Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Cefazedone sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
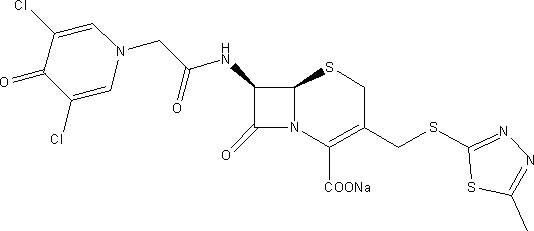
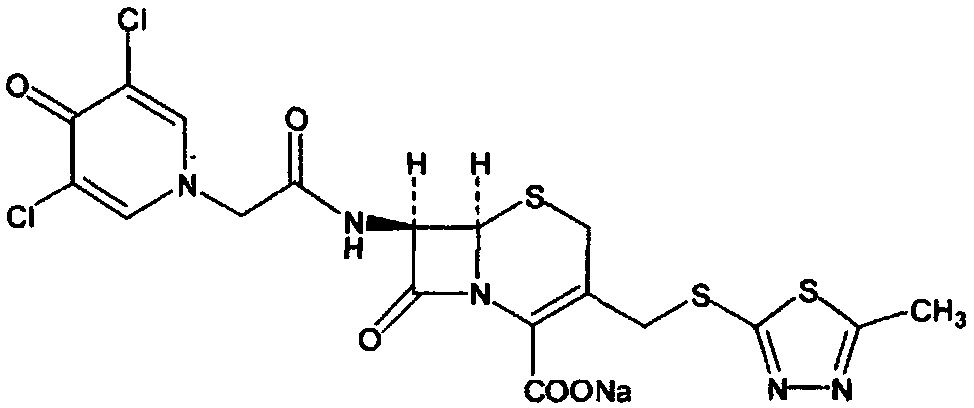
Cefazedone Sodium is the sodium salt form of cefazedone, a semi-synthetic first-generation, oral cephalosporin with antibacterial activity. from NCIt MeSH Pharmacological Classification
Cefazedone sodium medicament powder injection and method for synthesizing raw medicine of Cefazedone sodium
ActiveCN101584671ASingle componentImprove solubilityAntibacterial agentsPowder deliverySodium bicarbonateNitrogen gas
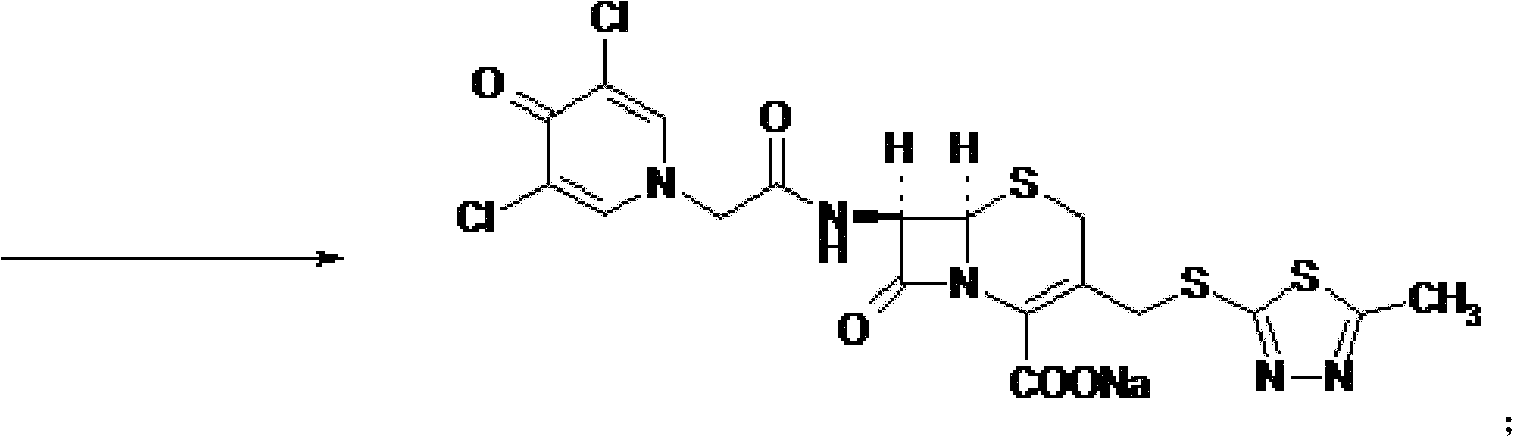
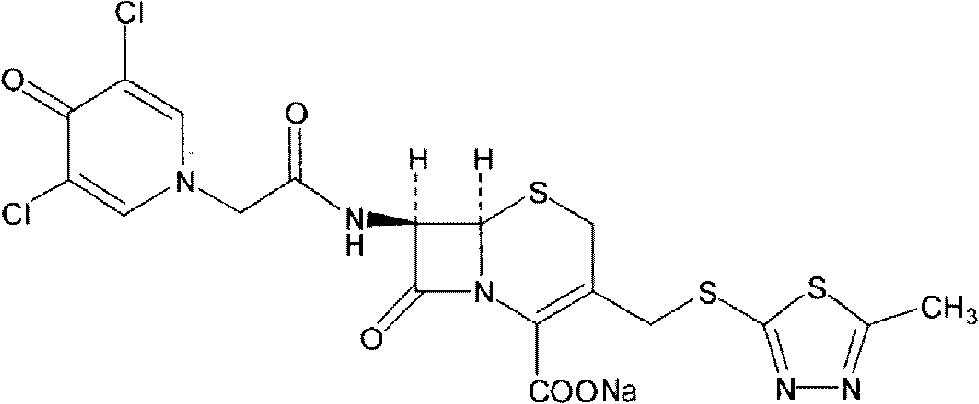
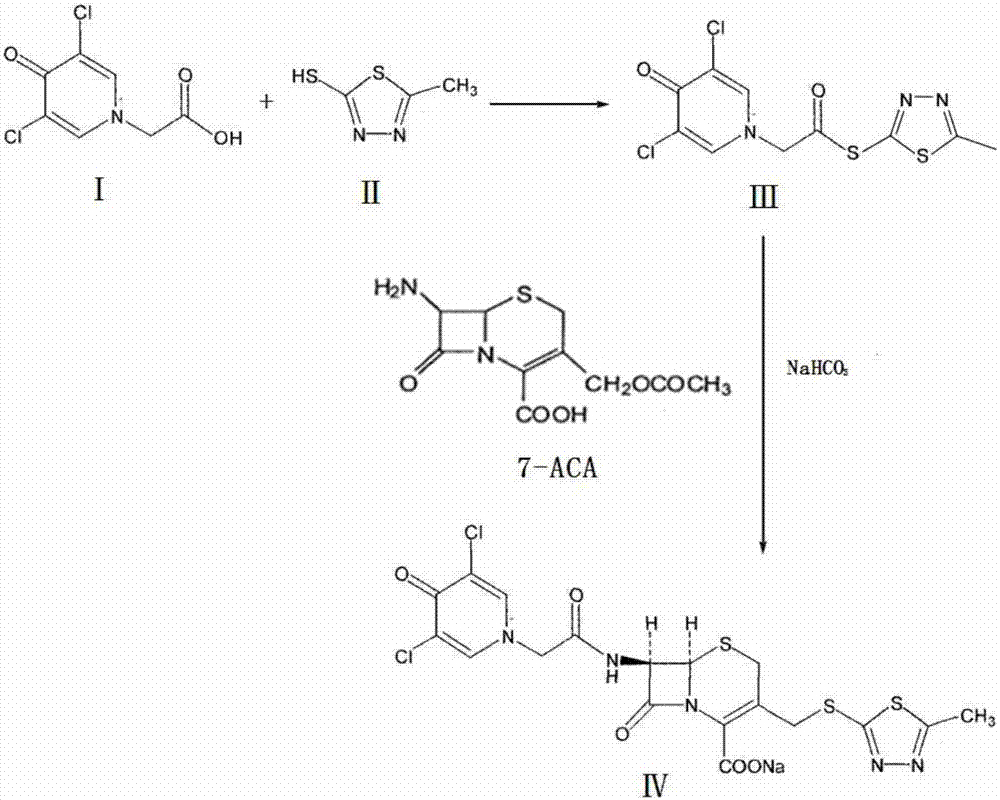
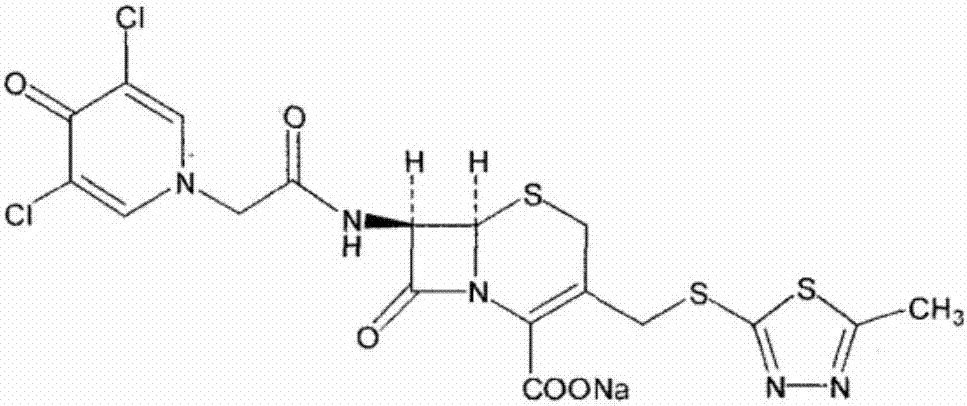
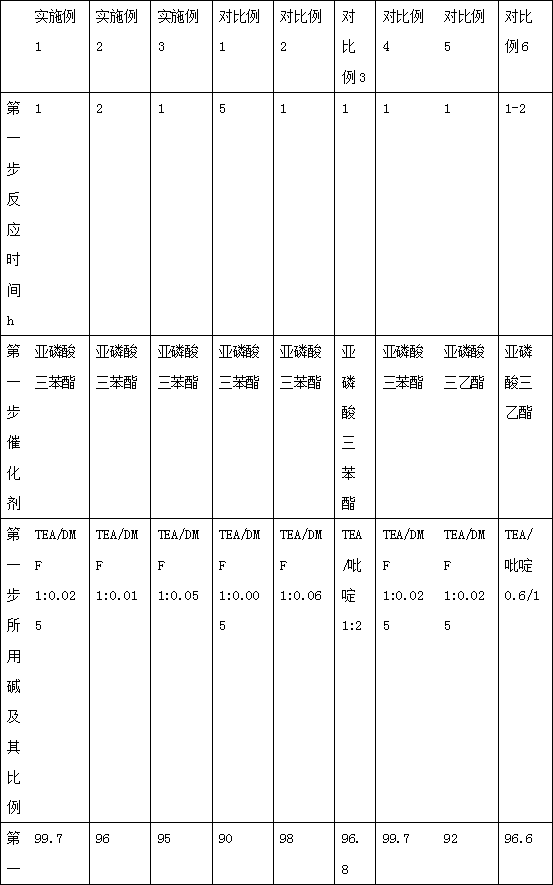
The present invention provides a cefazedone sodium medicament powder injection composed of 100% of cefazedone sodium. The cefazedone sodium is prepared by a method as follows: (1) 7-ACA and 3, 5-dichloro pyridine acetic acid react with each other with the action of an anhydrating agent, a mixture after the reaction is post-processed to obtain an intermediate product I; (2) the intermediate product I and 2-mercapto-5-methyl-1, 3, 4-thiadiazoles react with each other with the protection of nitrogen at a temperature of 50 to 90 DEG C, a mixture after the reaction is purified to obtain a water solution which is added with an inorganic acid to regulate pH value to be equal to 1 to 3, a precipitation is extracted from the water solution and is post-processed to obtain cefazedone; (3) the cefazedone and sodium hydrogen carbonate react with each other in water to obtain a cefazedone sodium solid body after an aftertreatment. The powder injection has single component and perfect dissolution performance, the raw medicine has a short synthetic route, the aftertreatment of the intermediate product or final product are all simple, and the yield and purity of the whole reaction process are all high.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
A purifying method of a novel antibiotic compound
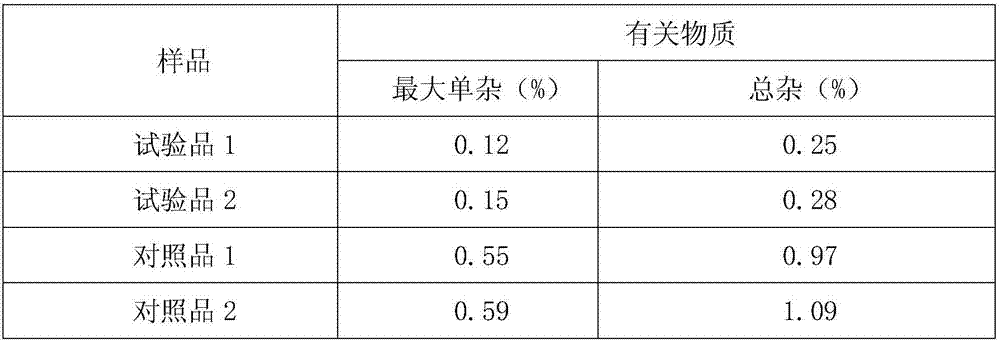
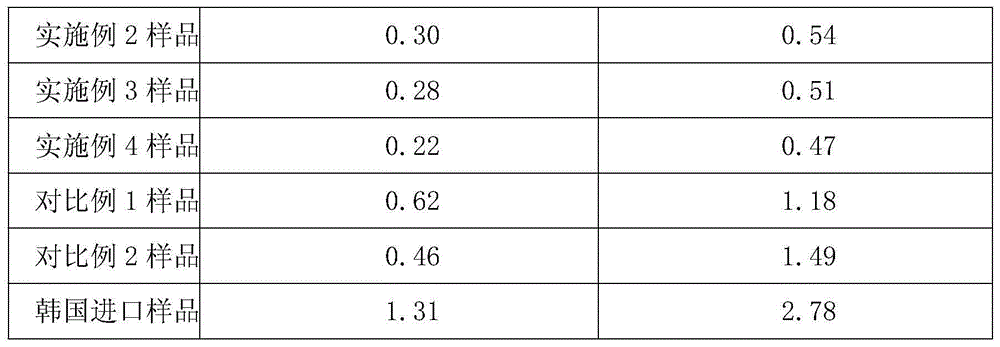
The invention relates to a purifying method of a novel antibiotic compound. The method includes: (1) a step of salifying, namely a step of adding cefazedone and sodium isooctoate into absolute methanol and performing a salifying reaction until the cefazedone is fully dissolved, with the temperature being controlled at 15-35 DEG C; (2) a step of decoloring, namely a step of adding active carbon into the reaction solution, stirring, decoloring, filtering to remove the active carbon, maintaining the temperature of the filtrate at 10-30 DEG C, and filtering again; (3) a step of crystallizing, namely a step of adding a certain amount of acetone and ethyl acetate into the filtrate after the