Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
477 results about "Drug synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthesis of Essential Drugs describes methods of synthesis, activity and implementation of diversity of all drug types and classes. With over 2300 references, mainly patent, for the methods of synthesis for over 700 drugs, along with the most widespread synonyms for these drugs,...
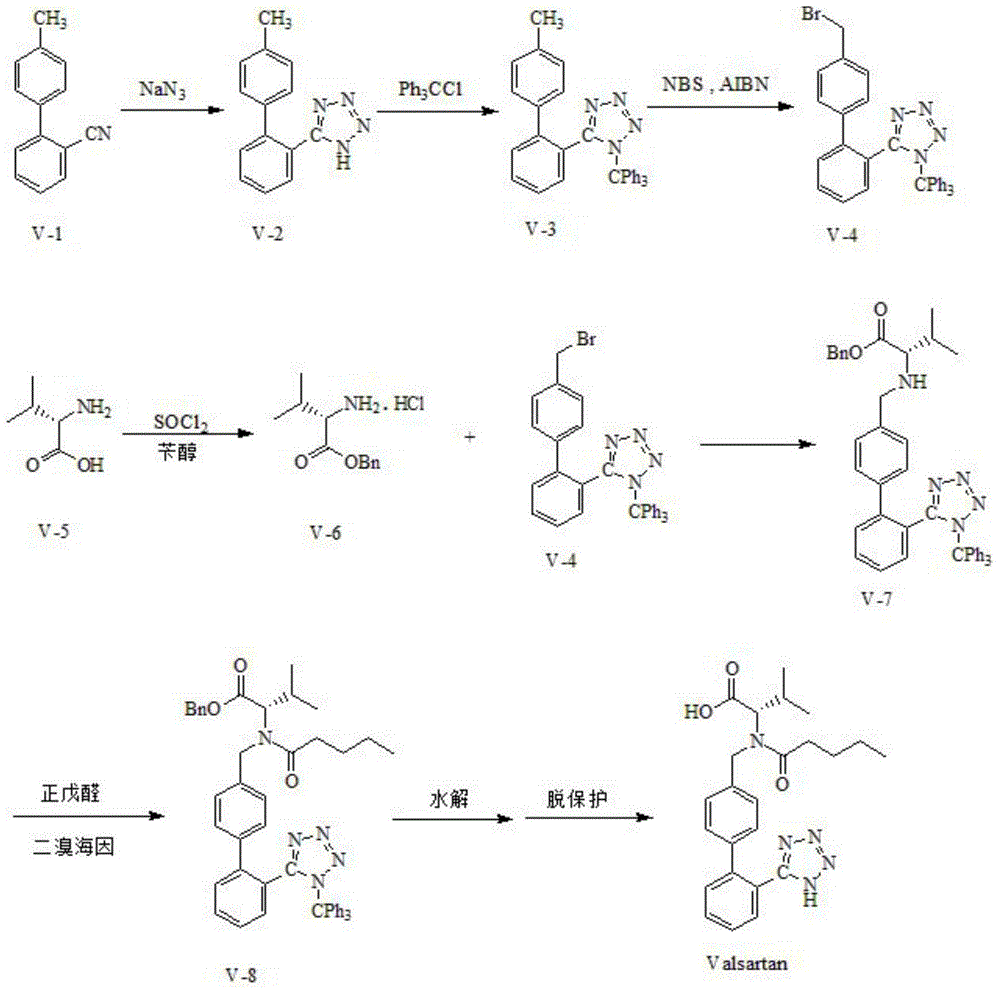
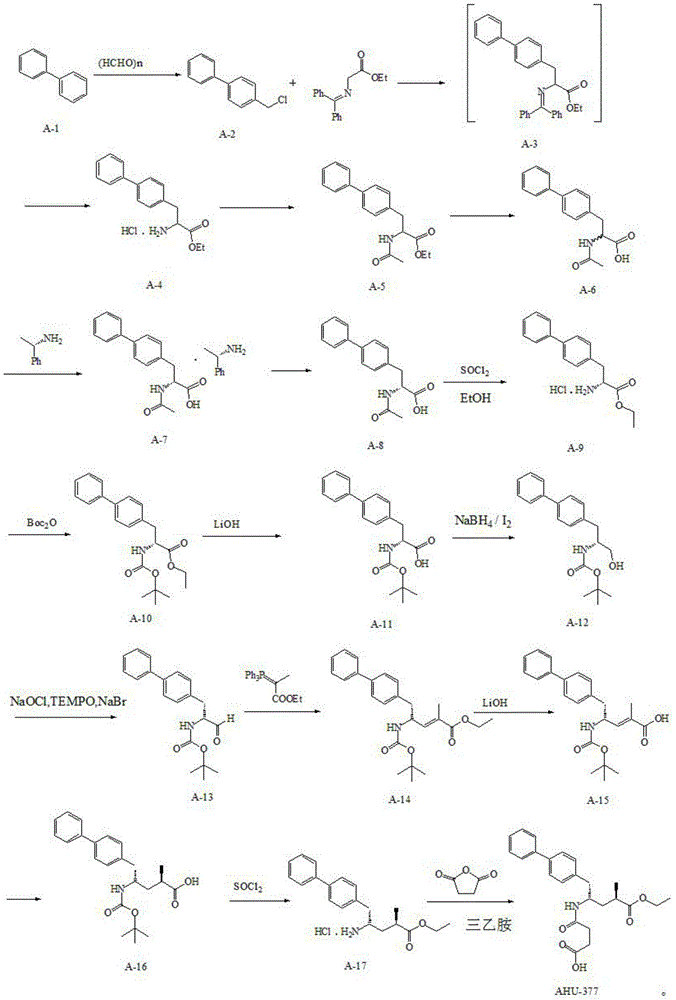
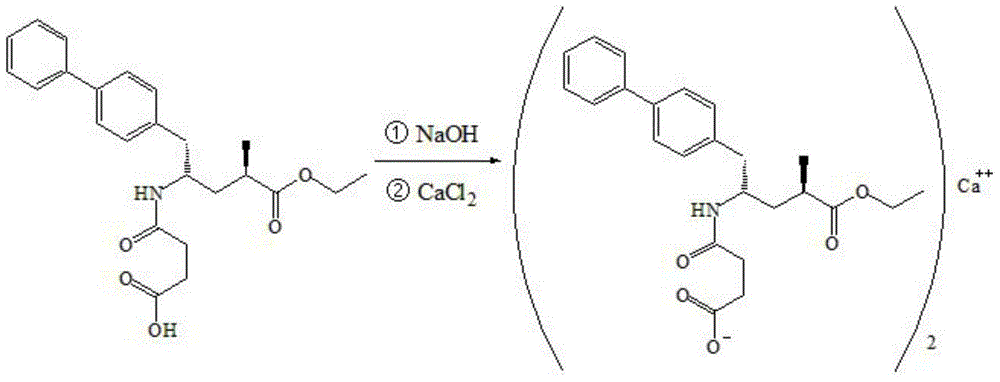
Preparation method for dual inhibitor LCZ696 of angiotensin II receptor and neprilysin
InactiveCN105168205AQuality improvementHigh purityOrganic compound preparationCarboxylic acid amides preparationValsartanHigh volume manufacturing
The invention specifically relates to a preparation method for a dual inhibitor LCZ696 of angiotensin II receptor and neprilysin, which belongs to the technical field of drug synthesis. The preparation method comprises the following steps: preparing valsartan and AHU-377 or AHU-377 calcium salt at first; and then mixing valsartan with AHU-377 or AHU-377 calcium salt under stirring so as to prepare LCZ696. The LCZ696 prepared in the invention has good quality and high purity; and the preparation method has the advantages of simplicity, low energy consumption, low cost and suitability for large batch production.
Owner:TAILITE MEDICINE HUBEI
Method for preparing cabazitaxel by taking 10-deacetylate-baccatin III as raw material
ActiveCN102417491AIncrease preparation meteringEasy to operateOrganic chemistryUrinary disorderAcetic acidCabazitaxel
The invention relates to a method for preparing cabazitaxel, particularly relates to a method for preparing the cabazitaxel by taking 10-deacetylate-baccatin III as a raw material and belongs to the technical field of drug synthesis. The method comprises the following steps: firstly, reacting 10-DAB with chlorocarbonate-2,2,2-trichloro ethyl ester, thereby obtaining a product; reacting the product with DMAP (dimethylamino pyridine), DCC (dicyclohexylcarbodiimide) and (4S, 5R)-2,2-dimethyl-4-phenyl-3-tert-butoxycarbonyl-3.5-oxazolidine formic acid, thereby obtaining a product; reacting the product with acetic acid and zinc powder, and then methylating the product; and lastly, adding a p-methyl benzenesulfonic acid, thereby reacting and obtaining a cabazitaxel product. The cabazitaxel prepared according to the method can be widely applied to the treatment of prostate cancer. Compared with a traditional technique for preparing the cabazitaxel, the method has the advantages that the preparation quantity is greatly increased, the operation steps are simplified, the manpower is saved and the method has industrial application value and prospect.
Owner:无锡紫杉药业股份有限公司
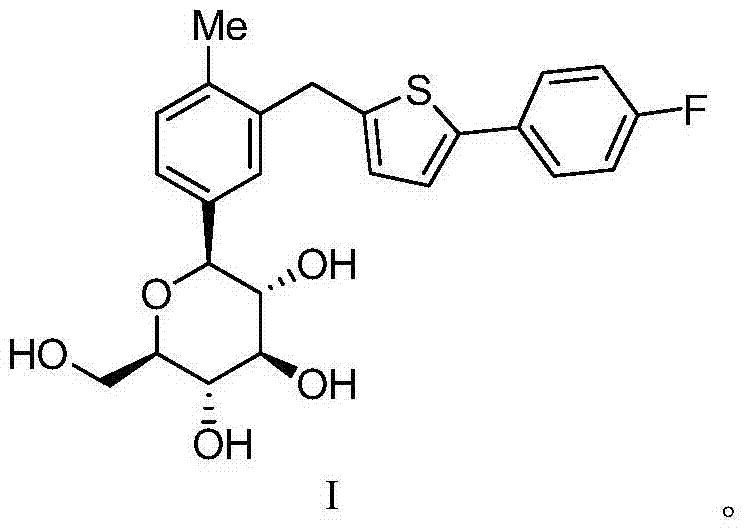
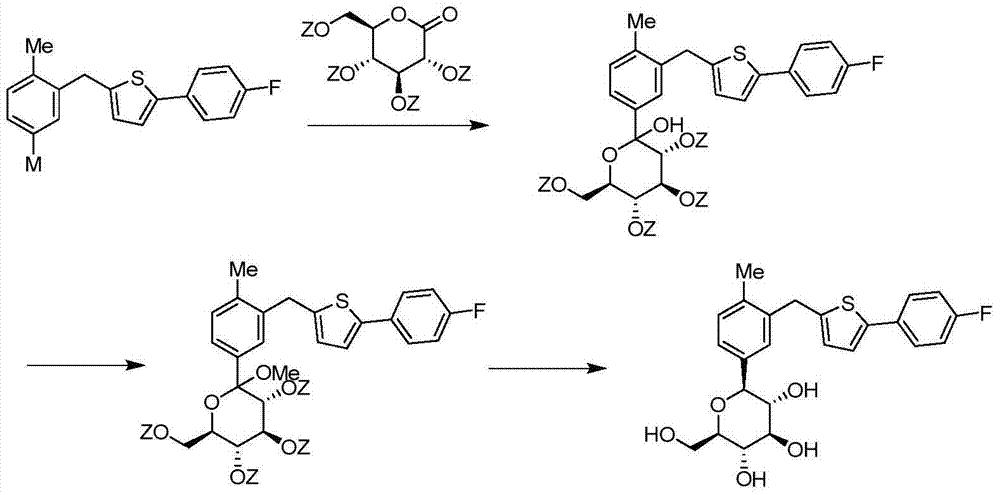
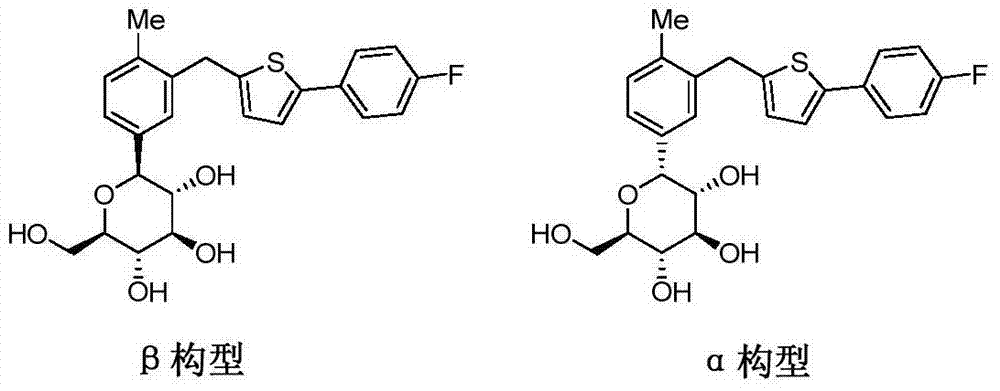
High-purity canagliflozin compound and preparation method thereof
The invention belongs to the field of drug synthesis and relates to a high-purity canagliflozin compound represented by a formula I shown in a drawing and a preparation method thereof. According to the canagliflozin compound provided by the invention, the content of an alpha-configuration impurity represented by a formula II shown in a drawing is lower than 1% and is further lower than 0.5%. The preparation method comprises the steps of preparing a eutectic substance from canagliflozin and amino acid in a solvent, separating the eutectic substance, and then, decomposing the eutectic substance, thereby obtaining the canagliflozin compound.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
