Beta-cyclodextrin derivative and preparation method and application thereof
A technology of cyclodextrin and derivatives, which is applied in the field of β-cyclodextrin derivatives and its preparation, can solve the problems of limited application and achieve the effects of complete series, high ee% value and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
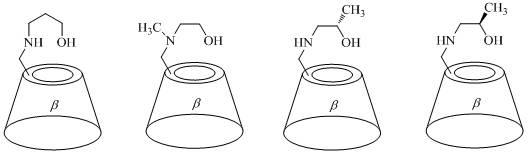
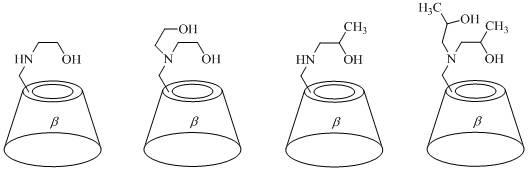
[0030] In a 100mL eggplant-shaped reaction flask, 6.4459g (5.0mmol) mono(6- O - p -tosyl)- β - Cyclodextrin was dissolved in 22.9050 g (375 mmol) ethanolamine, and stirred and reacted at 120° C. for 12.0 h. Cool to room temperature, add 20 mL of water to dilute, and inject the resulting reaction mixture into 400 mL of V 乙醇 :V 丙酮 = 1:1 mixed solution, a large amount of white solids precipitated, stood still, filtered with suction, and recrystallized the obtained solids in 10mL of water. The obtained crystals were vacuum-dried at 120°C for 12.0 hours to obtain 3.6875 g of white crystals with a purity of 96.09% and a yield of 60.15%.
[0031] CD-1: [α]25 D= +150.54° ( c = 0.8020, H 2 O); m.p. > 250°C (decomp); 1 H NMR (400MHz, D 2 O): δ = 5.11-5.09 (dd, J = 6.9, 3.4Hz , 7H), 4.02-3.88 (m, 26H), 3.76-3.58 (m, 14H), 3.49-3.44 (m, 1H), 3.12-3.09 (m, 1H), 2.90-2.75ppm ( m, 4H); 13 C NMR (400MHz, D 2 O): δMS (ESI): m / z : 1200.5 [ M +Na] + , 1178.4 [ M +H] + ....
Embodiment 2
[0033] In a 100mL eggplant-shaped reaction flask, 6.4459g (5.0mmol) mono(6- O - p -tosyl)- β - Cyclodextrin was dissolved in 39.4275g (375mmol) of diethanolamine, stirred and reacted at 120°C for 12.0h. Cool to room temperature, add 20 mL of water to dilute, and inject the resulting reaction mixture into 400 mL of V 乙醇 :V 丙酮 = 1:1 mixed solution, a large amount of white solids precipitated, stood still, filtered with suction, and recrystallized the obtained solids in 10mL of water. The obtained crystals were vacuum-dried at 120°C for 12.0 hours to obtain 2.6862 g of white crystals with a purity of 97.08% and a yield of 42.68%.
[0034] CD-2: [α]25 D= +140.90° ( c = 0.8060, H 2 O); m.p. > 260°C (decomp); 1 H NMR (400MHz, D 2 O): δ = 5.15-5.06 (m, 7H), 4.04-3.87 (m, 26H), 3.74-3.58 (m, 14H), 3.42 (t, J = 9.3Hz, 1H), 3.09-3.05 (m, 1H), 2.86-2.69ppm (m, 8H); 13 C NMR (400MHz, D 2 O): δ = 101.78, 100.95, 83.42, 81.11-80.87 (m), 80.27, 73.19-72.75 (m), 72.10-71.6...
Embodiment 3
[0036] In a 100mL eggplant-shaped reaction flask, 6.4459g (5.0mmol) mono(6- O - p -tosyl)- β - Cyclodextrin was dissolved in 28.1663g (375mmol) of isopropanolamine, stirred and reacted at 120°C for 12.0h. Cool to room temperature, add 20 mL of water to dilute, and inject the resulting reaction mixture into 400 mL of V 乙醇 :V 丙酮 = 1:1 mixed solution, a large amount of white solids precipitated, stood still, filtered with suction, and recrystallized the obtained solids in 10mL of water. The obtained crystals were vacuum-dried at 120° C. for 12.0 h to obtain 3.1365 g of white crystals with a purity of 95.41% and a yield of 50.21%.
[0037] CD-3: [α]25 D= +147.81° ( c = 0.4860, H 2 O); m.p. > 250°C (decomp); 1 H NMR (400MHz, D 2 O): δ = 5.09 (t, J = 3.6Hz, 7H), 4.02-3.88 (m, 26H), 3.69-3.58 (m, 14H), 3.46 (t, J = 9.4Hz, 1H), 3.14-3.06 (m, 1H), 2.89-2.77 (m, 1H), 2.67-2.55 (m, 2H), 1.21-1.17ppm (m, 3H); 13 C NMR (400MHz, D 2 O): δ = 101.85, 100.34, 83.58, 81.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com