filtration, controlling the temperature at 15-20 DEG C, growing the grain for 1-2 h, then adding a certain amount of the acetone and the ethyl acetate, stirring for crystallization with the stirring speed being maintained at 80-120 r / min, controlling the temperature at 15-25 DEG C, and growing the grain for 2-4 h; and (4) a step of drying, namely a step of filtering, washing the filter cake, and drying to obtain a purified product of the cefazedone sodium. Compared with the prior art, the method has characteristics of simple operation, low cost, high yield, and largely reduced pollution. The purified product of the cefazedone sodium has characteristics of low water content, low purity content and high stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Cefazedone sodium medicament powder injection and method for synthesizing raw medicine of Cefazedone sodium
ActiveCN101584671BSingle componentImprove solubilityAntibacterial agentsOrganic active ingredientsSodium bicarbonateNitrogen gas
The present invention provides a cefazedone sodium medicament powder injection composed of 100% of cefazedone sodium. The cefazedone sodium is prepared by a method as follows: (1) 7-ACA and 3, 5-dichloro pyridine acetic acid react with each other with the action of an anhydrating agent, a mixture after the reaction is post-processed to obtain an intermediate product I; (2) the intermediate productI and 2-mercapto-5-methyl-1, 3, 4-thiadiazoles react with each other with the protection of nitrogen at a temperature of 50 to 90 DEG C, a mixture after the reaction is purified to obtain a water solution which is added with an inorganic acid to regulate pH value to be equal to 1 to 3, a precipitation is extracted from the water solution and is post-processed to obtain cefazedone; (3) the cefazedone and sodium hydrogen carbonate react with each other in water to obtain a cefazedone sodium solid body after an aftertreatment. The powder injection has single component and perfect dissolution performance, the raw medicine has a short synthetic route, the aftertreatment of the intermediate product or final product are all simple, and the yield and purity of the whole reaction process are all high.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Preparation method of novel anti-infective drug
ActiveCN104086571AReduce moisture contentReduce the production of degradation impuritiesOrganic chemistrySodium acetateSolvent
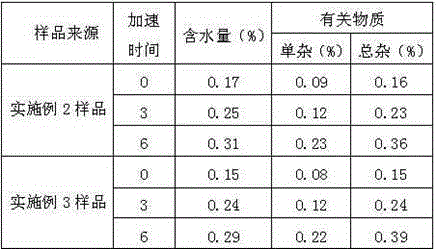
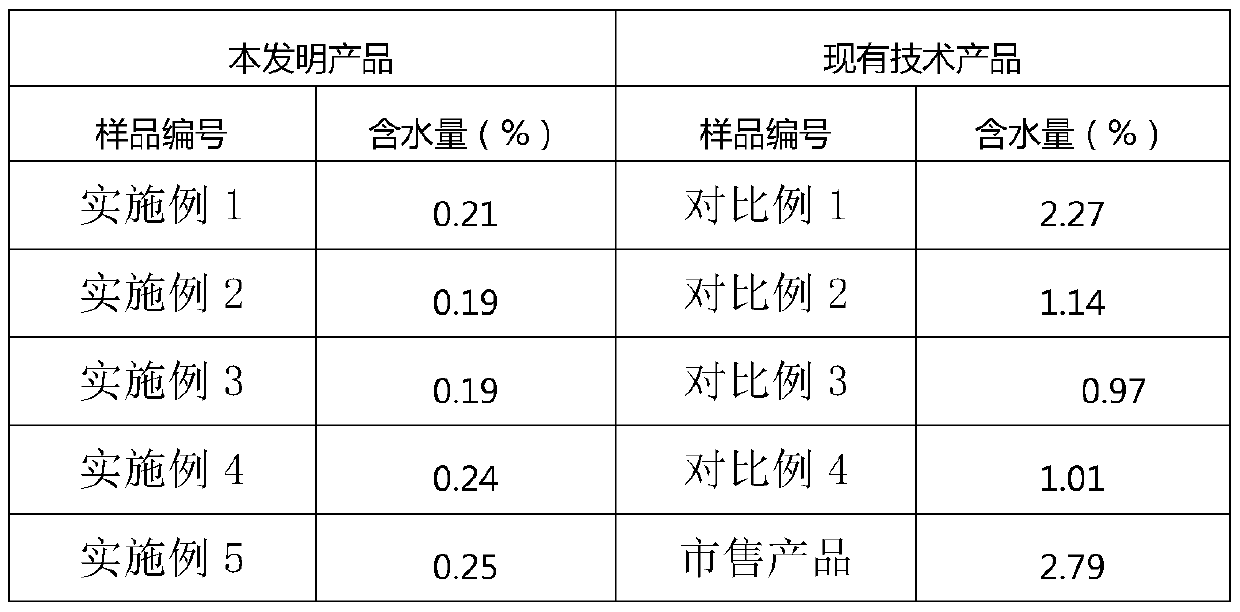