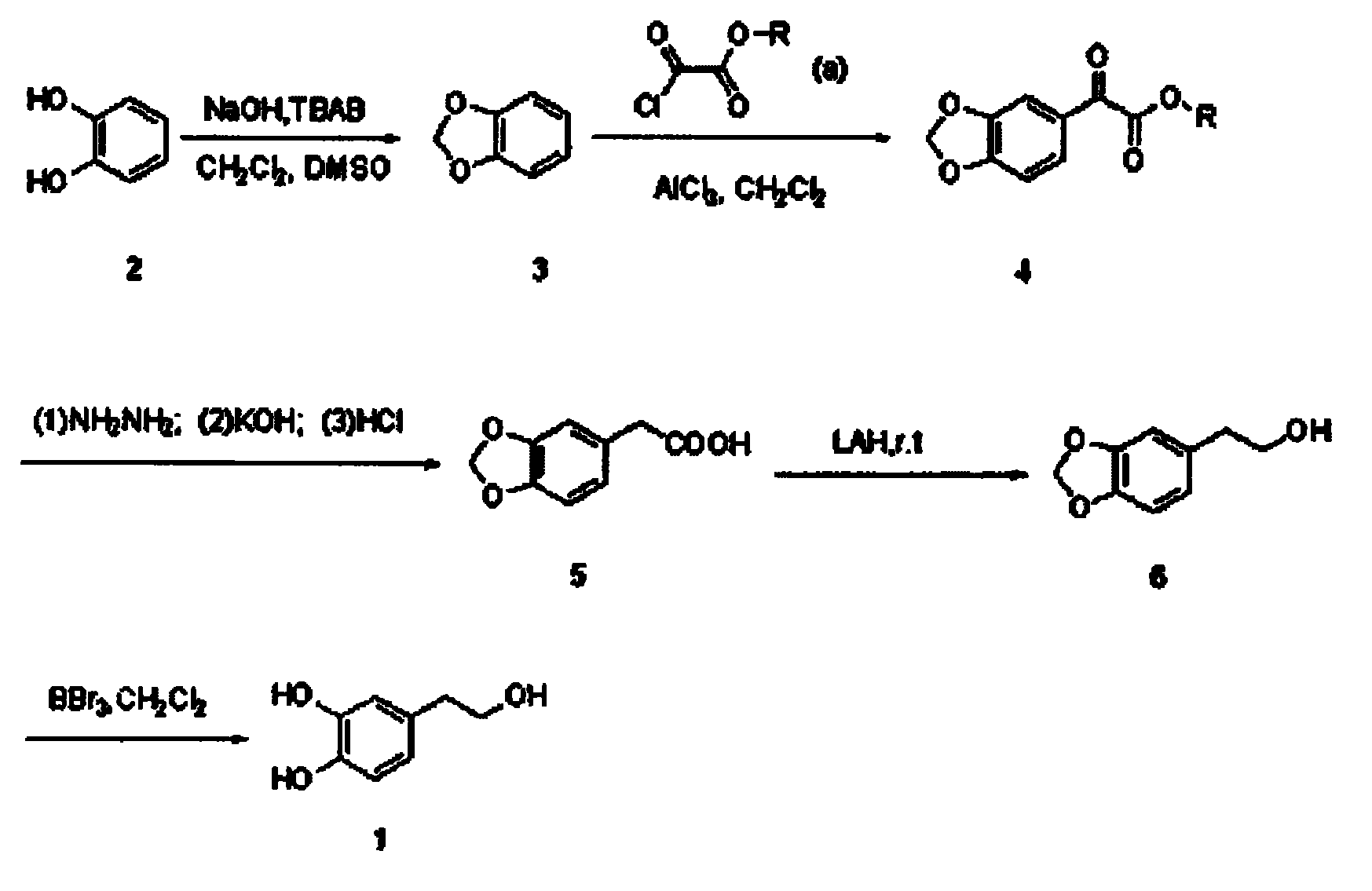
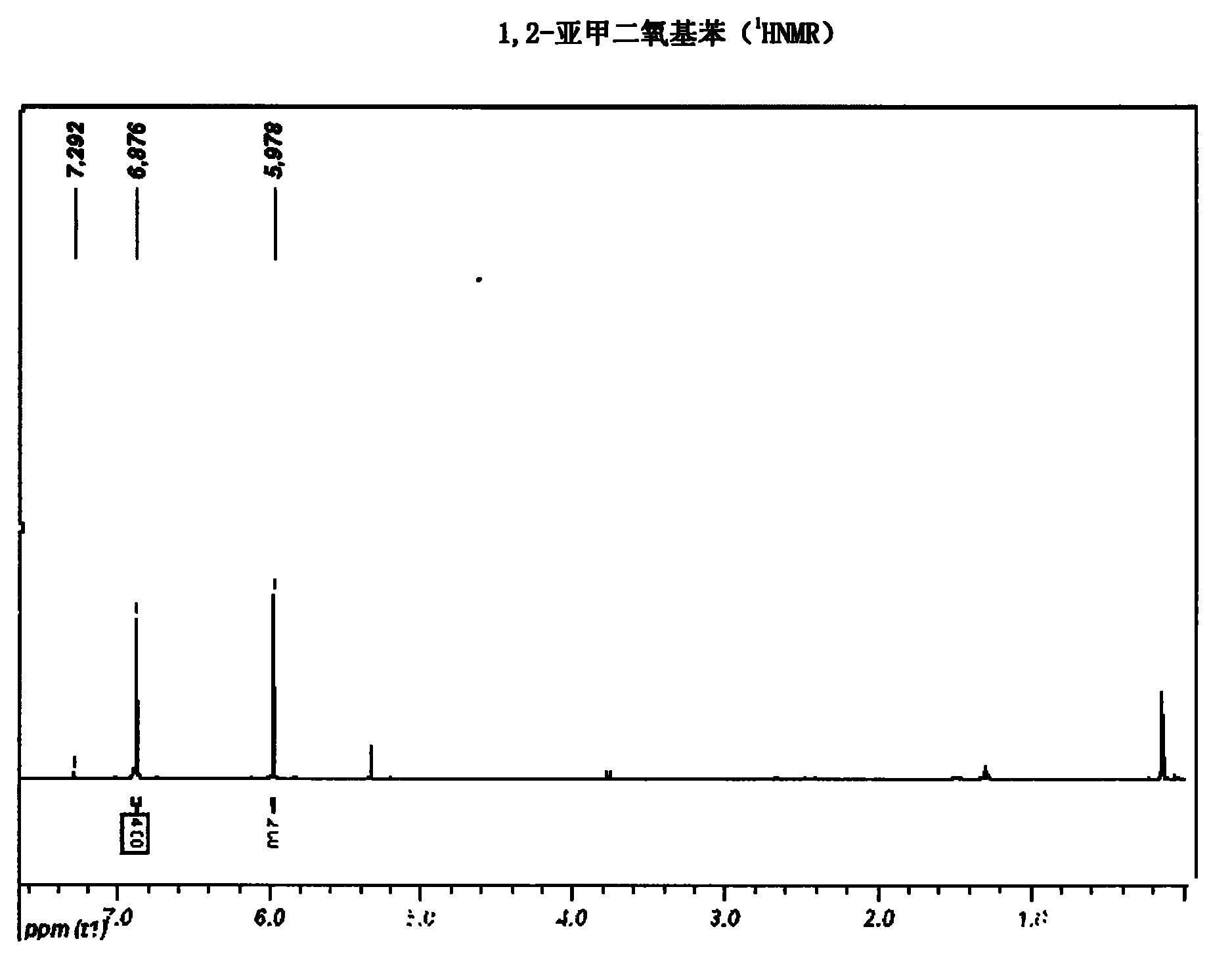
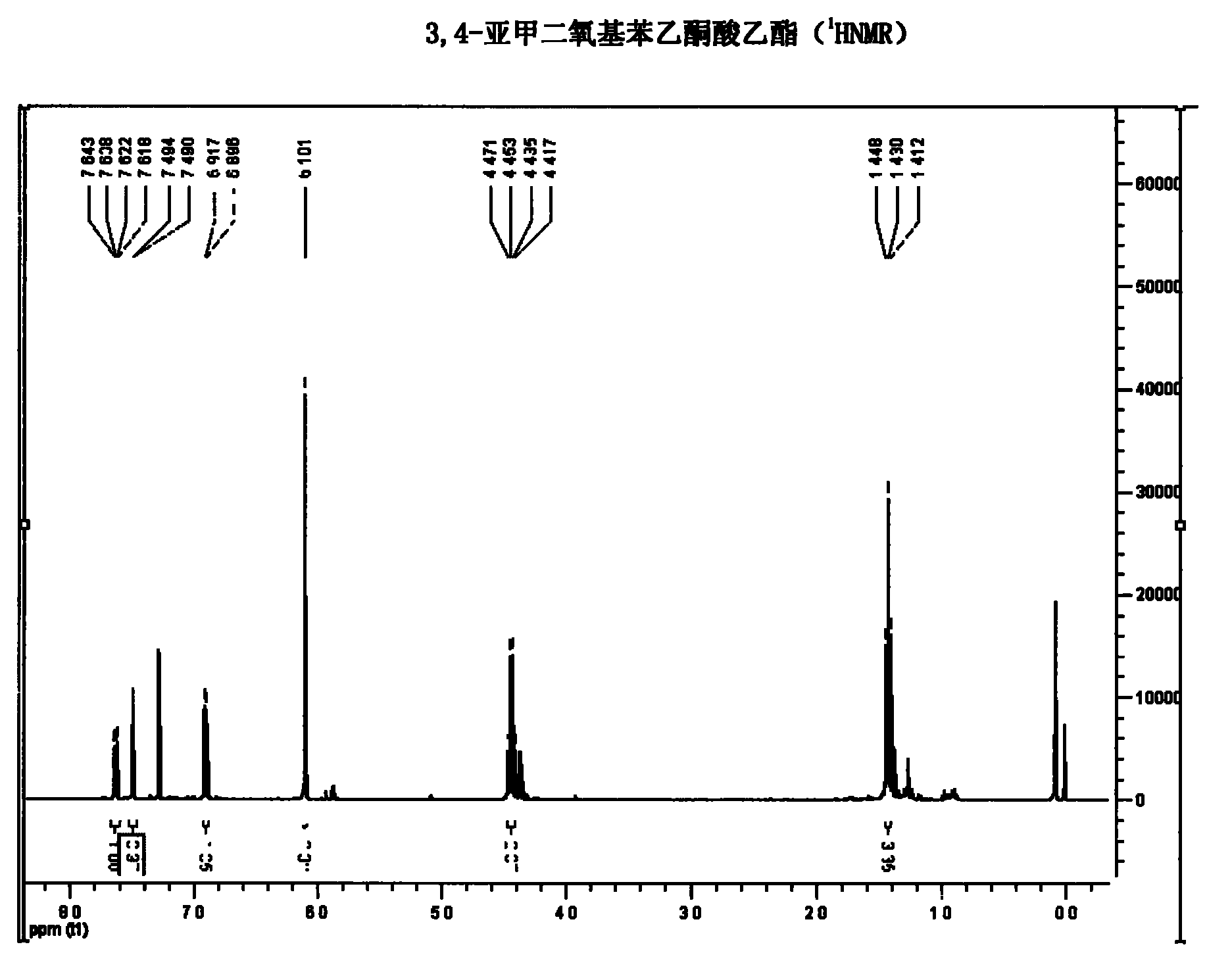
Synthetic method of hydroxytyrosol
InactiveCN103664536AMild reaction conditionsLower reaction costOrganic chemistryOrganic compound preparationChemical synthesisHydroxytyrosol
The invention belongs to the technical field of medicament synthesis, and in particular relates to a chemical synthetic method of hydroxytyrosol. The chemical synthetic method comprises the steps of (1) protecting two phenolic hydroxyl groups of catechol by using dichloromethane, and enabling catechol to react with dichloromethane to prepare 1,2-methylenedioxybenzene; (2) enabling 1,2-methylenedioxybenzene to react with various monoesters of oxalyl chloride to prepare 3,4-methylenedioxy phenylglyoxylic acid ester; (3) preparing 3,4-methylenedioxy phenylacetic acid by using 3,4-methylenedioxy phenylglyoxylic acid ester through a Wollff-kishner-Huang Minglong reduction reaction; and (4) reducing the 3,4-methylenedioxy phenylacetic acid by using lithium aluminum hydride, lithium borohydride or sodium borohydride to prepare 3,4-methylenedioxy phenethyl alcohol, and then removing methylene protection of the 3,4-methylenedioxy phenethyl alcohol by using boron tribromide or palladium on activated carbon to prepare hydroxytyrosol. A reactive reagent used in the synthetic method disclosed by the invention is easy to obtain and low in price, the reaction condition is mild, and the final yield of the whole reaction is 23%.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
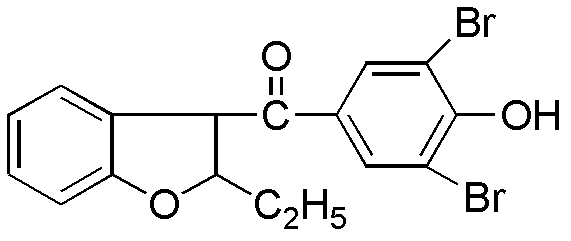
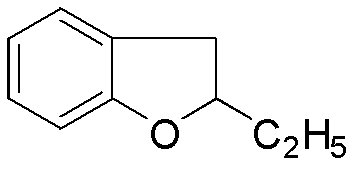
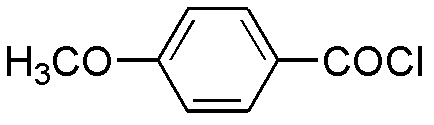
Preparation method of benzbromarone
ActiveCN102659727ASimple post-processingDoes not affect the quality of the finished productOrganic chemistryAnisoyl chlorideHalohydrocarbon
The invention relates to a preparation method of benzbromarone applied to the field of pharmaceutical synthesis, which comprises the following steps: taking 2-ethylbenzofuran and p-anisoyl chloride as starting raw materials, carrying out friedel-crafts acylation under the participation action of a catalyst and prepare 2-ethyl-3-p-methoxyphenyl formyl-benzofuran; carrying out demethylation reaction on the obtained 2-ethyl-3-p-methoxyphenyl formyl-benzofuran and pyridine hydrochloride, removing moisture in a reaction system by using a method that water is contained in toluene and preparing 2-ethyl-3-p-hydroxybenzene formyl-benzofuran; carrying out bromination reaction on the prepared 2-ethyl-3-p-hydroxybenzene formyl-benzofuran and bromide to prepare benzbromarone; and carrying out acidolysis with hydrochloric acid after the 2-ethylbenzofuran is fully reacted with the p-anisoyl chloride and extracting to obtain the 2-ethyl-3-p-methoxyphenyl formyl-benzofuran. The preparation method has the advantages that in the friedel-crafts acylation, methylene dichloride, trichloromethane and other halohydrocarbon are used for replacing carbon disulfide, and the post-processing process is simplified; and in the bromination reaction, bromine which is strong in corrosivity, generates great harm to human bodies and pollutes the environment is changed into the bromide.
