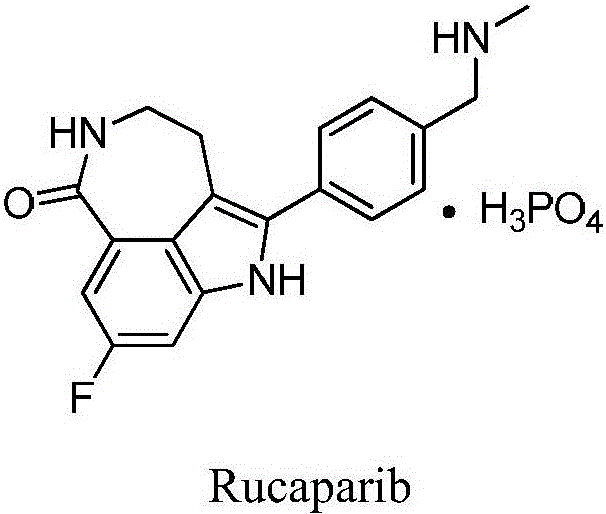
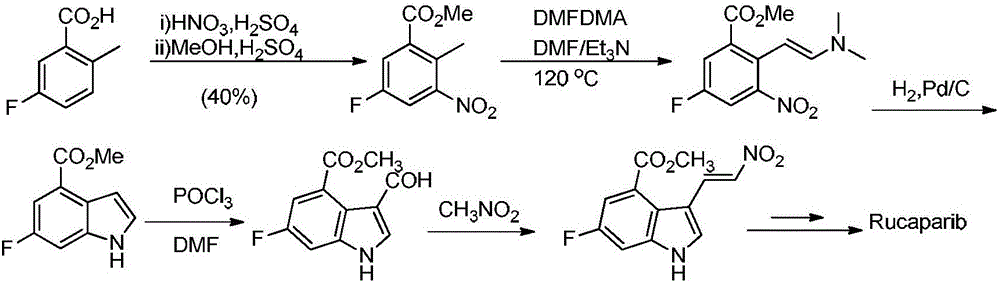
Preparation method of Rucaparib intermediate
An intermediate, indole technology, applied in the field of pharmaceutical synthesis, can solve the problems of low catalytic hydrogenation reaction yield, long reaction steps, unfavorable environmental protection and the like, and achieve the effects of shortening the preparation period, simplifying the route and improving the selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of 3-fluoro-5-methoxyacylbenzenediazotetrafluoroborate
[0036] Add 1.69g (10mmol) of methyl 3-amino-5fluorobenzoate to 10ml of 30% boron tetrafluoride solution, cool down to -5°C, and slowly add 4mol / L NaNO 2 2.9mL of the solution of 3-fluoro-5-methoxyacylbenzenediazotetrafluoroborate was obtained by keeping the temperature at -5—0°C during the dropwise addition for 30 minutes. %.
Embodiment 2
[0037] Example 2 Preparation of 3-fluoro-5-methoxyacylbenzenediazotetrafluoroborate
[0038] Add 1.69g (10mmol) of methyl 3-amino-5fluorobenzoate to 10ml of 30% boron tetrafluoride solution, cool down to -10°C, and slowly add 5mol / L NaNO 22.3mL of the solution of 3-fluoro-5-methoxyacylbenzenediazotetrafluoroborate was obtained by keeping the temperature at -5—0℃ during the dropwise addition for 30 minutes. %.
Embodiment 3
[0039] Example 3 Preparation of 3-fluoro-5-methoxyacylbenzenediazotetrafluoroborate
[0040] Add 1.69g (10mmol) of methyl 3-amino-5fluorobenzoate to 10ml of 40% boron tetrafluoride solution, cool down to -5°C, and slowly add 4mol / L NaNO 2 3.0mL of the solution, the temperature was kept at -5—0°C during the dropwise addition, reacted for 30 minutes, filtered, washed with methanol, and 2.47g of 3-fluoro-5-methoxyacylbenzenediazotetrafluoroborate was obtained, with a yield of 92.2 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com