The invention relates to a preparation method of a novel anti-infective drug. The preparation method comprises the following steps: (1) salifying, namely putting cefazedone and a sodium acetate solvent in absolute methanol to have a salifying reaction, and controlling the temperature in the range of 10-30 DEG C after the cefazedone is completely dissolved, (2) decoloring, namely adding active carbon to a reaction liquid, stirring and decoloring, and removing the active carbon by filtering, keeping the temperature of the filtrate in the range of 10-30 DEG C, and then re-filtering; (3) crystalizing, namely slowly and dropwise adding a certain amount of ethanol to the filtered filtrate at a rate of 20L / H under a stirring condition until the system is turbid, growing crystals for 0.5 to 1 hour, next, adding a certain amount of acetone, growing crystals for 0.5 to 1 hour, and then reducing the temperature to 5 DEG C at a temperature reducing rate of 5-15 DEG C per hour, and stirring by keeping the stirring speed in the range of 80-120r / min for crystallization, and growing the crystals for 1-3 hours; and (4) drying, namely filtering and washing the filter cake, and drying to obtain refined cefazedone sodium. Compared with the prior art, the preparation method is simple to operate, low in cost and high in yield, and pollution is greatly reduced; and the obtained refined cefazedone sodium is low in moisture content, low in impurity content and high in stability.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Preparation method for cephalosporin anti-infective drug
ActiveCN105017286ASimple preparation processReduce generationOrganic chemistry7-ACACefazedone sodium
The invention relates to a preparation method for a cephalosporin anti-infective drug-cefazedone sodium, belonging to the field of pharmaceutical synthesis. According to the invention, the method uses GCLE as a raw material to substitute 7-ACA and overcomes the defects of low yield, high pollution and the like in prior art; the preparation method with mild reaction conditions, little side reaction and simple process is provided; meanwhile, the method has the advantages of cheap and easily-available raw materials, low cost, high product yield, high product purity and applicability to industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for detecting toxicity of cefazedone sodium impurities and intermediates
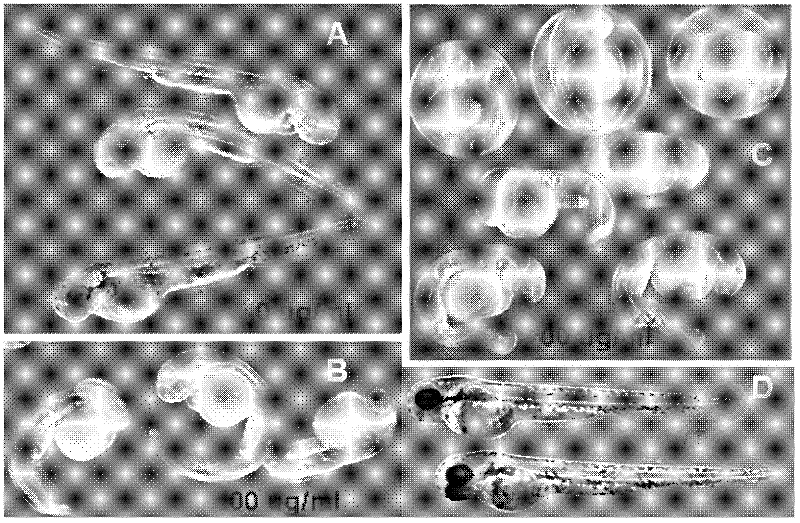
InactiveCN102288735AEnsure safetyAvoid product qualityComponent separationTesting medicinal preparationsCefazedone sodiumPharmacotoxicology
The invention relates to a method for detecting toxicity of cefazedone sodium impurities. TU line Zebra fish, Danio rerio Tuebingen serves as a model animal of pharmocotoxicology evaluation. The invention relates to toxicity evaluation of intermediate impurities, byproducts and various degraded impurities of cefazedone sodium in the whole synthetic process. The toxicity of trace impurities in cefazedone is evaluated by chiefly adopting a novel model animal 'zebra fish' of the pharmocotoxicology evaluation, the product quality can be effectively controlled through a zebra fish toxicity test, the safety of clinical use is guaranteed, and a reliable basis is provided for administration safety of a patient.