Owner:NORTHEAST PHARMA GRP
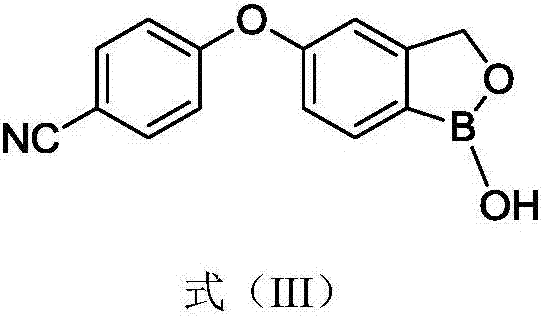
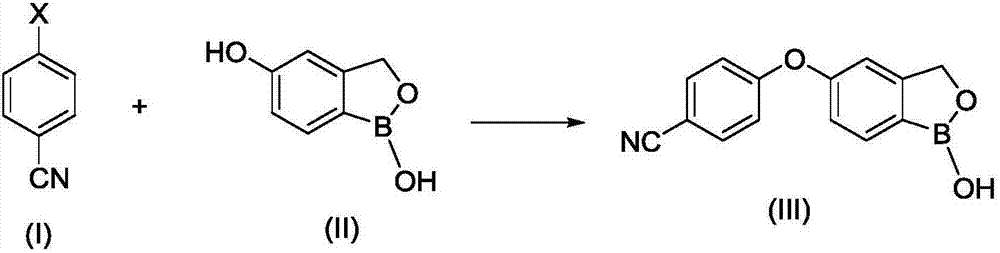
Synthesis method of crisaborole
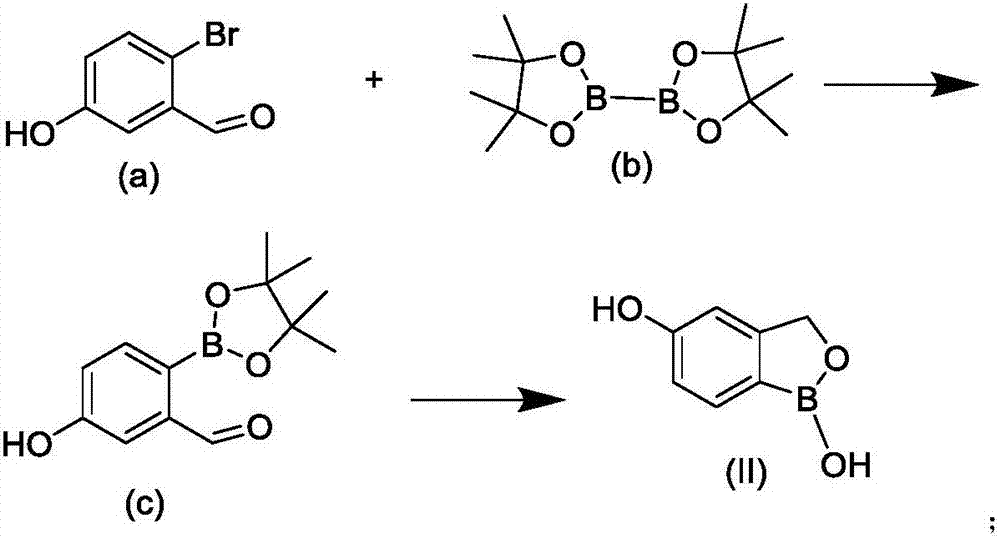
InactiveCN106928264AHigh yieldHigh purityGroup 3/13 element organic compoundsOrganic solventSynthesis methods
The invention provides a synthesis method of crisaborole, belongs to the technical field of drug synthesis, and relates to a synthesis method of a novel boron-containing anti-inflammatory preparation, namely crisaborole. 4-halobenzonitrile and benzo[c][1,2] oxaborolane-1,5(3H)-diol are reacted in an organic solvent in the presence of alkali, and the crisaborole is obtained. The synthesis methoid is simple, and the crisaborole is high in yield and high in purity. (The reaction formula is shown in the description).
Owner:湖南中智优库科技有限公司
Synthetic process of vildagliptin
InactiveCN104326961AReduce pollutionSimple preparation processOrganic chemistryBulk chemical productionPyrrolidineVildagliptin
The invention belongs to the field of drug synthesis and discloses a novel method for preparing vildagliptin. The process comprises the following steps: dehydrating N-fluorenylmethoxy carbony-L-prolinamide (raw material) to form nitrile; removing the Fmoc- protective group and carrying out N-chloracetylation to obtain an intermediate (S)-1-(chloracetyl)-2-cyan pyrrolidine; further carrying out condensation on the intermediate and 3-amino-1-adamantanol to obtain a coarse vildagliptin product; and recrystallizing to obtain a refined vildagliptin product. The process flow used for synthesizing vildagliptin is simple in method and low in impurity content, thereby facilitating industrial production of vildagliptin.
Owner:HAINAN ZHONGHE PHARM CO LTD
Preparing method for brexpiprazole
The invention belongs to the technical field of chemical drug synthesis and particularly relates to a preparing method for brexpiprazole. According to the preparing method, crystalline brexpiprazole is prepared through five steps. The preparing method has the advantages that the synthetic yield is high, operation is simple, purification is easy, and the end product is stable. In addition, a metal catalyst is effectively avoided, and the number of reaction steps is greatly reduced, so that the reaction difficulty is lowered, post-treatment is simplified, the yield is increased, the reaction cost is greatly reduced, and thus industrialized mass production is facilitated.
Owner:NANJING CORE TECH CO LTD
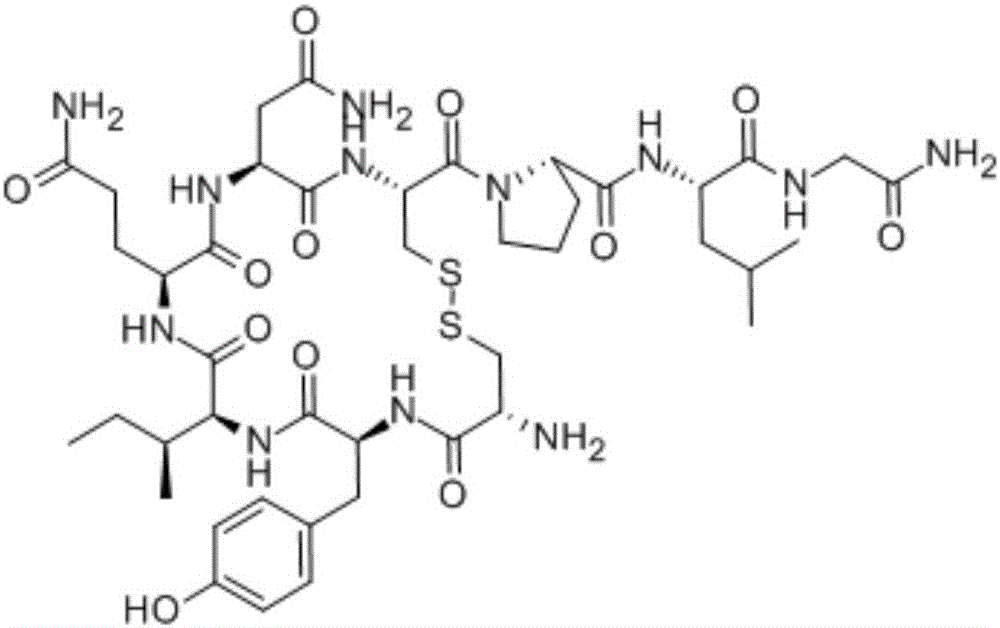
Synthesis method of Bremelanotide
ActiveCN106589111AEasy to removeRaw materials are cheap and easy to getPeptide preparation methodsDepsipeptidesSynthesis methodsBremelanotide
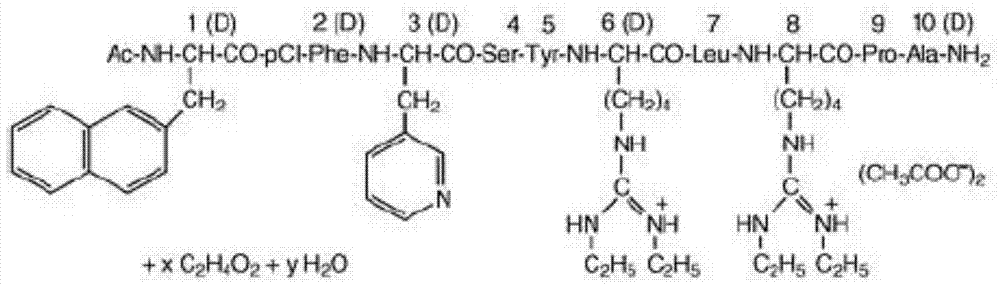
The invention discloses a preparation method of polypeptide, particularly relates to a synthesis method of Bremelanotide and belongs to the field of drug synthesis. The synthesis method comprises the following steps: two fragments of Bremelanotide are synthesized firstly and comprise a fragment with two amino acids and a fragment with five amino acids, p-hydroxymethylbenzoic acid is used for protecting the right side of a Bremelanotide sequence, namely, a carboxyl functional group of lysine, and the final product Bremelanotide is obtained with a counter-clockwise ring-forming synthetic method. The synthesis method has the advantages of being short in synthesis route, high in yield and low in synthesis difficulty and cost, and adopting cheap and available raw materials, and is suitable for industrial production.
Owner:江苏中泰药业有限公司
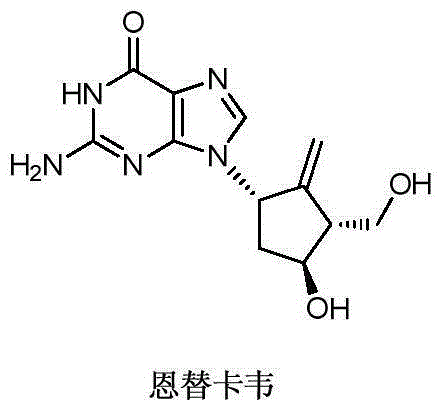
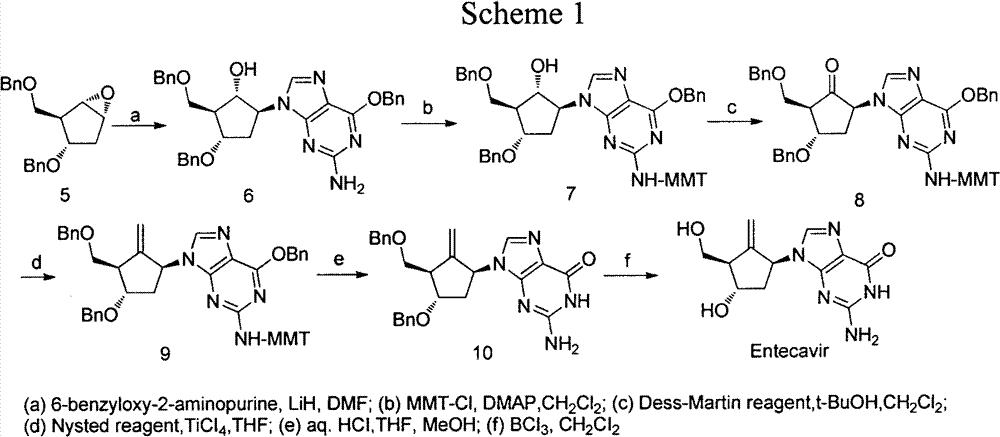
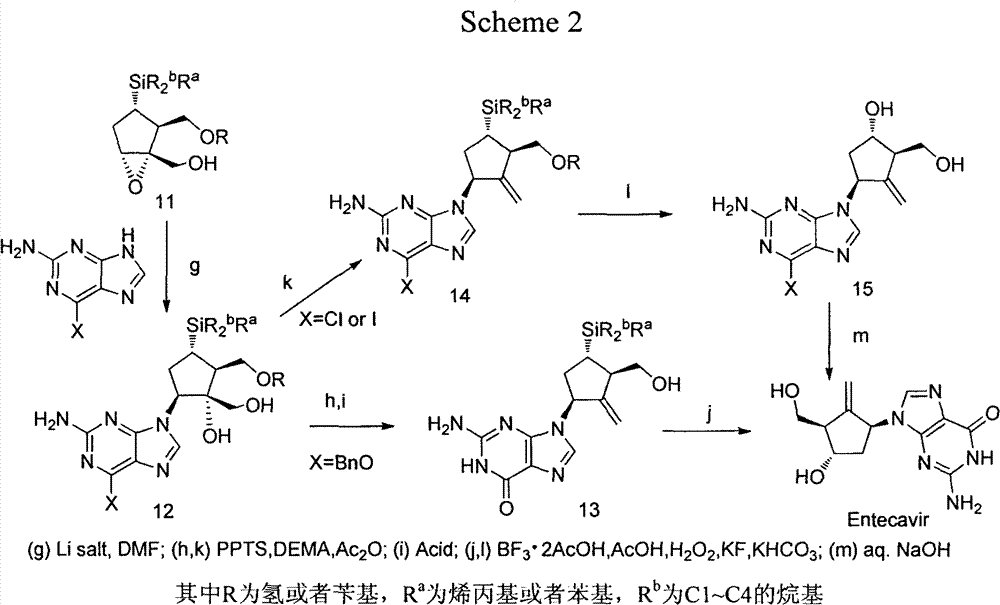
Novel synthetic method for entecavir compound
ActiveCN105037363ASolve rare problemsDosage controllableGroup 4/14 element organic compoundsKetoneAdipate
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
A kind of preparation method of amoxicillin sodium
