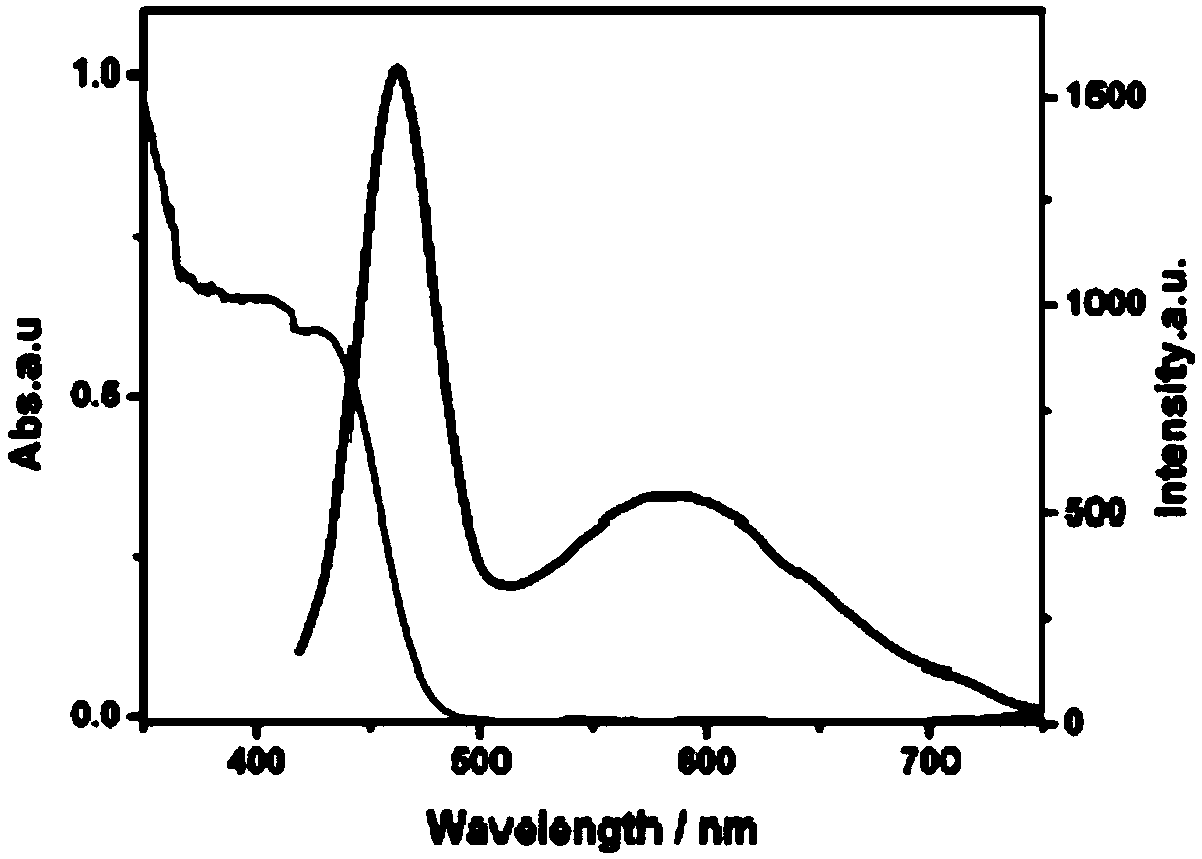
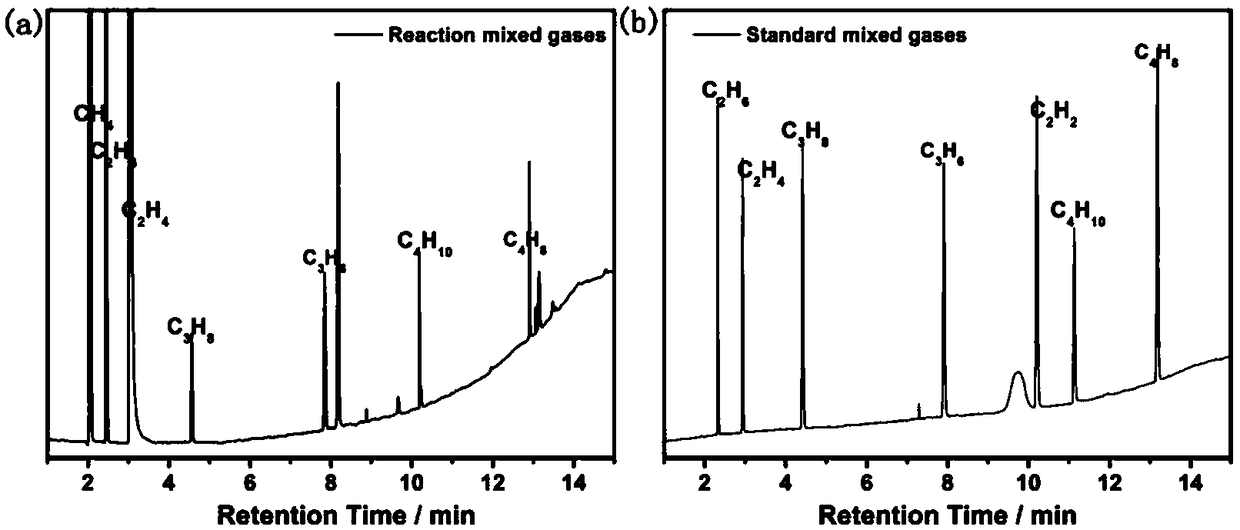
Photocatalytic halohydrocarbon dehalogenation conversion method
A halogenated hydrocarbon and photocatalytic technology, which is applied in the field of catalytic synthesis and the sustainable development of the environment and energy, can solve the problems of precious metals and narrow use range of substrates, and achieve the effect of avoiding by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Using CdSe QDs as the photocatalyst, add the photocatalyst to 4mL of water, and the solubility of the catalyst is 2×10 -5 M, add 50μL of dichloromethane (66.3mg, 0.78mmol) and 300μL of triethylamine (218.1mg, 2.16mmol), replace the air with Ar atmosphere, 1mL N 2 As an internal standard, at room temperature, LED lights (λ> 460nm) irradiation for 4h. After the reaction, use GC (TCD) to detect CH 4 Yield. The methane yield is 80%.
Embodiment 2
[0058] Using CdSe QDs as the photocatalyst, adding the photocatalyst to 4mL of water, the solubility of the catalyst is 2×10 -5 M, add 55μL of dibromomethane (135.6mg, 0.78mmol) and 300μL of triethylamine (218.1mg, 2.16mmol), replace the air with Ar atmosphere, 1mL N 2 As an internal standard, at room temperature, LED lights (λ> 460nm) irradiation for 8h. After the reaction, use FID-GC to detect CH 4 And ethylene selectivity and yield. The selectivity for methane is 22%, and the selectivity for ethylene and ethane is as high as 77%.
Embodiment 3
[0060] Using CdSe QDs as the photocatalyst, adding the photocatalyst to 4mL of water, the solubility of the catalyst is 2×10 -5 M, add 63μL of diiodomethane (208.9mg, 0.78mmol) and 300μL of triethylamine (218.1mg, 2.16mmol), replace the air with Ar atmosphere, 1mL N 2 As an internal standard, at room temperature, LED lights (λ> 460nm) irradiation for 8h. After the reaction, use FID-GC to detect CH 4 And ethylene selectivity and yield. The selectivity of ethylene and ethane is as high as 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com