Owner:天津新丰制药有限公司
Preparation method of cefazedone sodium compound
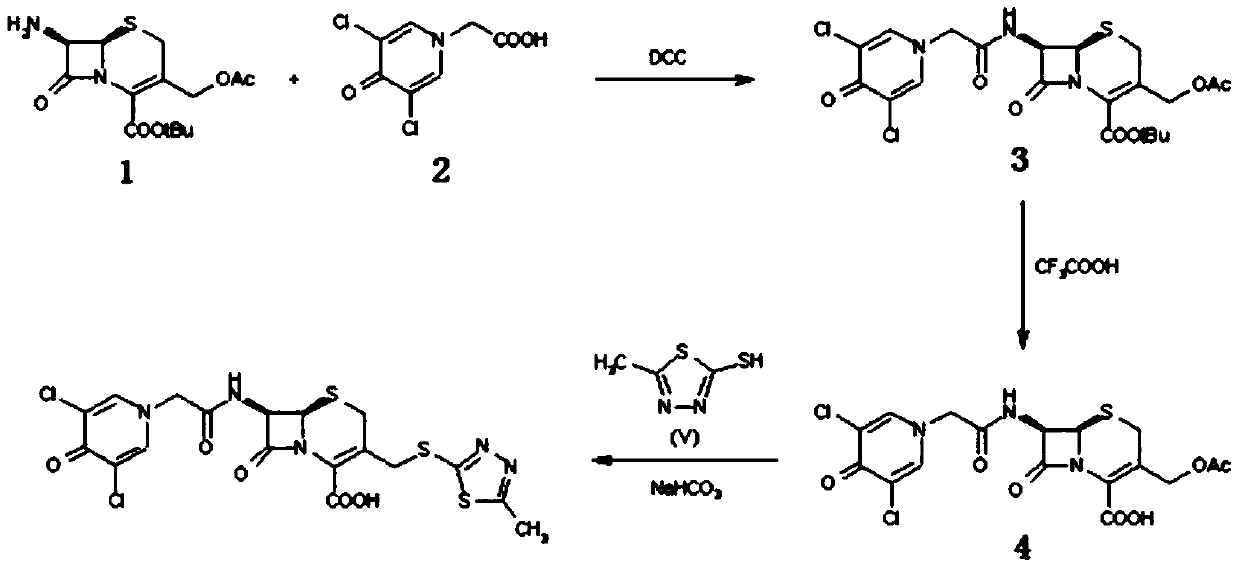
The invention discloses a preparation method of a cefazedone sodium compound. 7-ACA and a compound III react to prepare a compound IV, and the compound IV and a compound V have an amidation reaction,and are salified and refined to obtain a competitive product of cefazedone sodium (I). The process route of the reaction is simple, the total yield and the purity are high, and the method is suitablefor industrial production.
Owner:SHANDONG YUXIN PHARMA CO LTD
A kind of synthesis technique of cephalosporin anti-infective drug
ActiveCN105017285BNo pollution in the processThe synthesis process is simpleOrganic chemistryAcetic acidThiol
The invention relates to a synthetic process of a novel cephalosporin anti-infective drug. A thioester compound (formula III) synthesized by adopting 3,5-dichloro-4-pyridone-1-acetic acid and 2-thiol-5-methyl-1,3,4-thiadiazole as raw materials is directly reacted with 7-ACA in one step to successfully prepare cefazedone sodium. According to the synthetic process disclosed by the invention, the operating steps are simplified, so that the production conditions are milder, the generation of byproducts is reduced, and the production technology is simpler; and moreover, the product yield and purity are greatly improved, the cost is reduced, the environmental pollution is little, and the synthetic process is higher in environmental protection, so that the process accords with the requirement of industrialization.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