The present invention relates to the field of drug synthesis, in particular to a method for preparing amoxicillin sodium. The method of the present invention comprises the following steps: (1) preparation of acid anhydride; (2) preparation of 6-APA solution; (3) condensation: Move the 6-APA salt solution into the mixed acid anhydride reaction bottle, and time it for 3 hours; (4) Hydrolysis: add the condensation solution of step (3) to the hydrolyzed solution; (5) Crystallization: Leave the crystal to grow for 1 hour and then stir to grow the crystal for 1 hour (6) Suction drying: wash with water first, then wash with acetone, and dry in vacuum at 40° C. for 3 hours to obtain amoxicillin.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
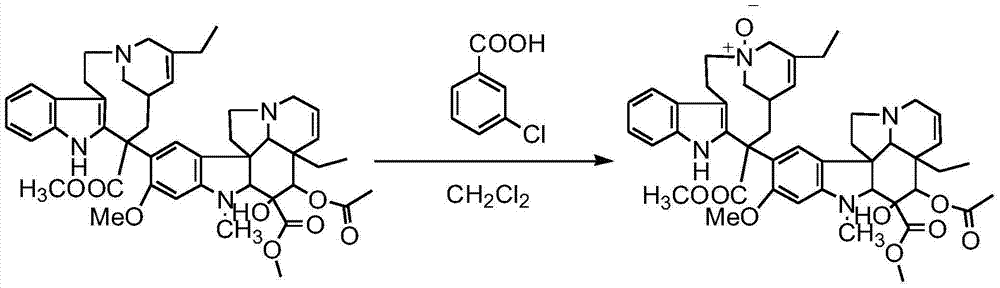
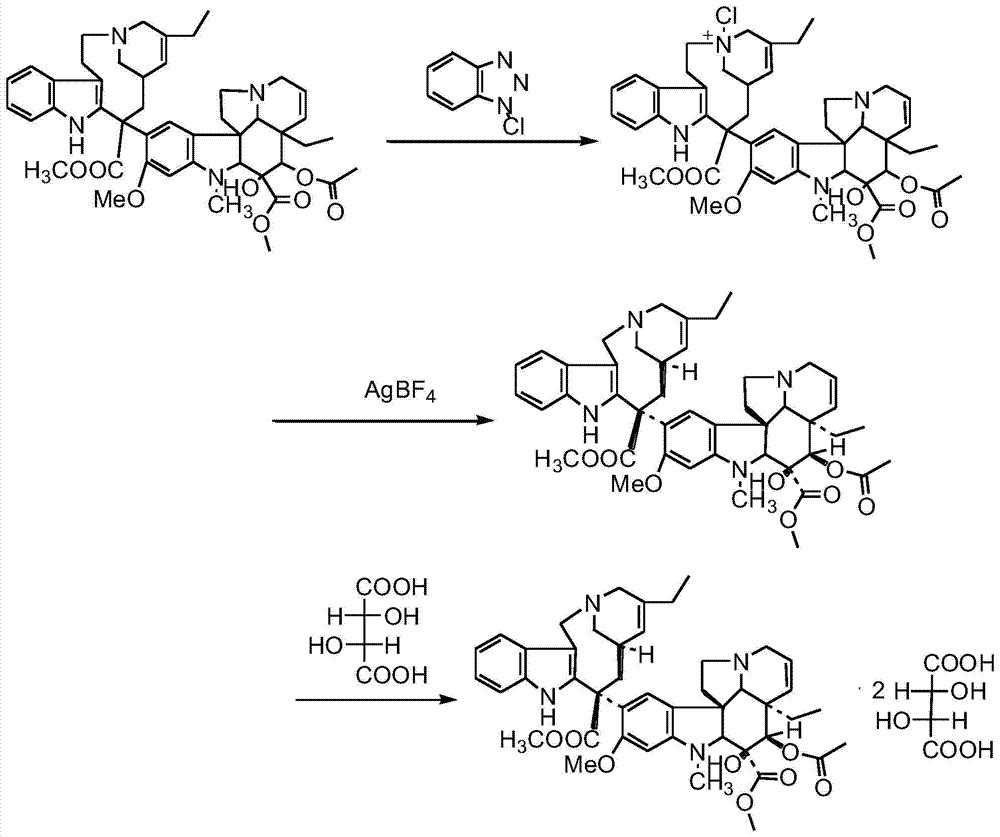
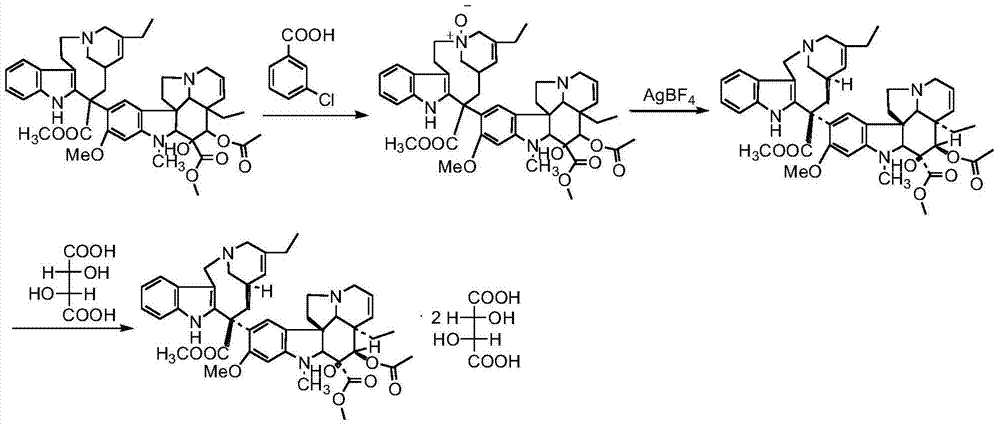
Preparation method of vinorelbine
The invention belongs to the field of pharmaceutical synthesis, and provides a novel method used for synthesizing vinorelbine tartrate. According to the novel method, catharanthine tartrate and vindoline are taken as initial raw materials, vinorelbine crude products are obtained via a two-step one-pot procedure reaction instead of an original four-step reaction, synthesis steps are simplified, and yield is increased. The vinorelbine crude products are subjected to normal phase silica gel column purification and reverse phase silica gel column purification, so that purity of obtained vinorelbine purified products is increased substantially; and at the same time, increasing of vinorelbine purity possesses significant influences on salifying effects of vinorelbine with tartaric acid, so that after recrystallization, purity of the obtained vinorelbine tartrate is higher than 99.5%, and accords with requirements of United States Pharmacopeia; competitive advantage is more excellent; all of the operation processes are normal operation processes; and it is more beneficial for industrialized production.
Owner:NAT INST OF PHARMA R & D CO LTD
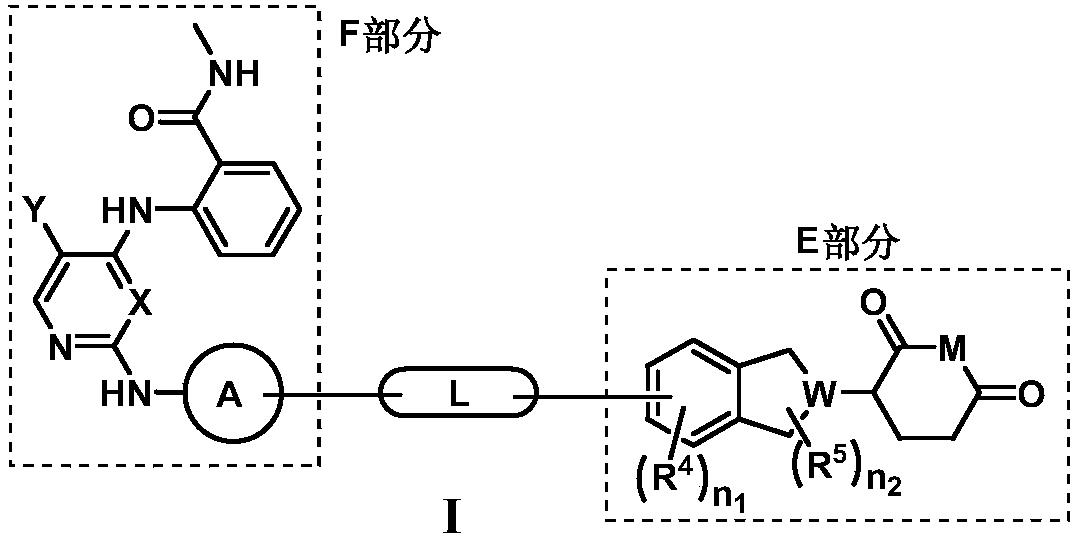
Compounds for targeted degradation of focal adhesion kinase and application of the compounds in medicine
InactiveCN111285851APromote degradationOrganic active ingredientsOrganic chemistryDiseaseFocal adhesion
The invention relates to the field of biomedicine and drug synthesis, in particular to compounds for targeted degradation of focal adhesion kinase (FAK) protein, pharmaceutically acceptable salts, hydrates, solvates or prodrugs of the compounds, preparation methods of the compounds and the pharmaceutically acceptable salts, hydrates, solvates or prodrugs, and application of the compounds as therapeutic agents, especially as FAK degradation agents. The structures of the compounds, and the geometric isomers or pharmaceutically acceptable salts, hydrates, solvates or prodrugs thereof are shown inthe specification. The compounds provided by the invention have a good degradation effect on FAK kinase, and can be used for preventing, treating or adjunctively treating various diseases related tothe expression or activity of FAK kinase.
Owner:SHENYANG PHARMA UNIVERSITY
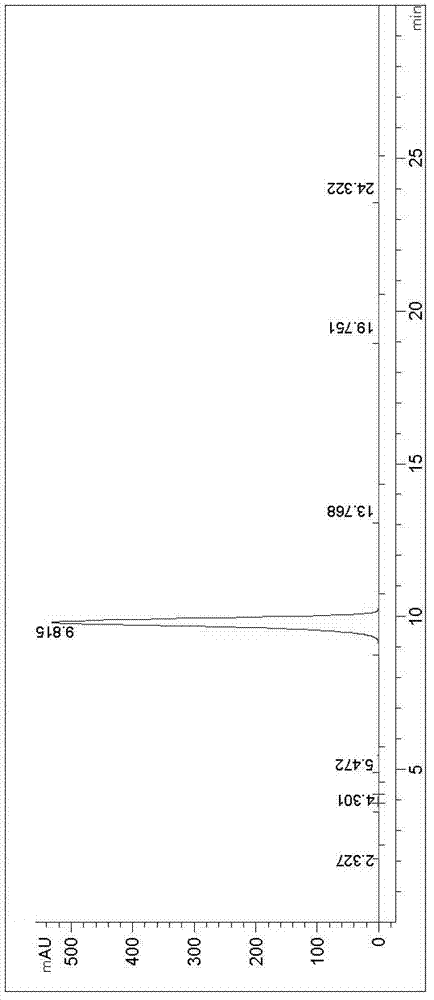
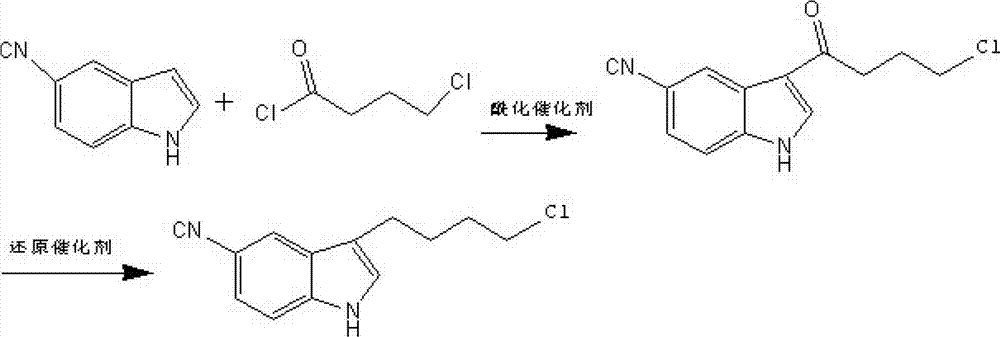
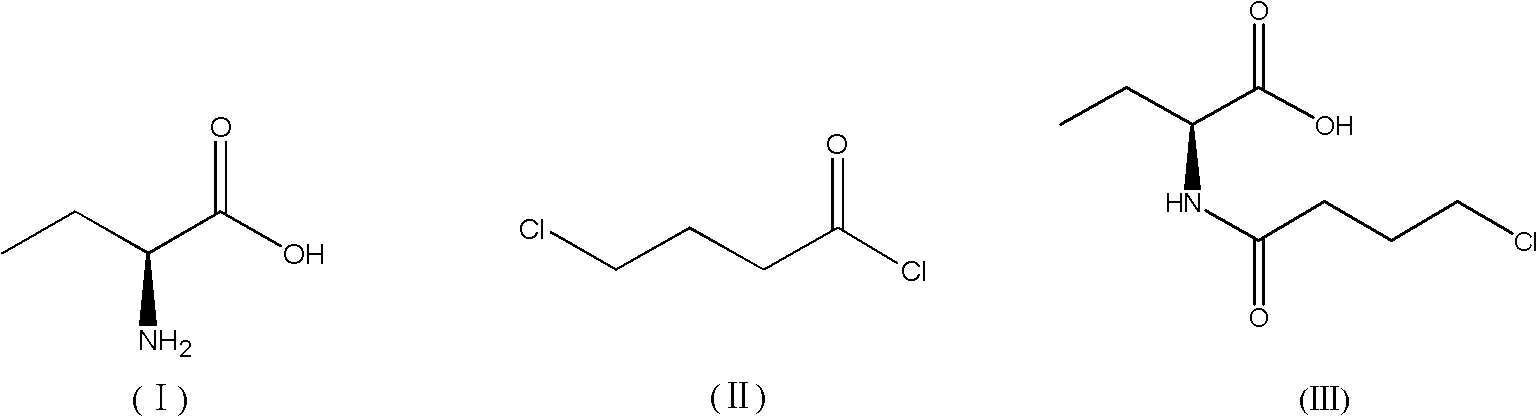
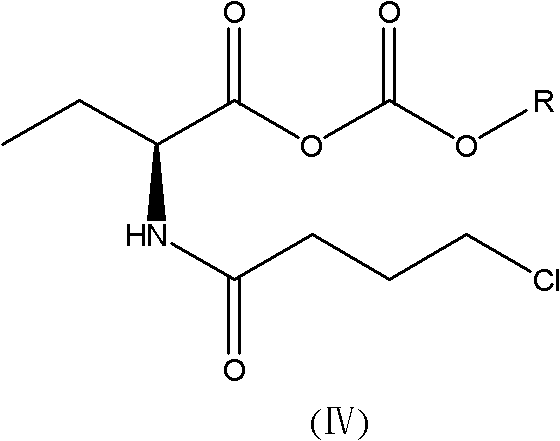
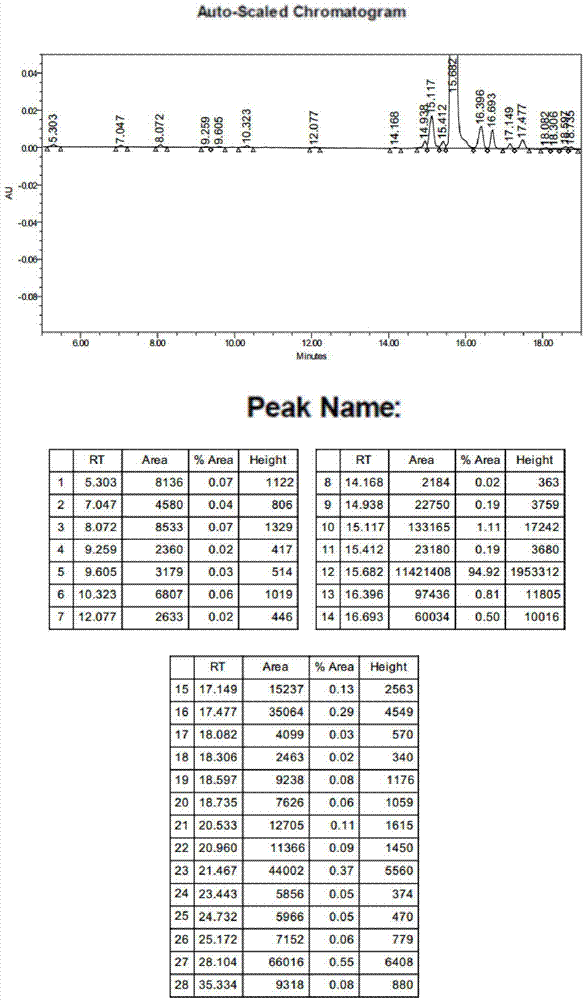
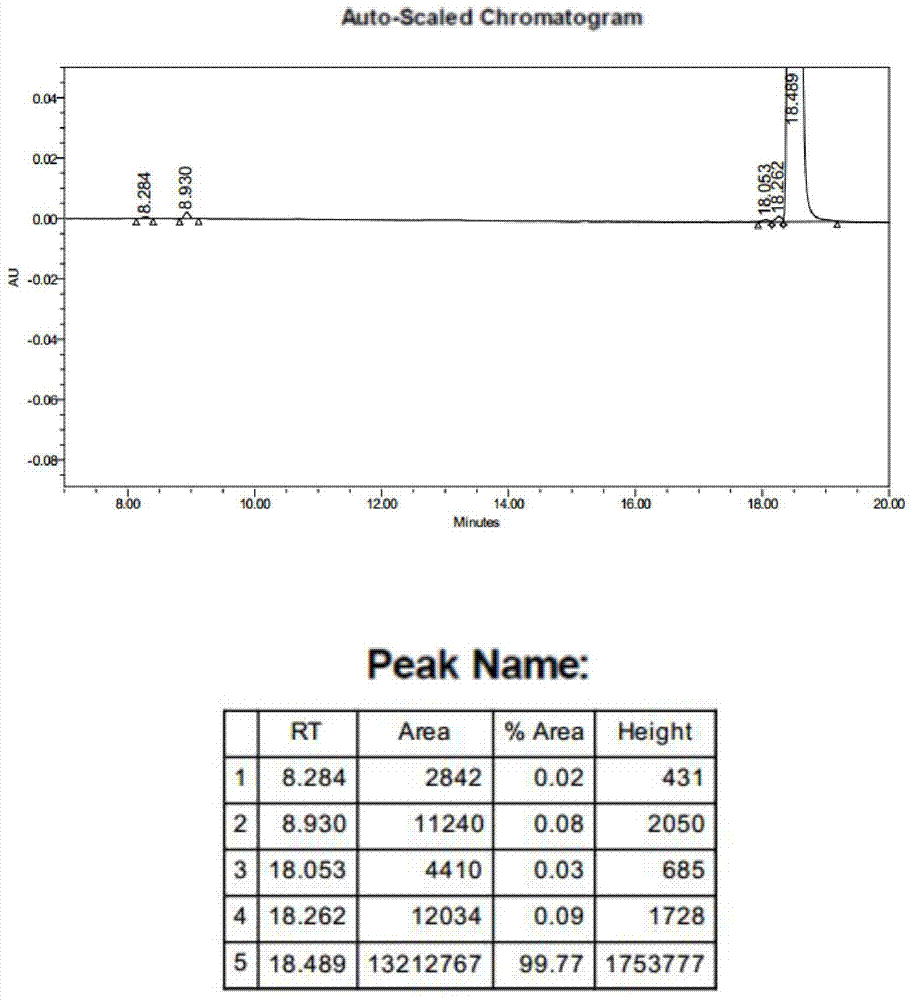
New synthesis method of 3-(4-chlorobutyl)-5-cyanoindole
The invention provides a new synthesis method of a vilazodone hydrochloride intermediate 3-(4-chlorobutyl)-5-cyanoindole, belonging to the technical field of medicine synthesis. The invention solves the following problems in the prior art: the synthesis yield of the 3-(4-chlorobutyl)-5-cyanoindole is low, and dangerous sodium borohydride and anhydrous aluminum trichloride are used as reduction catalysts. The synthesis method comprises the following steps: (1) dissolution of alcohol: in a nitrogen or inert gas protective atmosphere, dissolving 1,1-dimethoxy-6-chlorohexane in an alcohol-water mixed solution, and heating to dissolve the 1,1-dimethoxy-6-chlorohexane; and (2) Fischer indole cyclization reaction: slowly and dropwisely adding 4-cyanophenylhydrazine hydrochloride and an alcohol-pure water mixed solvent into the reaction solution in the step (1) to carry out Fischer indole cyclization reaction, carrying out vacuum filtration, and recrystallizing to obtain the 3-(4-chlorobutyl)-5-cyanoindole. The method has the advantage of mild reaction and is simple to operate; and the final product has the advantages of high yield and good quality.
