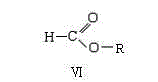
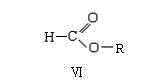
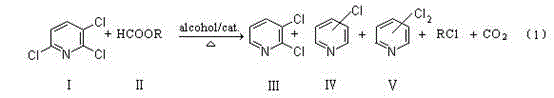
Preparation method of 2,3-dichloropyridine
A technology of dichloropyridine and trichloropyridine is applied in the field of preparation of 2,3-dichloropyridine, can solve unseen problems and the like, and achieve the effects of good yield, safe preparation process and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Add 300ml of absolute ethanol and 0.125mol of 2,3,6-trichloropyridine (23g, 99.2% GC content) into a 500ml four-necked reaction flask equipped with a stirrer, thermometer, reflux condenser and feeder, and then Add 5.55g of ammonium formate, start stirring and heat slowly at the same time. When the temperature of the feed liquid reaches 50-55°C, start to add 2g of 5% Pd / C catalyst (containing 65% of water base), after a while, dense bubbles will escape, and continue to slowly Slowly raise the temperature to slight reflux reaction for 3 hours, then add 10.20 g of ammonium formate in 6 batches within 6 hours, after the addition, react at the same temperature for about 1 hour, monitor the reaction by GC until the conversion rate meets the requirements, filter while hot , the filter cake is washed with water, and the catalyst recovered after desalination is used for the next reaction. GC analysis of the filtrate showed:
[0052] 3-chloropyridine: 4.95%,
[0053] 2,5-D...
Embodiment 2
[0060] Operate in the same manner as in Example 1, but change the amount of ammonium formate added to 8 g with 2.5 g of reclaimed catalyst instead, and then add 16 g of ammonium formate evenly in 8 batches within 7 hours. GC analysis of the filtrate showed:
[0061] 3-chloropyridine: 3.55%,
[0062] 2,5-dichloropyridine: 1.12%,
[0063] 2,3-Dichloropyridine: 40.83%,
[0064] 2,6-dichloropyridine: 2.52%,
[0065] 2,3,6-trichloropyridine: 51.98%.
[0066] After vacuum distillation and comprehensive treatment, the yield of 2,3-dichloropyridine is 81.46% based on the reacted 2,3,6-trichloropyridine.
Embodiment 3
[0068] Operate in the same way as in Example 1, but use methanol as a solvent, and when the reaction finishes, the GC analysis result of the reaction mixture is:
[0069] 3-chloropyridine: 7.56%,
[0070] 2,5-Dichloropyridine: 2.01%,
[0071] 2,3-Dichloropyridine: 34.61%,
[0072] 2,6-dichloropyridine: 3.01% %,
[0073] 2,3,6-trichloropyridine: 52.76%.
[0074] After vacuum distillation and comprehensive treatment, the yield of 2,3-dichloropyridine is 69.10% based on the converted 2,3,6-trichloropyridine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com