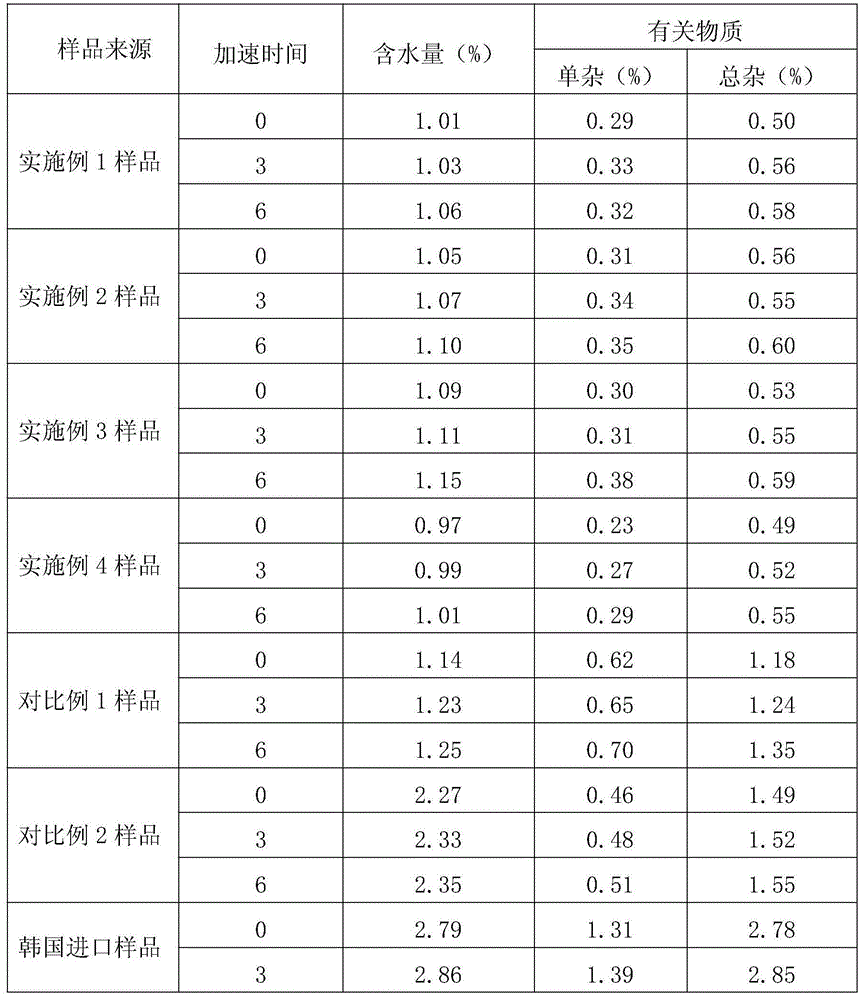
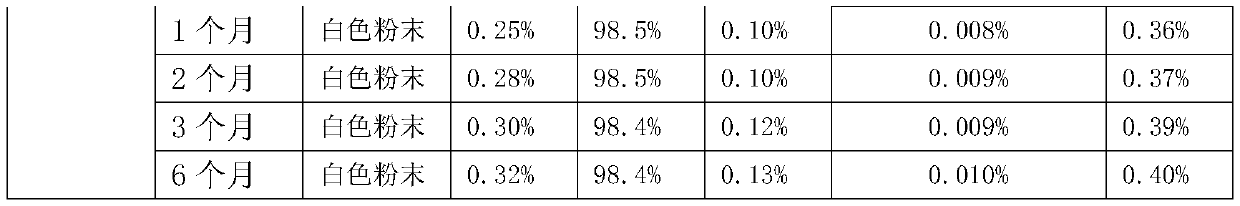
Method for determining moisture limit of cefazedone sodium sterile
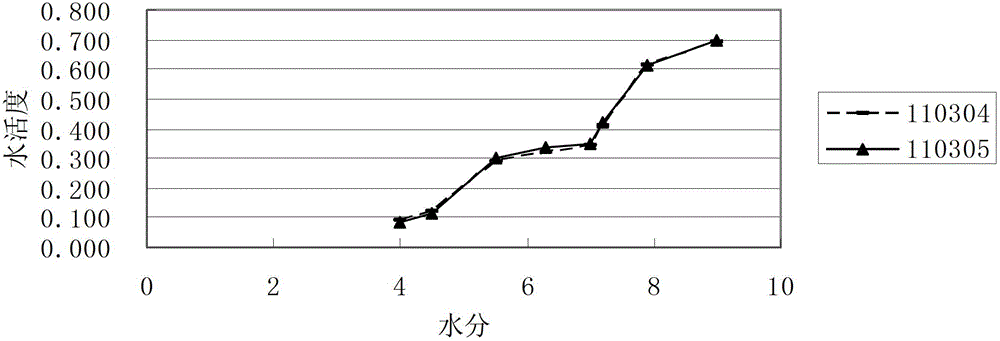
InactiveCN102914626AEfficient determination of moisture limit valuesGuaranteed stabilityTesting medicinal preparationsWater activityCefazedone sodium
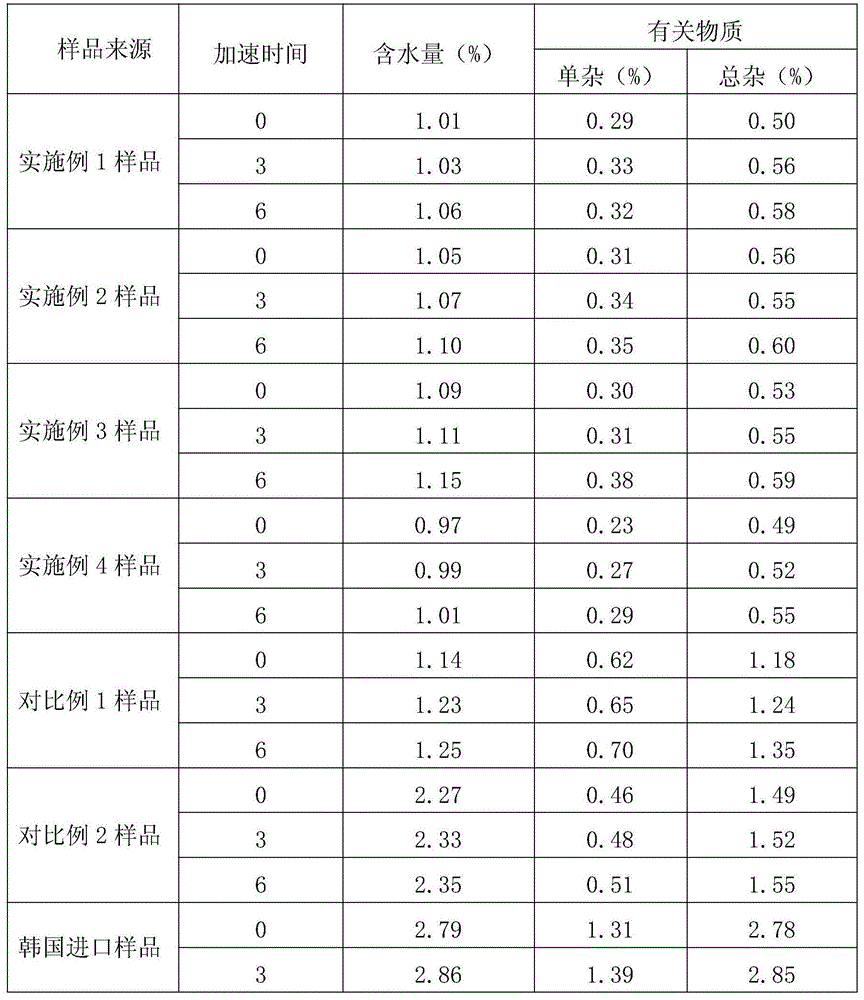
The invention relates to a method for determining a moisture limit of cefazedone sodium sterile. The method comprises the steps of: (1) measuring water activity of a cefazedone sodium sterile sample; (2) measuring a moisture content of the cefazedone sodium sterile sample; and (3) drawing a correlation curve of the water activity and the moisture content through the two values and finally determining the moisture control limit of the cefazedone sodium sterile. By measuring the moisture content in the cefazedone sodium sterile solid and the water activity thereof, drawing an isothermal adsorption curve of the cefazedone sodium sterile sample and determining the moisture control limit of the cefazedone sodium sterile based on the relationship between the two indexes, the invention provides a method for determining the moisture limit more simply and more directly.