Owner:SUZHOU UUGENE BIOPHARMA
Synthesis method for medicine entecavir for treating hepatitis B
The invention belongs to the field of medicine synthesis, and provides a method for synthesising entecavir by taking (1R,2S,3S,5R)-3-(1-phenyl cyclo-silicon)-6-oxa-bicyclo[3.1.0] hexane-1,2-dimethyl carbinol as a raw material. The synthesis method comprises the following steps of: combining the raw material with purine compounds at first, then building a chiral five-membered exocyclic double bond via deoxidization reaction, and finally removing a protecting group to obtain entecavir. Via the cyclosilane structure of the raw material, the rigidity of molecules is enhanced, so that the product obtained via the method is easier to crystallize and purify; and the tar generated during the reaction is less, and the yield is high. Therefore, the synthesis method is good in industrialization prospect.
Owner:NANJING UNIV OF TECH
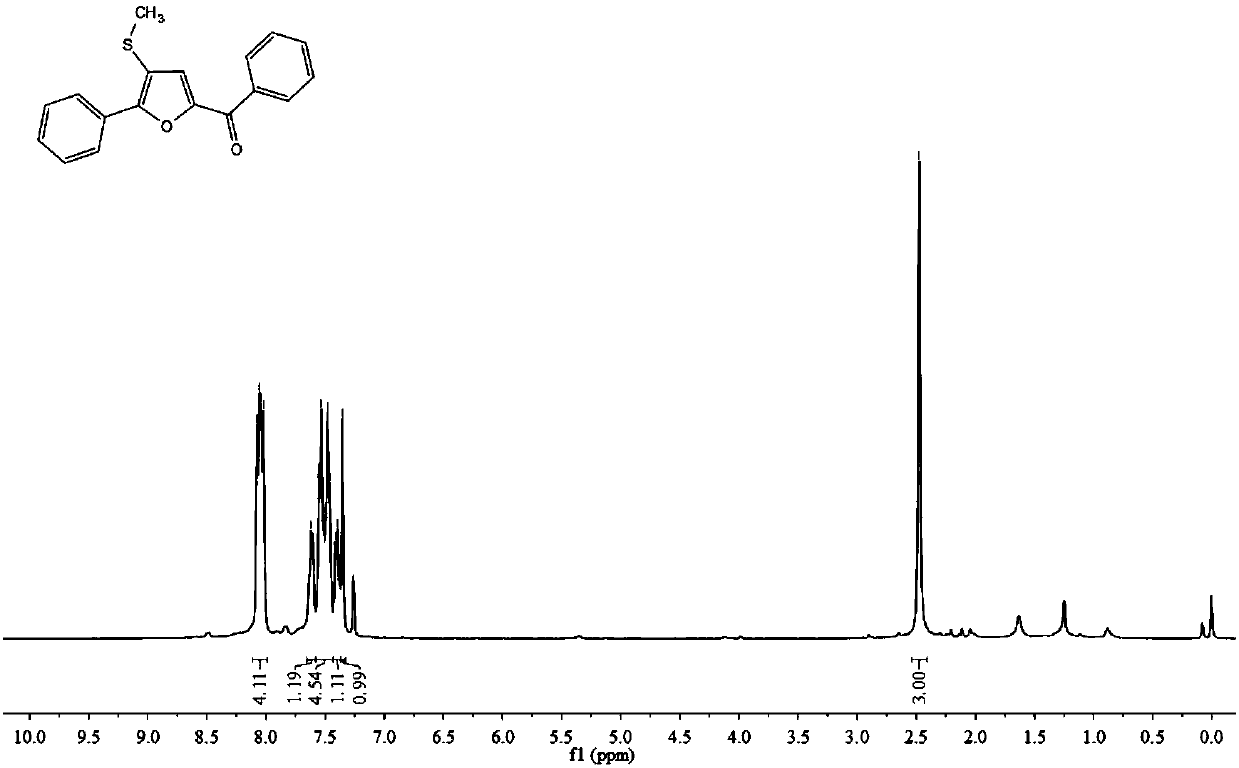
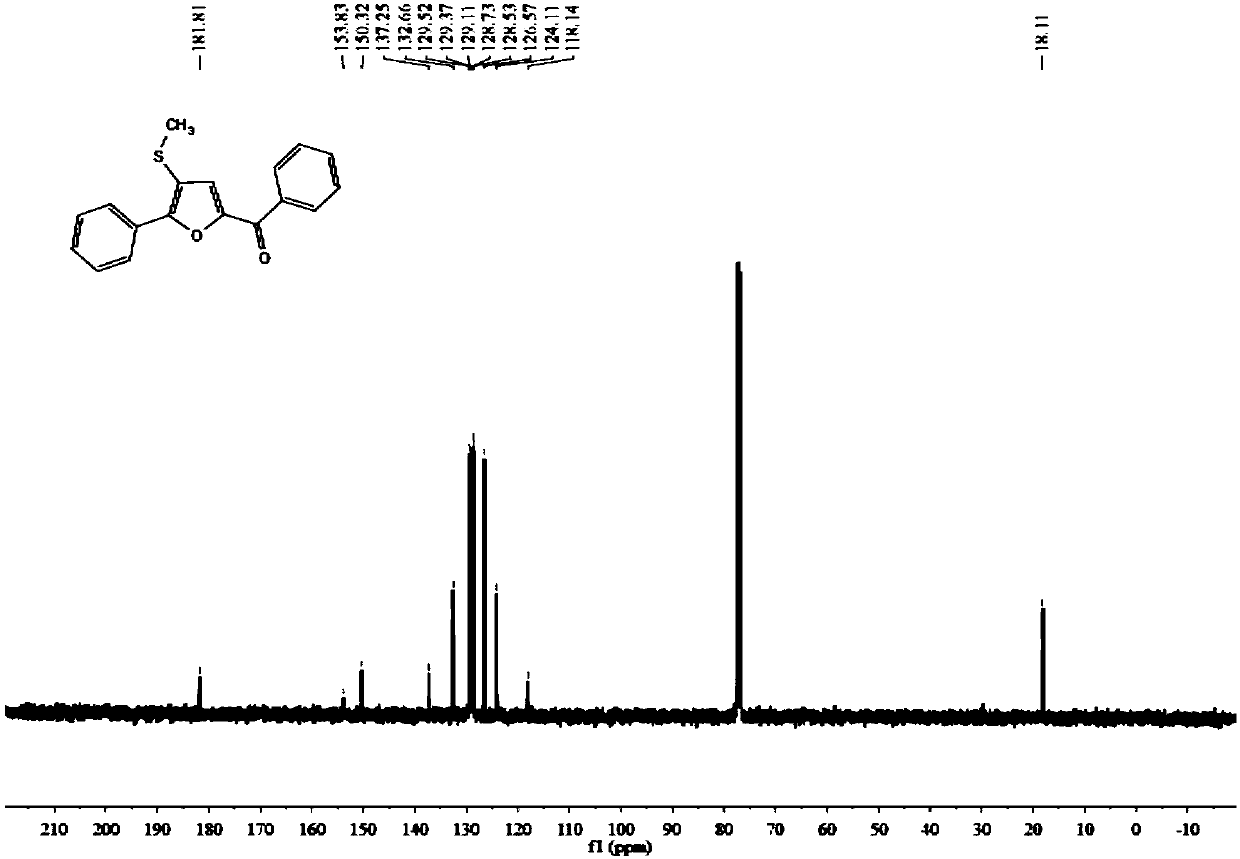
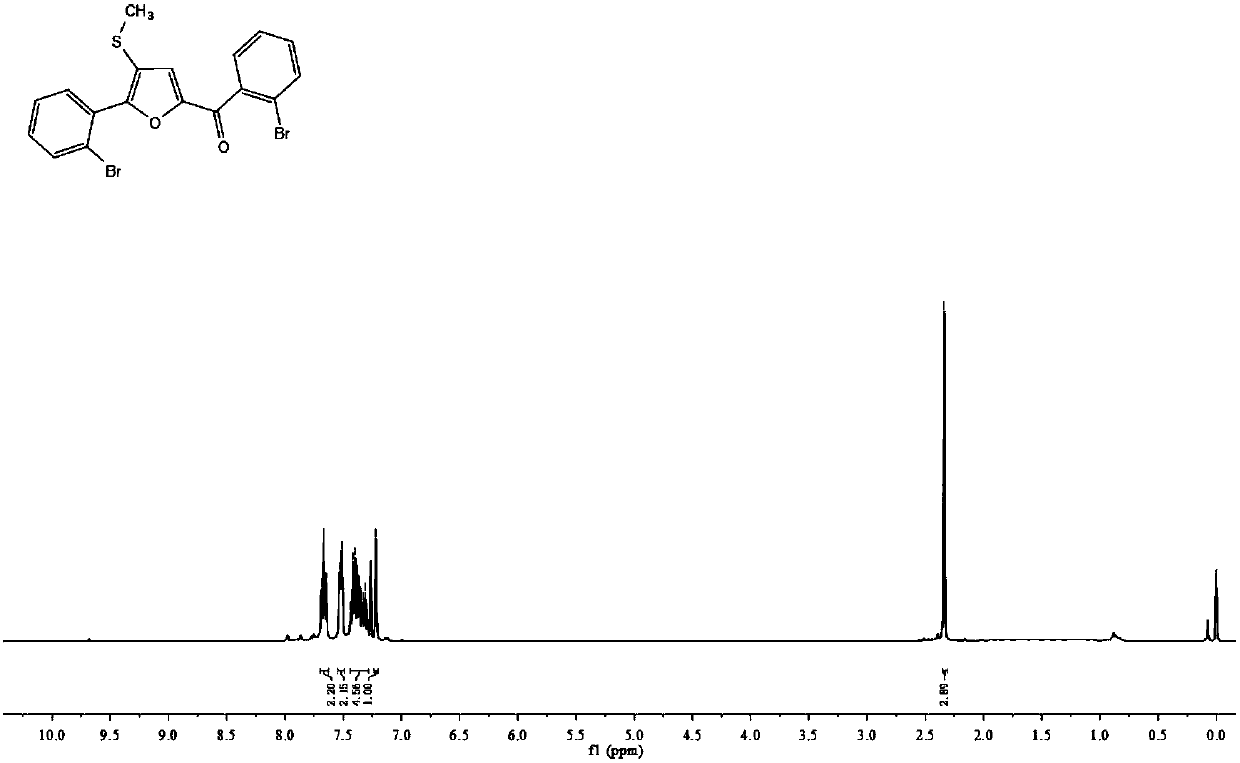
Synthetic method of furan derivatives containing methylthio group
The invention discloses a synthetic method of furan derivatives containing methylthio group. The synthetic method comprises: subjecting acetophenone compounds and dimethyl sulfoxide to one-pot reaction in the presence of an elemental iodine catalyst and potassium persulfate oxidant to obtain furan derivatives containing methylthio group. The synthetic method has the advantages that richer types offuran derivatives are provided, more intermediates are provided for drug synthesis, sources of materials are wide, the steps are simple, reaction conditions are mild, the yield is high, and industrial production is facilitated.
