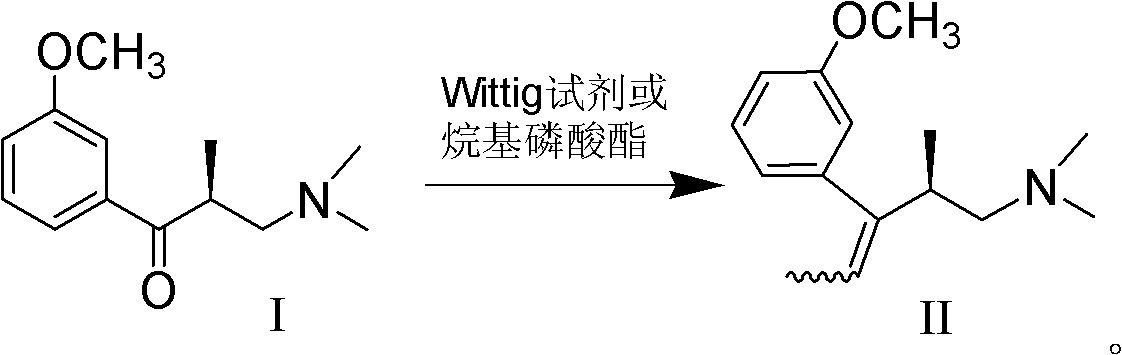
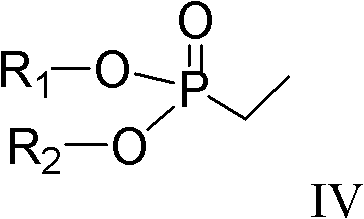
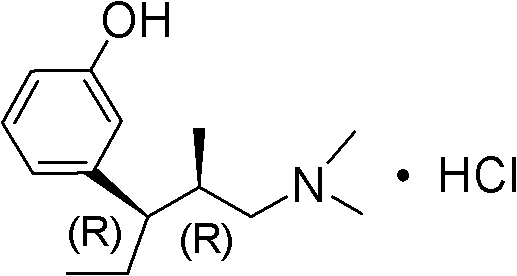
Preparation method of (R)-3-(3-methoxy phenyl)-N,N,2-trimethylpent-3-ene-1-amine
A technology of methoxyphenyl and trimethylpentane, applied in the field of drug synthesis, can solve the problems of cumbersome operation, many steps, and inability to obtain high optical purity, achieve mild reaction conditions, short synthesis route, and avoid reagents and solvents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1, 3-dimethylamino-1-(3-methoxyphenyl)-2-methyl-1-propanone (compound A)
[0035]
[0036] N, N, N', N'-tetramethylmethylenediamine (46.3g, 0.45mol) was added into methyl tert-butyl ether (100ml), and acetyl chloride (37.0g, 0.47mol) was added dropwise under ice-cooling A solution of methyl tert-butyl ether (100ml) was stirred at room temperature for 1h, and the solvent was removed under reduced pressure. Add isopropanol (150ml) to dissolve the residue, continue to add 1-(3-methoxyphenyl)-1-propanone (50.9g, 0.31mol), heat up to reflux under stirring, react for 5h, cool to room temperature, Add water and toluene. The organic layer was discarded, 30% NaOH aqueous solution (50 ml) was added to the aqueous phase, stirred for 10 minutes, 200 ml of ethyl acetate was added, and the layers were separated. The organic layer was washed once with saturated brine, dried over anhydrous sodium sulfate, and concentrated to give oily 3-dimethylamino-1-(3-methoxyphenyl)-2-...
Embodiment 2
[0037] Example 2, (S)-3-(dimethylamino)-1-(3-methoxyphenyl)-2-methylpropan-1-one (I)
[0038]
[0039]Add L-(-)-dibenzoyl tartaric acid monohydrate (111.7g, 0.29mol) into 30ml of methanol and 180ml of acetone, stir to dissolve, then add compound A (64.0g, 0.29mol), at 38°C Stir for 48h, cool to room temperature, precipitate a large amount of white solid salt, filter with suction, wash the filter cake with acetone, and obtain 126.0g (S)-3-(dimethylamino)-1-(3-substituted oxybenzene) after drying Base)-2-methylpropan-1-one·L-(-)-dibenzoyl tartrate, the yield was 75%.
[0040] The above white solid was added to 500ml of methyl tert-butyl ether, 67ml of diethylamine was added under stirring at room temperature, and stirring was continued for 1h. Concentrate to dryness by suction filtration to obtain 45.5g light yellow oil, compound of formula I, its MS-ESI (m / z): 222 (M+1); 1 H-NMR (400MHz, CDCl 3 )δ: 7.56(d, J=8.0Hz, 1H), 7.52(s, 1H), 7.38(t, J=8.0Hz, 1H), 7.10(d, J=8.0...
Embodiment 3
[0041] Example 3, (R)-3-(3-methoxyphenyl)-N,N,2-trimethylpent-3-en-1-amine (II)
[0042]
[0043] At room temperature, ethyltriphenylphosphine bromide (26.7g, 72mmol) was added to methyl tert-butyl ether (150ml), potassium tert-butoxide (8.1g, 72mmol) was added under stirring, and stirring was continued for 1h. After cooling in an ice bath to below 20°C, a solution of I (13.3g, 60mmol) in methyl tert-butyl ether (50ml) was added dropwise. After stirring at room temperature for 2 hours, cool down in an ice bath, slowly add 100ml of water, separate the liquids, concentrate the organic phase, then extract with petroleum ether, wash with water, dry over anhydrous sodium sulfate, filter, and concentrate to obtain 13.5g of the crude compound of formula II in the form of light yellow oil .
[0044] The crude compound of formula II above was dissolved in 200ml of ethyl acetate, and 12ml of ethyl acetate solution of hydrogen chloride with a concentration of 6mol / L was added dropwis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com