Owner:天津新丰制药有限公司
Cefazedone sodium frozen powder injection and preparation method thereof
InactiveCN101732264ASimple preparation processQuality is easy to controlAntibacterial agentsPowder deliveryArginineExcipient
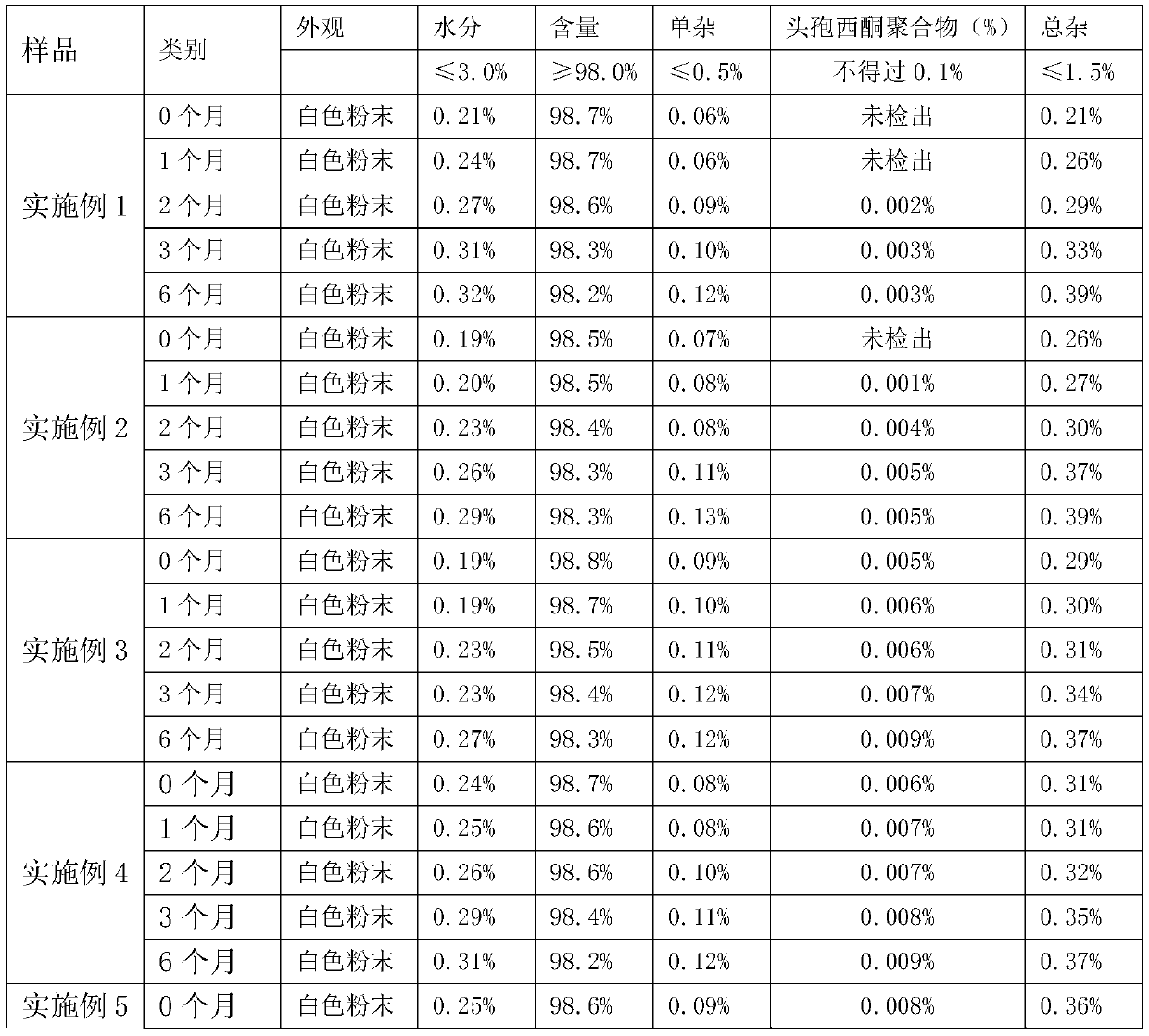
Belonging to the field of pharmaceutical preparation, the invention relates to cefazedone sodium frozen powder injection which comprises 45-90% of cefazedone, 5-52% of excipient, 0.02-5% of antioxygen; wherein the excipient is composed of one or more of mannitol, sorbic alcohol or lactose, the antioxygen is composed of one or more of arginine, cysteine hydrochloride or glutathione. The cefazedone sodium frozen powder injection features stable physical and chemical properties, controllable quality; in addition, polymer impurities of the cefazedone sodium frozen powder injection are obviously reduced in the process of storage, thus improving safety of using the injection.
Owner:湖南万健康品生物科技有限公司
Synthetic process of novel cephalosporin anti-infective drug
ActiveCN105017285ANo pollution in the processThe synthesis process is simpleOrganic chemistryAcetic acidThiol
The invention relates to a synthetic process of a novel cephalosporin anti-infective drug. A thioester compound (formula III) synthesized by adopting 3,5-dichloro-4-pyridone-1-acetic acid and 2-thiol-5-methyl-1,3,4-thiadiazole as raw materials is directly reacted with 7-ACA in one step to successfully prepare cefazedone sodium. According to the synthetic process disclosed by the invention, the operating steps are simplified, so that the production conditions are milder, the generation of byproducts is reduced, and the production technology is simpler; and moreover, the product yield and purity are greatly improved, the cost is reduced, the environmental pollution is little, and the synthetic process is higher in environmental protection, so that the process accords with the requirement of industrialization.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Synthetic method of cefazedone sodium
ActiveCN109553630AImprove acylation activityGuaranteed stabilityOrganic chemistryAcetic acidCefazedone sodium
The invention belongs to the technical field of medicines, and discloses a synthetic method of cefazedone sodium. Active ester is synthesized by taking 3,5-dichloro-4-pyridone-1-acetic acid and 2,2'-dithiobis(benzothiazole) as raw materials, then the active ester reacts with an intermediate generated from 7-aminocephalosporanic acid and mercaptoterazole, and the cefazedone sodium is obtained through salifying. According to the invention, a mixed solvent is used in 3-position substitution, so that the reaction is more stable and soft, and generation of by-products is reduced; the active ester is used in an acylation reaction, so that activity is high, and a 7-position acylation reaction is facilitated; and the yield and purity of the cefazedone sodium are relatively high.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Enzymatic synthesis technology of novel cephalo-type anti-infection drug
ActiveCN105002253AEmission reductionReduce pollutionFermentationEnzymatic synthesisCefazedone sodium
The invention relates to an enzymatic synthesis technology of a novel cephalo-type anti-infection drug. The technology comprises the following steps: preparing TDA hydrochloride from TDA, purifying, processing to prepare TDA sodium salt, and directly carrying out enzymatic synthesis of cefazedone sodium in a water phase under the action of immobilized penicillin acylase. The enzymatic synthesis technology has the advantages of operating step simplification, implementation of a reaction in the water phase, mild reaction conditions, few byproducts, great reduction of energy consumption and organic wastewater discharge, cost reduction, small pollution to environment, good environmental protection property, and industrialization requirement meeting; and the technology greatly improves the product yield and the product yield, the product purity is greater than 99.9%, and the total yield is greater than 90%.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Pharmaceutical composition for treating infectious diseases and preparation thereof
InactiveCN104825458AImprove stabilityEasy to degradeAntibacterial agentsOrganic active ingredientsInfective disorderBiology
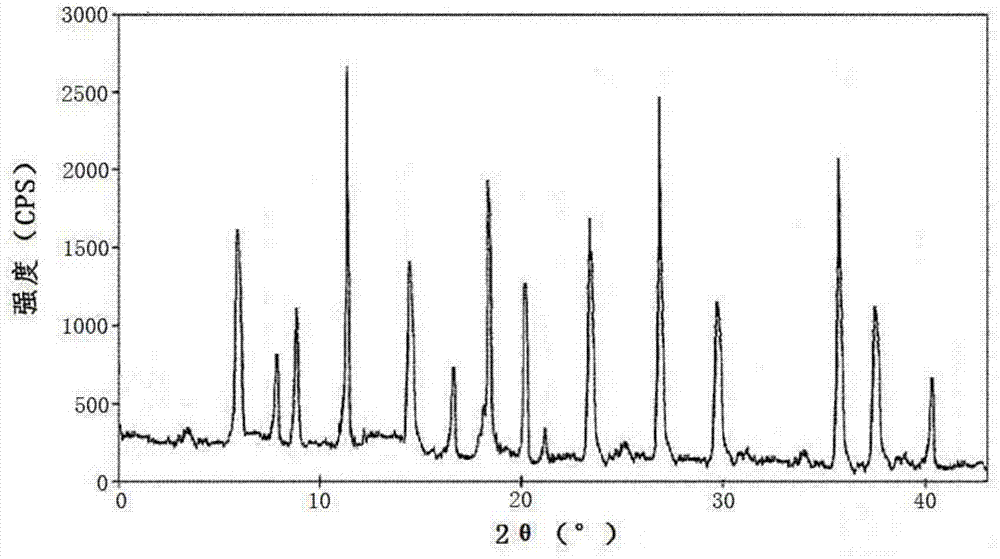
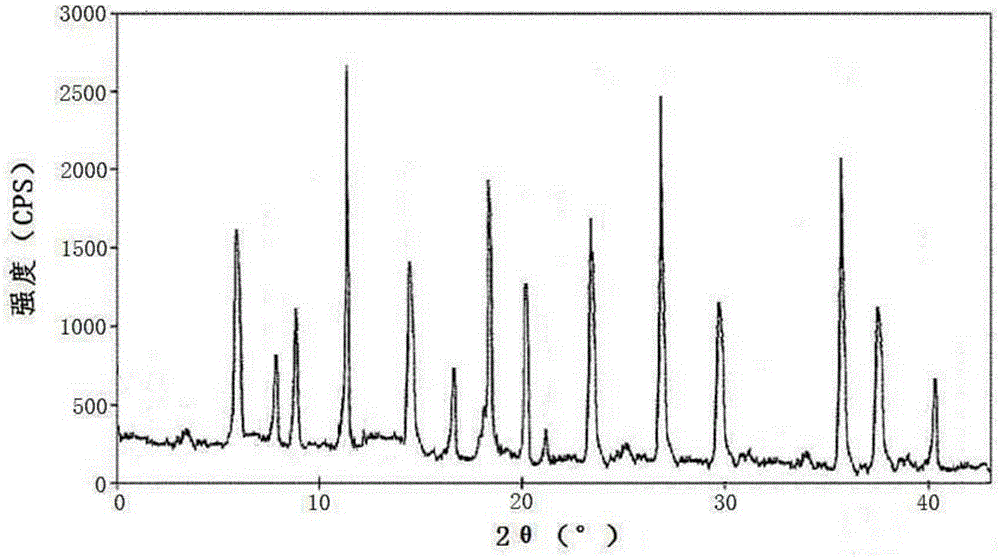
The invention relates to a pharmaceutical composition for treating infectious diseases and a preparation thereof, specifically to a composition of cefazedone sodium for injection. The composition comprises cefazedone sodium and anhydrous sodium carbonate, wherein the cefazedone sodium is a crystal, and in the X-ray powder diffraction pattern of the crystal measured by using Cu-K alpha rays, cefazedone sodium has characteristic peaks at diffraction angles 6.5 DEG, 8.2 DEG, 9.5 DEG, 16.4 DEG, 17.0 DEG, 19.2 DEG, 23.1 DEG, 23.9 DEG, 26.0 DEG, 27.4 DEG, 29.8 DEG, and 32.1 DEG. According to verification results of test, it is found out that compared with prior art, a powder injection prepared from the compound with the novel crystal form has good fluidity, effectively reduced impurity and moisture contents, good security and high stability.