Owner:YUANJIANG HUALONG CATALYST TECH
Synthesis method of levetiracetam
ActiveCN102558012AAvoid prescriptive requirementsMeet production requirementsOrganic chemistrySynthesis methodsPollution
The invention relates to a synthesis method of levetiracetam and belongs to the technical field of medicine synthesis. The invention provides a synthesis method of levetiracetam for the purpose of solving the technical problems that in the prior art, a process in which thionyl chloride is used as a raw material or benzene is used for splitting is large in environment pollution, complicated in splitting and is disadvantageous to production. The method comprises the following steps of: carrying out alkylation reaction on (S)-2-reanal which is used as the raw material and 4-chlorobutyryl chloride; carrying out acylation reaction on a product obtained from the former step and an acylation agent; and then carrying out cyclization reaction through ammonolysis in the presence of a phase transfer catalyst to obtain the levetiracetam. The invention provides a bran-new synthesis method of levetiracetam. In the method, a splitting process is omitted so as to avoid the problem existing when benzene is used as a splitting agent; a thionyl chloride reagent is not used, so as to reduce human damage and environment pollution; and the yield and the quality of a product are high, the total molar yield of the product is more than 81%, the HPLC (high performance liquid chromatography) purity of the product is more than 98%, and the optical purity of the product is more than 99.0%.
Owner:江苏八巨药业有限公司
Ganirelix acetate preparation method
InactiveCN104844694AHigh purityLess impuritiesLuteinising hormone-releasing hormonePeptide preparation methodsOrganic solventPurification methods
The present invention belongs to the technical field of drug synthesis, and discloses a ganirelix acetate preparation method, wherein Fmoc-Lys (Boc)-OH and Fmoc-D-Lys (Boc)-OH are adopted to respectively replace Fmoc-HArg (Et)2-OH and Fmoc-D-HArg (Et)2-OH, a ganirelix precursor I is previously synthesized, and the side chain amino of Lys and D-Lys in the precursor I is modified and treated so as to obtain the ganirelix acetate. According to the present invention, water is adopted to replace the organic solvent and is adopted as the reaction solvent, such that the reaction can be performed within the established pH value range, and the product content in the crude peptide is significantly improved; and the product yield can be significantly improved through the HPLC two-step purification method.
Owner:HYBIO PHARMA
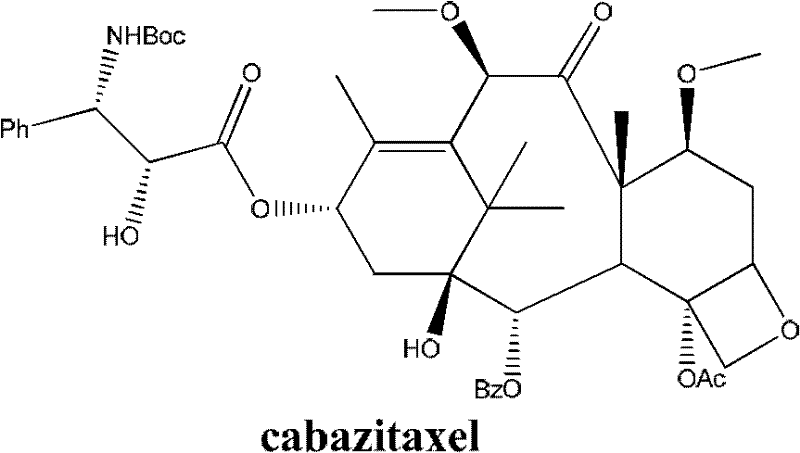
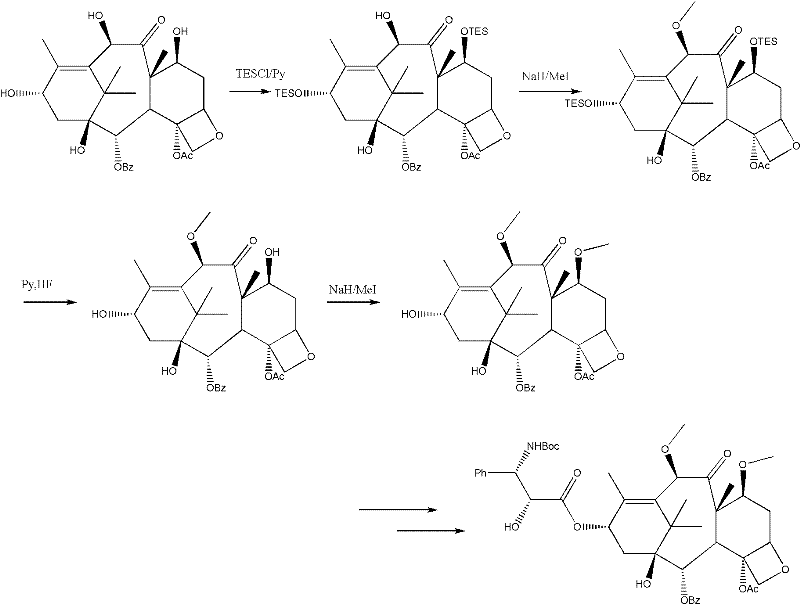
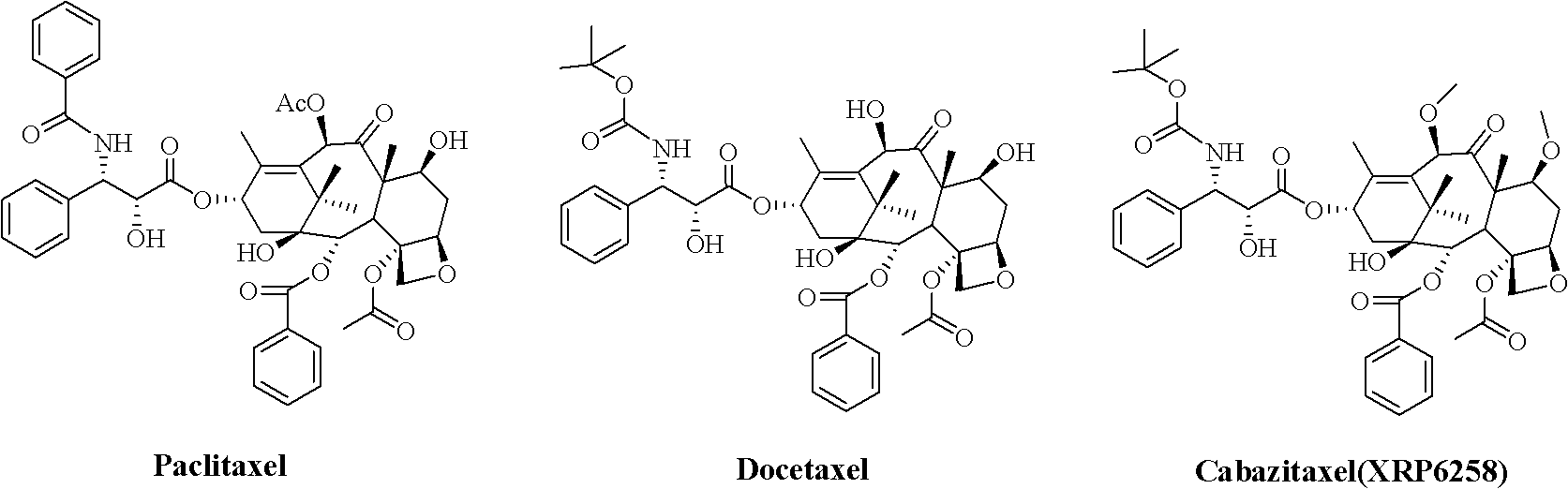
Method for preparing second-generation taxol anticancer drug Cabazitaxel
InactiveCN103012328AEasy to manufactureEasy to makeOrganic chemistryBulk chemical productionCabazitaxelChemical compound
The invention belongs to the field of medicament synthesis, and relates to a method for synthesizing a second-generation taxol anticancer drug Cabazitaxel. The method comprises obtaining a key intermediate (II): a 10-deacetyl baccatin III (I) with C-7 and C-10 hydroxy-methylthio-methylene and C-13 hydroxy-oxidization, completing a de-methylthio operation for C-13 carbonyl and C-7 and C-10 methylthio methylene (MTM) of the compound II by a one-pot method to obtain a nucleus (IV) of an XRP6258, connecting the nucleus with various side chains, and then removing side chain protecting groups to obtain the product Cabazitaxel (V). The method has advantages of high efficiency in the preparation process, simple process, high yield, relatively low cost and easy operation, and is suitable for large-scale production and preparation of anti-cancer drugs XRP6258.
Owner:FUDAN UNIV +1
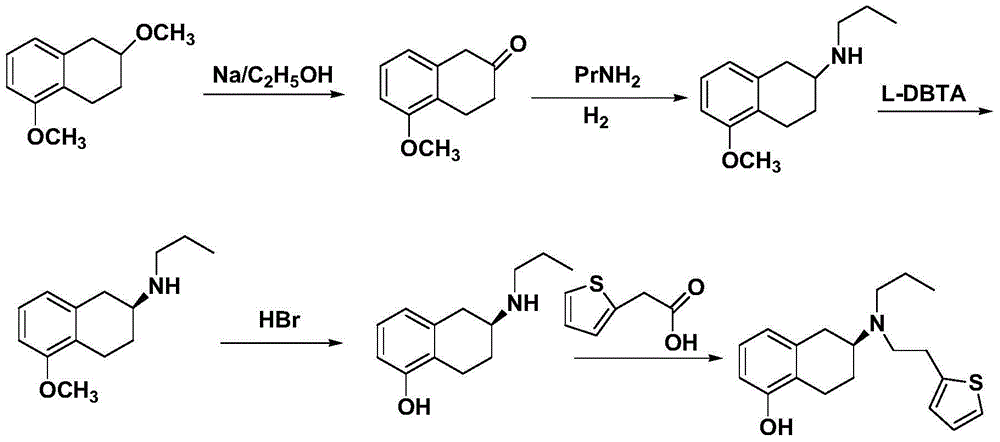
Preparation method of rotigotine
ActiveCN104130238AFew synthetic stepsHigh yieldOrganic compound preparationAmino-hyroxy compound preparationChemical reactionTetralin
The invention discloses a preparation method of rotigotine, and belongs to the technical field of medicine synthesis. According to the method, 5-methoxy-2-tetralone is used as a raw material for amination, asymmetric reduction, halogenation and methoxyl group removal four step reaction for synthesis of chiral rotigotine {(-)-(S)-2-(N-propyl-N-(2-(2-thiophene) ethyl] amino]-5-hydroxy-1, 2, 3, 4-tetralin}. According to the method, a simplex stereoscopic structural compound is synthesized by stereo selective chemical reaction, in the asymmetric reduction process, hantzsch ester 1, 4-dihydropyridine (HEH) is used as a reducing agent, and chiral phosphoric acid is used as a catalyst to synthesize an important intermediate (S)-2-(N-n-propyl) amido-5-methoxy tetralin (II) with a simplex stereoscopic structure, the use of a chiral reagent for splitting to obtain the simplex stereoscopic structural compound is avoided, the synthesis procedure is shortened, the yield is improveds, and the method is favorable for industrialized production.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
Preparation method for cephalosporin anti-infective drug
ActiveCN105017286ASimple preparation processReduce generationOrganic chemistry7-ACACefazedone sodium
The invention relates to a preparation method for a cephalosporin anti-infective drug-cefazedone sodium, belonging to the field of pharmaceutical synthesis. According to the invention, the method uses GCLE as a raw material to substitute 7-ACA and overcomes the defects of low yield, high pollution and the like in prior art; the preparation method with mild reaction conditions, little side reaction and simple process is provided; meanwhile, the method has the advantages of cheap and easily-available raw materials, low cost, high product yield, high product purity and applicability to industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
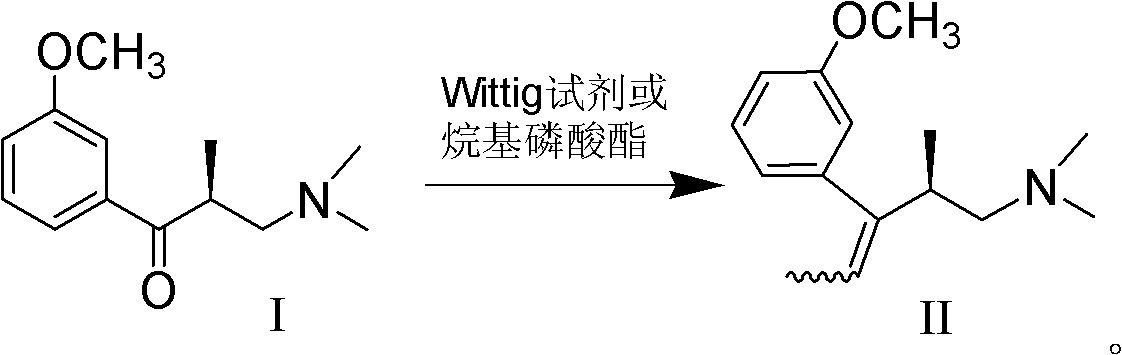
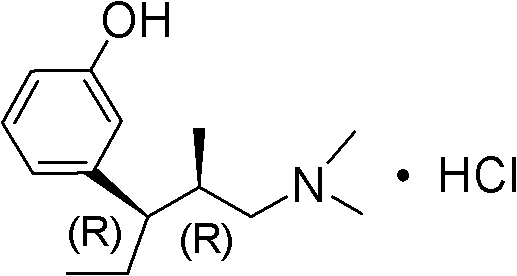
Preparation method of (R)-3-(3-methoxy phenyl)-N,N,2-trimethylpent-3-ene-1-amine
ActiveCN102320984AHigh purityReduce usageOrganic compound preparationAmino-hyroxy compound preparationWittig reactionSynthesis methods
The invention belongs to the technical field of drug synthesis, and discloses a preparation method of (R)-3-(3-methoxy phenyl)-N,N,2-trimethylpent-3-ene-1-amine. The preparation method comprises the following step of: carrying out wittig reaction or Horner-Emmons-Wittig reaction on an alkaline substance and a compound shown in formula I at a certain temperature in an inert solvent under alkaline conditions to obtain a compound shown in formula II; or further preparing the compound shown in the formula II into a salt. The preparation method has the advantages of short synthesis route, mild reaction conditions in each step and no need for special equipment, is simple to operate, and is suitable for industrial production; the product is easy to separate and purify; and by using the synthesis method, a high-purity finished product can be obtained, and strong-toxicity reagents and solvents are not used.