Owner:苗怡文
Anti-infective medicinal cefazedone sodium sterile composition
InactiveCN105055422AEasy to degradeImprove stabilityAntibacterial agentsOrganic active ingredientsPhysical chemistryCefazedone sodium
The invention discloses an anti-infective medicinal cefazedone sodium composition, and belongs to the technical field of medicines. The composition consists of cefazedone sodium and sodium chloride; the cefazedone sodium is crystals; an X-ray powder diffraction pattern obtained by measuring the crystals by using Cu-K alpha rays is shown as figure 1; the main particle size of the crystals of the cefazedone sodium is 150 to 180 mu m, and the distribution width is 135 to 195 mu m. The new crystal form of the cefazedone sodium provided by the invention is different from the crystal form structure in the prior art; through experimental verification, people surprisingly find that powder-injection prepared by utilizing the new crystal form compound is high in mobility compared with the prior art; moreover, the content of impurities and the content of water are effectively reduced; the product is high in safety and high in stability.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
A compound of anti-infective drug and its preparation method
ActiveCN104945419BImprove stabilityImprove liquidityAntibacterial agentsOrganic chemistryState of artCefazedone sodium
The invention belongs to the technical field of medicine and particular relates to a novel compound of an anti-infection drug and a preparation method thereof. According to the compound, characteristic peaks are displayed at diffraction angles of 3.5 degrees, 4.6 degrees, 8.4 degrees, 12.5 degrees, 13.8 degrees, 16.2 degrees, 19.0 degrees, 19.5 degrees, 20.0 degrees, 21.1 degrees, 27.1 degrees and 33.1 degrees in an X-ray powder diffraction pattern obtained through measurement by Cu-KAlpha rays. The invention further relates to powder injection containing 100% cefazedone sodium. The new crystal from of the compound is different from that in the prior art, through experimental verification, it is surprised to find that compared with the prior art, the powder injection obtained by direct subpackage of the compound of the novel crystal form does not need any auxiliary material and is good in liquidity, the content of impurities and moisture is reduced effectively, the safety of products is good, and the stability is high.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Antibacterial agent cefazedone sodium composition
InactiveCN105055421AEasy to degradeImprove stabilityAntibacterial agentsOrganic active ingredientsArginineChemical compound
The invention discloses an antibacterial agent cefazedone sodium composition and belongs to the technical field of medicines. The composition comprises cefazedone sodium and arginine. Cefazedone sodium is crystal, an X-ray powder diffraction diagram obtained by measurement through Cu-Kalpha rays is as shown in . 1, the main particle size of the cefazedone sodium crystal is 200-300 [mu]m, and the distribution width is 185-315 [mu]m. The new crystal form of cefazedone sodium is different from a crystal structure in the prior art. Through test verification, it is found surprisingly pleasantly that compared with the prior art, powder injections prepared by utilizing the new crystal form composition are good in mobility, the content of impurities and moisture is effectively reduced, the product safety is good, and the stability is high.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Synthesis process of cefazedone sodium
InactiveCN111187283ANo pollution in the processThe synthesis process is simpleOrganic chemistryAcetic acidMeth-
The invention relates to a synthesis process of cefazedone sodium, which is characterized in that a thioester compound synthesized by taking 3,5-dichloro-4-pyridone-1-acetic acid and 2-sulfydryl-5-methyl-1,3,4-thiadiazole as raw materials is directly subjected to a one-step reaction with 7-ACA to prepare the cefazedone sodium. The process has the advantages of simplified operation steps, mild production conditions, reduced generation of byproducts, simple production process, improved product yield and purity, reduced cost, small environmental pollution and better environmental friendliness, and enables the process to meet the requirements of industrialization.
Owner:陶志泽
A kind of preparation method of cephalosporin anti-infection medicine
ActiveCN105017286BSimple preparation processReduce generationOrganic chemistry7-ACACefazedone sodium
The invention relates to a preparation method for a cephalosporin anti-infective drug-cefazedone sodium, belonging to the field of pharmaceutical synthesis. According to the invention, the method uses GCLE as a raw material to substitute 7-ACA and overcomes the defects of low yield, high pollution and the like in prior art; the preparation method with mild reaction conditions, little side reaction and simple process is provided; meanwhile, the method has the advantages of cheap and easily-available raw materials, low cost, high product yield, high product purity and applicability to industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Cefazedone sodium frozen powder injection and preparation method thereof
InactiveCN101732264BImprove securityPhysical stabilityAntibacterial agentsPowder deliveryArginineExcipient
Belonging to the field of pharmaceutical preparation, the invention relates to cefazedone sodium frozen powder injection which comprises 45-90% of cefazedone, 5-52% of excipient, 0.02-5% of antioxygen; wherein the excipient is composed of one or more of mannitol, sorbic alcohol or lactose, the antioxygen is composed of one or more of arginine, cysteine hydrochloride or glutathione. The cefazedonesodium frozen powder injection features stable physical and chemical properties, controllable quality; in addition, polymer impurities of the cefazedone sodium frozen powder injection are obviously reduced in the process of storage, thus improving safety of using the injection.
Owner:湖南万健康品生物科技有限公司
A kind of preparation method of cefazedone sodium compound
ActiveCN108084213BHigh yieldHigh reaction yieldOrganic chemistryCefazedone sodiumMedicinal chemistry
The invention discloses a preparation method of a cefazedone sodium compound. 7-ACA and a compound III react to prepare a compound IV, and the compound IV and a compound V have an amidation reaction,and are salified and refined to obtain a competitive product of cefazedone sodium (I). The process route of the reaction is simple, the total yield and the purity are high, and the method is suitablefor industrial production.