Owner:NHWA PHARMA CORPORATION
Purification method of oxytocin
InactiveCN106831951AHigh purityHigh recovery rateOxytocins/vasopressinsPeptide preparation methodsChemical synthesisOrganic solvent
The invention discloses a purification method of oxytocin, relating to the technical field of pharmaceutical synthesis and purification. The method comprises the following steps: by using an oxytocin water solution prepared by a chemical synthesis process as a raw material, concentrating the oxytocin water solution, adding an organic solvent for dissolution, cooling to crystallize, filtering and drying, thereby achieving the goal of purifying and refining the oxytocin. The method is simple to operate, lowers the production cost, and enhances the production efficiency of the enterprise. The prepared oxytocin has high purity. The method uses the single organic solvent, so the organic solvent has low toxicity, can be easily recovered and reutilized, and has small influence on the environment. Thus, the method is suitable for industrialized application.
Owner:BENGBU BBCA MEDICINE SCI DEV
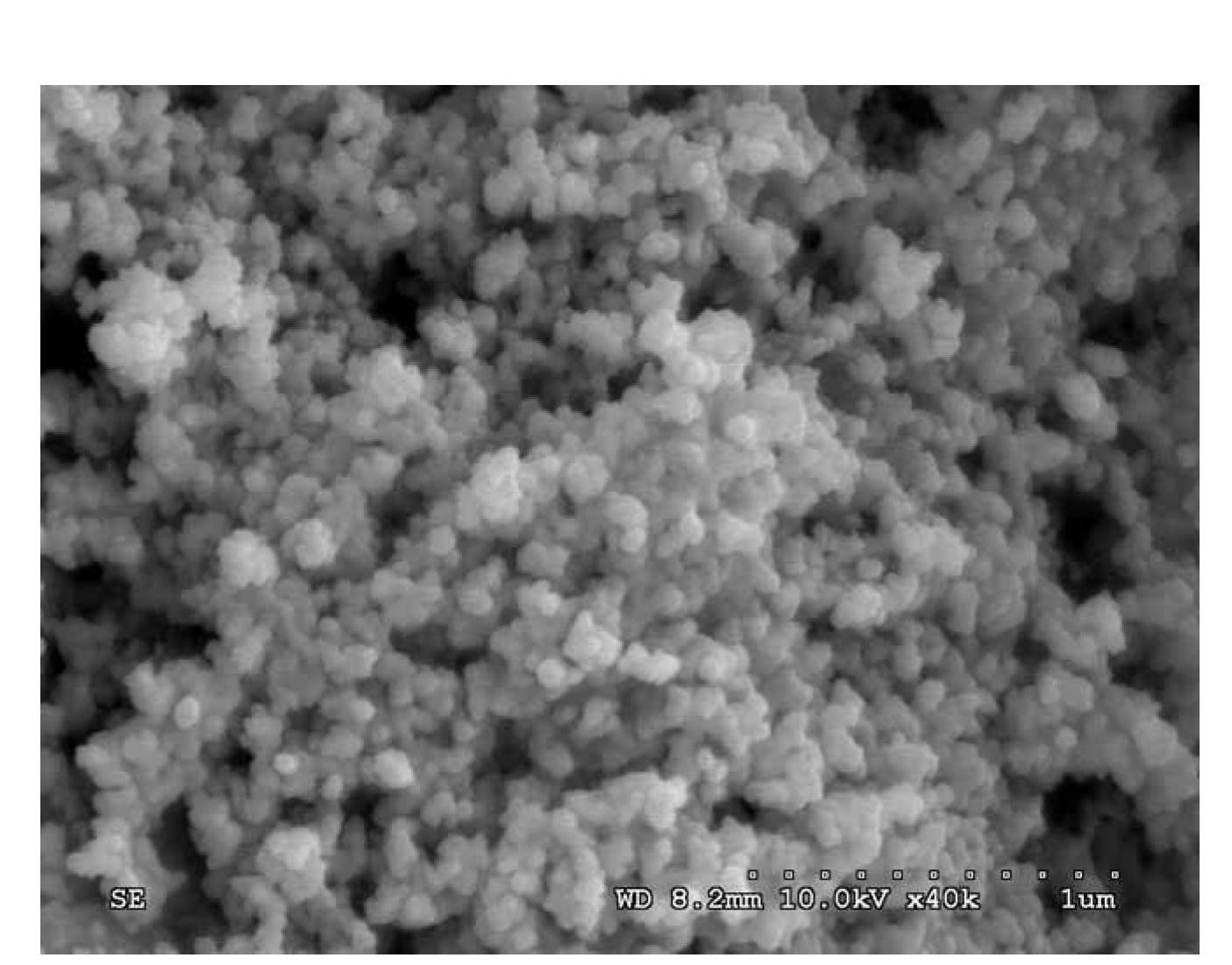
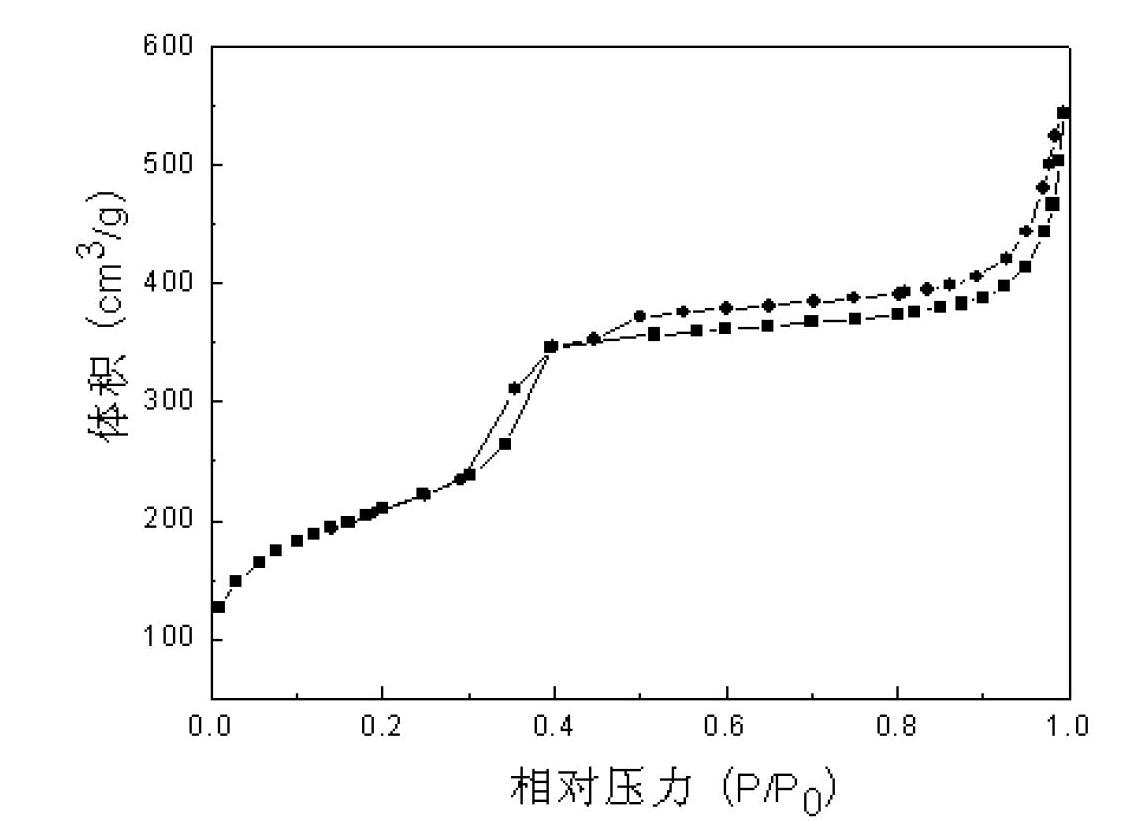
Method for preparing mesoporous titanium silicon molecular sieve nanoparticles
InactiveCN102633282ASmall sizeIncrease the effective collision frequencyNanotechnologyCrystalline aluminosilicate zeolitesMuffle furnaceMaterials science
The invention relates to a method for preparing mesoporous titanium silicon molecular sieve nanoparticles, aiming at solving the technical problem that the mesoporous titanium silicon material particles prepared by hydro-thermal synthesis are larger in size. The method comprises the steps of: sequentially adding hexadecyl trimethyl ammonium bromide (HTAB), polyquaternium-6 and ammonia water into deionized water, and evenly mixing to obtain a mixed solution; then, adding tetraethoxysilane and tetrabutyl titanate into the mixed solution dropwise, and continuously stirring to obtain precursor solution; carrying out hydrothermal treatment on the precursor solution, separating out solid phase substance, washing and drying; and finally, putting the dried solid phase substance in a muffle furnace for calcination to obtain the mesoporous titanium silicon molecular sieve nanoparticles. The mesoporous titanium silicon molecular sieve nanoparticles have the average size of less than 100nm, the mesoporous aperture of 2-3.5nm and the specific surface area of 300-850m<2> / g, and can be used for macromolecular catalytic fine chemical engineering and pharmaceutical synthesis fields.
Owner:HARBIN INST OF TECH
Cefodizime sodium composition and preparation method thereof
InactiveCN102258521AImprove stabilityStable recipe processAntibacterial agentsOrganic active ingredientsCefodizime SodiumFreeze-drying
The invention relates to the field of drug synthesis and preparation thereof, relates to a preparation method of an antibacterial drug, in particular to a stable cefodizime sodium composition preparation and a preparation method thereof. The present invention directly dissolves sterile cefodizime sodium in water, adds sterile potassium clavulanate to dissolve it completely, and obtains an aqueous solution of cefodizime sodium / clavulanate potassium, and adds hydroxypropyl-β to the aqueous solution. -Cyclodextrin (HP-β-CD) inclusion, sub-package, and freeze-drying to obtain Cefodizime Sodium / Clavulanate Potassium for Injection. The preparation method provided by the invention is simple, and the cefodizime sodium / clavulanic acid potassium salt is clathrated with hydroxypropyl-β-cyclodextrin, which increases the stability of the sterile cefodizime sodium drug and reduces its toxic and side effects , improve drug availability, and the preparation process is simple, suitable for industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD +1
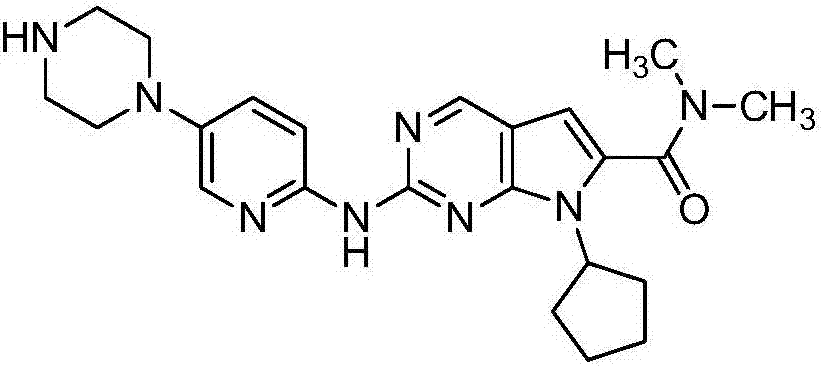
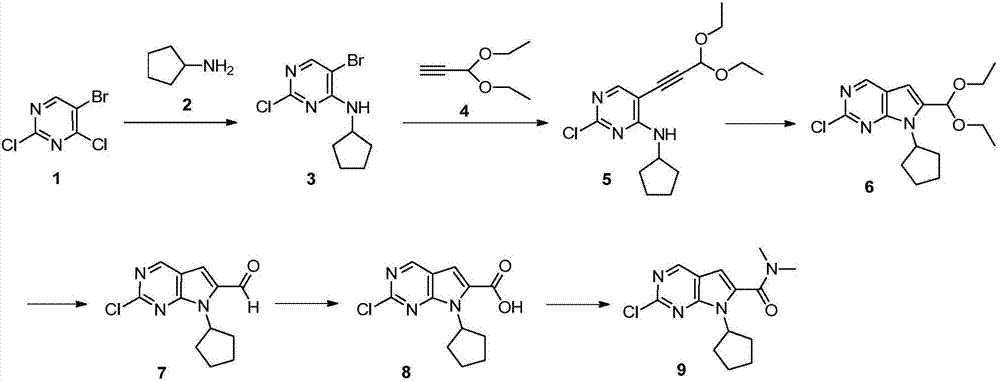
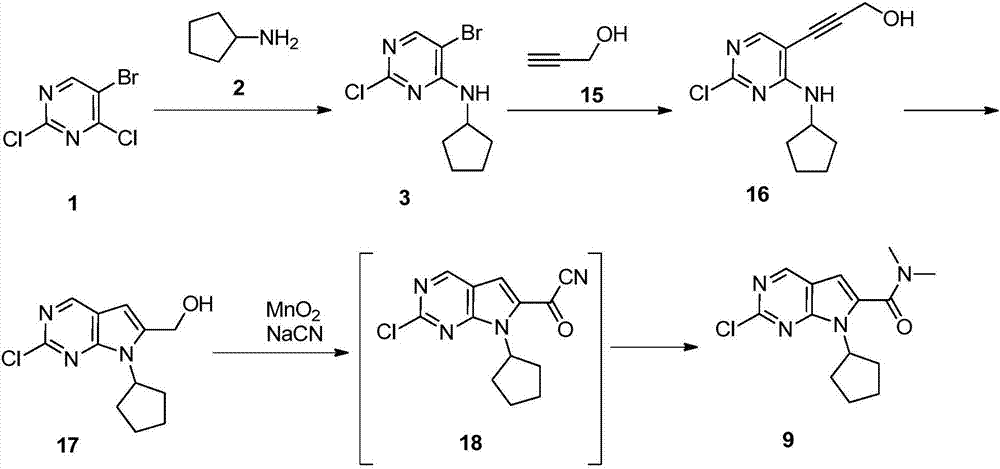
Preparation method of ribociclib intermediate
The invention belongs to the field of organic synthesis and pharmaceutical synthesis and particularly relates to a preparation method of a ribociclib intermediate. According to the preparation method, after 2-halo-7-cyclopentyl-6-(((tetrahydro-2H-pyran-2-yl)oxy)methyl)-7H-pyrrolo[2,3-d] pyrimidine is obtained, the ribociclib intermediate, namely, 2-halo-7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-formamide is obtained through three steps of reactions, and high yield and high purity are realized in each reaction step, so that the total yield of the overall route is high and is remarkably better than that in the prior art; besides, raw materials are easy to obtain, the production cost is low, the preparation is simple and easy to operate, reaction reagents are environmentally friendly, and the preparation method of the ribociclib intermediate is particularly suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
New polypeptide fragment of somalutide and preparation method thereof
ActiveCN110317258ALess impuritiesHigh purityPeptide preparation methodsBulk chemical productionCombinatorial chemistryDrug synthesis
The invention relates to the field of polypeptide drug synthesis, in particular to a somalutide polypeptide fragment with a protective group and a preparation method thereof. When the new polypeptidefragment is used for synthesizing somalutide, impurities in the fragment are reduced, and the purity of the final product is improved.