Owner:SHANDONG YUXIN PHARMA CO LTD
Novel compound of anti-infection drug and preparation method thereof
ActiveCN104945419AControl the crystallization processImprove stabilityAntibacterial agentsOrganic chemistryState of artCefazedone sodium
The invention belongs to the technical field of medicine and particular relates to a novel compound of an anti-infection drug and a preparation method thereof. According to the compound, characteristic peaks are displayed at diffraction angles of 3.5 degrees, 4.6 degrees, 8.4 degrees, 12.5 degrees, 13.8 degrees, 16.2 degrees, 19.0 degrees, 19.5 degrees, 20.0 degrees, 21.1 degrees, 27.1 degrees and 33.1 degrees in an X-ray powder diffraction pattern obtained through measurement by Cu-KAlpha rays. The invention further relates to powder injection containing 100% cefazedone sodium. The new crystal from of the compound is different from that in the prior art, through experimental verification, it is surprised to find that compared with the prior art, the powder injection obtained by direct subpackage of the compound of the novel crystal form does not need any auxiliary material and is good in liquidity, the content of impurities and moisture is reduced effectively, the safety of products is good, and the stability is high.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
A kind of preparation method of novel anti-infective drug
ActiveCN104086571BReduce moisture contentReduce the production of degradation impuritiesOrganic chemistrySodium acetateSolvent
The invention relates to a preparation method of a novel anti-infective drug. The preparation method comprises the following steps: (1) salifying, namely putting cefazedone and a sodium acetate solvent in absolute methanol to have a salifying reaction, and controlling the temperature in the range of 10-30 DEG C after the cefazedone is completely dissolved, (2) decoloring, namely adding active carbon to a reaction liquid, stirring and decoloring, and removing the active carbon by filtering, keeping the temperature of the filtrate in the range of 10-30 DEG C, and then re-filtering; (3) crystalizing, namely slowly and dropwise adding a certain amount of ethanol to the filtered filtrate at a rate of 20L / H under a stirring condition until the system is turbid, growing crystals for 0.5 to 1 hour, next, adding a certain amount of acetone, growing crystals for 0.5 to 1 hour, and then reducing the temperature to 5 DEG C at a temperature reducing rate of 5-15 DEG C per hour, and stirring by keeping the stirring speed in the range of 80-120r / min for crystallization, and growing the crystals for 1-3 hours; and (4) drying, namely filtering and washing the filter cake, and drying to obtain refined cefazedone sodium. Compared with the prior art, the preparation method is simple to operate, low in cost and high in yield, and pollution is greatly reduced; and the obtained refined cefazedone sodium is low in moisture content, low in impurity content and high in stability.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
A kind of cephalosporin compound and preparation method thereof
ActiveCN105153199BImprove stabilityImprove liquidityOrganic chemistryCefazedone sodiumCombinatorial chemistry
The present invention belongs to the technical field of medicine, and particularly relates to a novel cephalosporin cefazedone sodium crystal form compound and a preparation method thereof. According to the present invention, the cefazedone sodium crystal form compound is a cefazedone sodium hydrate, is different from the cefazedone sodium reported in the prior art, and has the X-ray powder diffraction pattern represented by a Figure 1; and the test results show that the cefazedone sodium hydrate has characteristics of significantly increased stability and good fluidity compared with the cefazedone sodium hydrate in the prior art.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
A kind of preparation method of cefoxizone sodium
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Enzymatic synthesis process of a new cephalosporin anti-infective drug
The invention relates to an enzymatic synthesis technology of a novel cephalo-type anti-infection drug. The technology comprises the following steps: preparing TDA hydrochloride from TDA, purifying, processing to prepare TDA sodium salt, and directly carrying out enzymatic synthesis of cefazedone sodium in a water phase under the action of immobilized penicillin acylase. The enzymatic synthesis technology has the advantages of operating step simplification, implementation of a reaction in the water phase, mild reaction conditions, few byproducts, great reduction of energy consumption and organic wastewater discharge, cost reduction, small pollution to environment, good environmental protection property, and industrialization requirement meeting; and the technology greatly improves the product yield and the product yield, the product purity is greater than 99.9%, and the total yield is greater than 90%.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Preparation method of Cefazedone sodium salt
ActiveCN109535182AImprove stabilitySubstance reductionOrganic chemistrySodium acetateSodium acetrizoate
The invention provides a preparation method of Cefazedone sodium salt, comprising the following main steps: adding Cefazedone acid into a methanol-isopropanol mixed solvent, adding methanol / triethylamine to carry out a salt forming reaction, regulating pH by adding acetic acid, adding sodium acetate and seed crystals to precipitate out sodium salt, growing the grain, adding acetone to completely precipitate out crystals, carrying out programmable heating and vacuum drying to obtain a finished product. an anhydrous system is adopted in the method without the need of humidification, and moisturecan reach 0.5% and below; the content of residual solvent is low, and the contents of methanol, isopropanol and acetone are all below 0.1%; the production cycle is shortened and the production cost is lowered; and there are few crystal impurities and the purity is high.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
A kind of preparation technology of cefoxizone sodium
The invention belongs to the technical field of medicine, and discloses a preparation technology of cefazedone sodium. 3,5-dichloropyridone acetic acid and pivaloyl chloride are taken as the raw materials to synthesize mixed acid anhydrides; and then cefazedone sodium is obtained after salt forming reactions between mixed acid anhydrides and an intermediate prepared from 7-aminocephalosporanic acid and thiol tetrazole. A mixed solvent is used in 3-substitution, the reaction becomes more stable and softer, the byproducts are reduced; in acylation reactions, mixed acid anhydrides are used, the activity is high, 7-acylation reactions are promoted, and thus the yield and purity of cefazedone sodium are high.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Novel cephalosporin compound and preparation method thereof
The present invention belongs to the technical field of medicine, and particularly relates to a novel cephalosporin cefazedone sodium crystal form compound and a preparation method thereof. According to the present invention, the cefazedone sodium crystal form compound is a cefazedone sodium hydrate, is different from the cefazedone sodium reported in the prior art, and has the X-ray powder diffraction pattern represented by a Figure 1; and the test results show that the cefazedone sodium hydrate has characteristics of significantly increased stability and good fluidity compared with the cefazedone sodium hydrate in the prior art.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
A kind of refining method of novel antibiotic compound
The invention relates to a purifying method of a novel antibiotic compound. The method includes: (1) a step of salifying, namely a step of adding cefazedone and sodium isooctoate into absolute methanol and performing a salifying reaction until the cefazedone is fully dissolved, with the temperature being controlled at 15-35 DEG C; (2) a step of decoloring, namely a step of adding active carbon into the reaction solution, stirring, decoloring, filtering to remove the active carbon, maintaining the temperature of the filtrate at 10-30 DEG C, and filtering again; (3) a step of crystallizing, namely a step of adding a certain amount of acetone and ethyl acetate into the filtrate after the filtration, controlling the temperature at 15-20 DEG C, growing the grain for 1-2 h, then adding a certain amount of the acetone and the ethyl acetate, stirring for crystallization with the stirring speed being maintained at 80-120 r / min, controlling the temperature at 15-25 DEG C, and growing the grain for 2-4 h; and (4) a step of drying, namely a step of filtering, washing the filter cake, and drying to obtain a purified product of the cefazedone sodium. Compared with the prior art, the method has characteristics of simple operation, low cost, high yield, and largely reduced pollution. The purified product of the cefazedone sodium has characteristics of low water content, low purity content and high stability.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com