Owner:QILU PHARMA
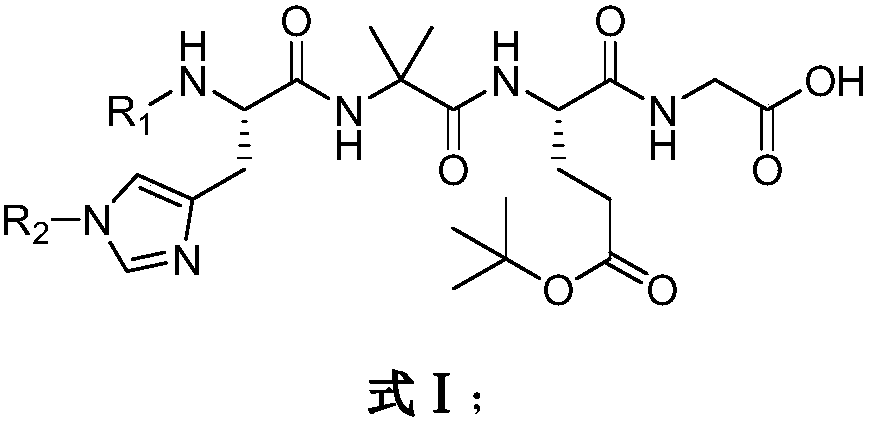
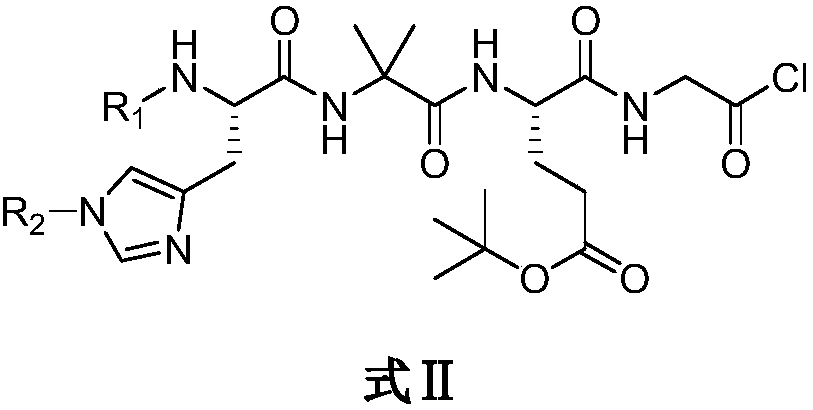
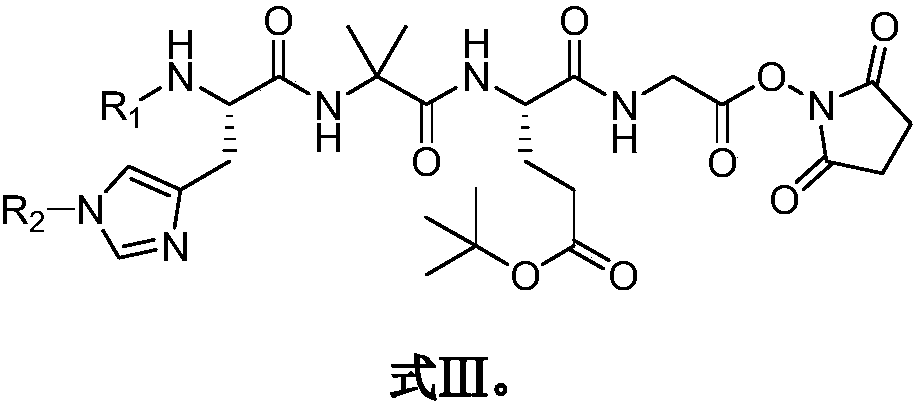
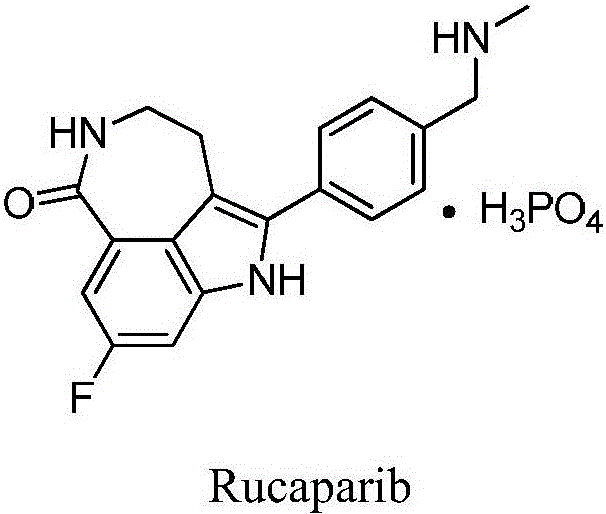
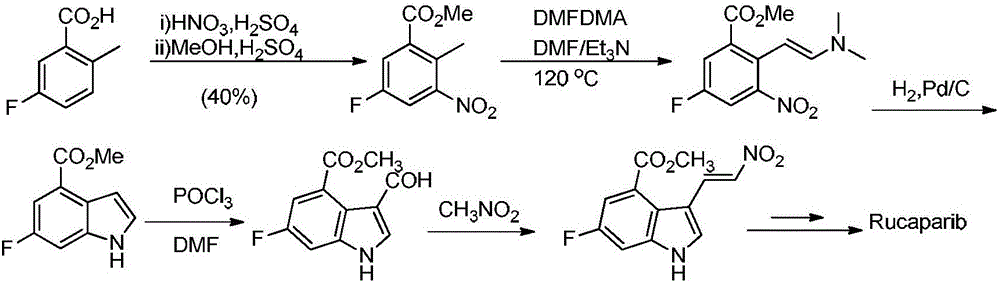
Preparation method of Rucaparib intermediate
The invention belongs to the field of medicine synthesis, and in particular relates to a preparation method of a Rucaparib intermediate 6-fluoro-3-[(E)-2-nitroethylene]-1H-indole-4-methyl formate. 3-amino-5-methyl fluorobenzoate is used as a raw material in the method, which reacts with a zinc reagent of 2-(2-bromoethyl)-1,3-dioxolane after being diazotized, then is hydrolyzed, and reacts with nitromethane. The preparation method has cheap and easily available materials, a refined reaction route, high reaction selectivity and high yield.
Owner:伦俊杰
4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs
The invention belongs to the technical field of new drug synthesis, in particular to a class of 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-L-xylofuranose)pyrrolo [2,3-d]pyrimidine, its similar derivatives, and drugs for preparing anti-human liver cancer cells, human lung cancer cells, human breast cancer cells, human cervical cancer cells, and human gastric cancer cells. The compounds of the present invention are directed against 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-L-xylofuranose)pyrrolo[2,3 -d] The C6 position of pyrimidine is modified and synthesized by chemical methods. Experiments have shown that this type of compound can significantly inhibit the proliferation of various cancer cells, effectively induce apoptosis of cancer cells, including highly metastatic cancer cells, and can be used as drugs and drug components for treating cancer.
Owner:JILIN UNIV
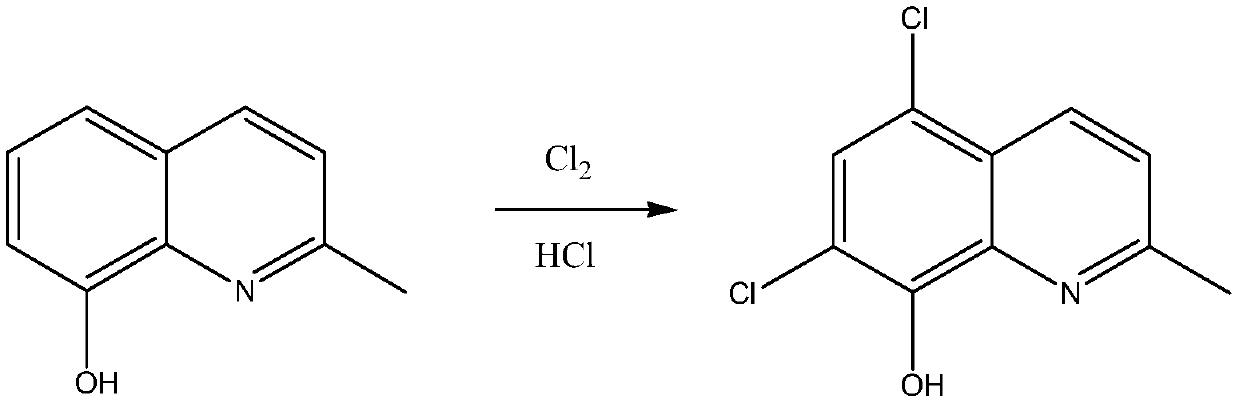
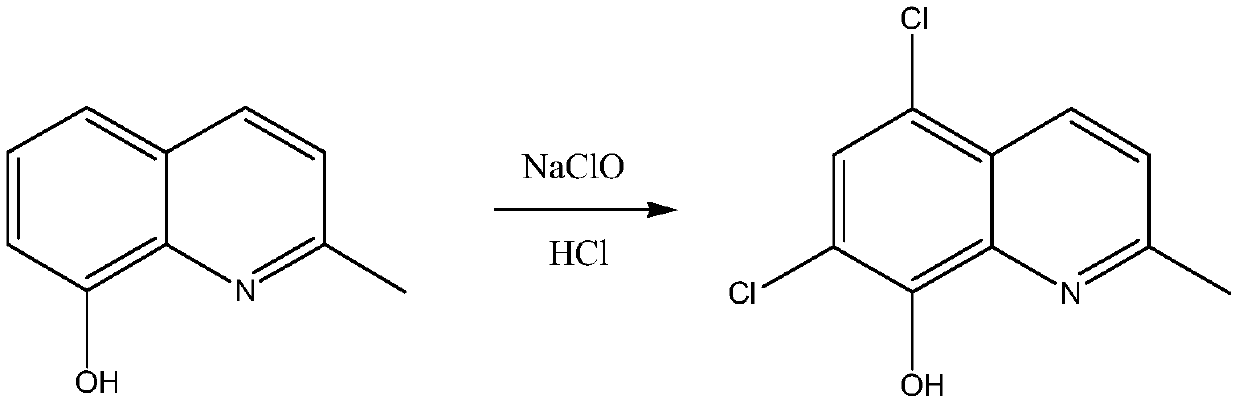
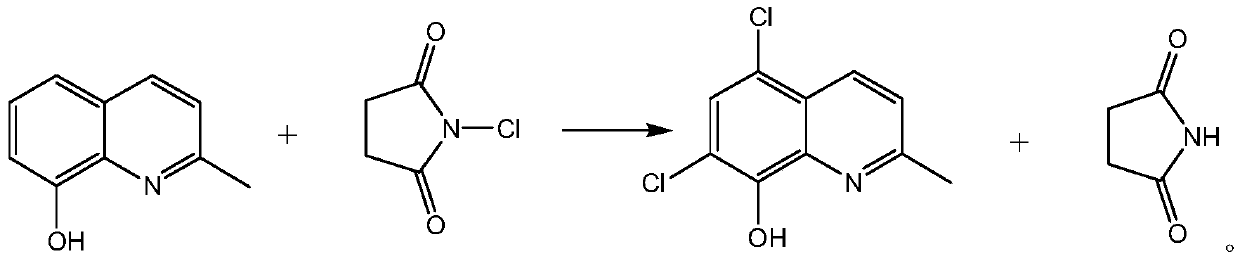
Synthetic technology of chlorquinaldol
ActiveCN110143919AReduce pollutionQuality assuranceOrganic chemistryEnvironmental resistanceN-Chlorosuccinimide
The invention belongs to the field of pharmaceutical synthesis technology and specifically relates to a synthetic technology of chlorquinaldol. By using Lewis acid as a catalyst, 8-hydroxy-2-methylquinoline and N-Chlorosuccinimide, which are used as raw materials, are subjected to a one-step chlorination reaction to generate chlorquinaldol; and after the reaction, chlorquinaldol is refined to obtain the chlorquinaldol. By using N-Chlorosuccinimide to replace chlorine as the raw material of the chlorination reaction, the selectivity is good, side reaction is reduced, conversion rate and yield are increased, yield reaches 98.2% and above, purity is 99.90% and above, quality of the chlorquinaldol is guaranteed, generation of spend liquor is decreased, environmental pollution is reduced, the cost is saved, water dissolution of the reaction product is avoided, and the yield is increased. The technology of the invention is a green and environmentally-friendly technology, and is suitable forindustrial production.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com













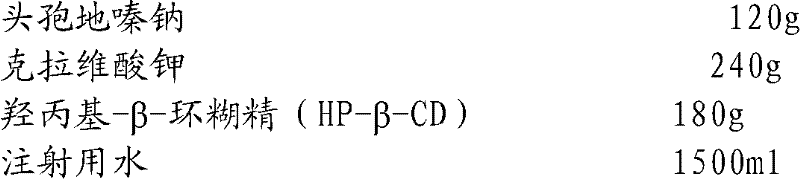
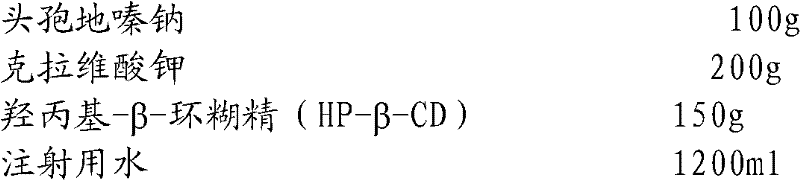
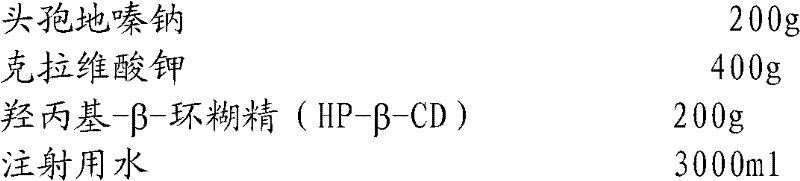
![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000011.png)
![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000012.png)
![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000013.png)