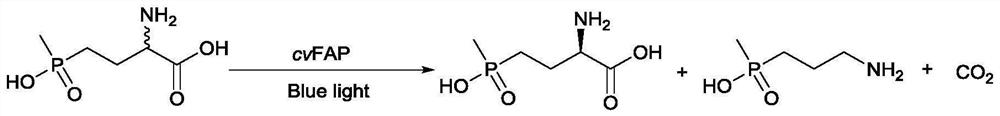
Fatty acid photodecarboxylase mutant and application thereof to synthesis of L-phosphinothricin
A photodecarboxylase, fatty acid technology, applied in the direction of application, enzyme, lyase, etc., can solve the problems of low substrate concentration, low L-PPT activity, etc., and achieve the effect of good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Construction and screening of fatty acid photodecarboxylase mutant library
[0041] The fatty acid photodecarboxylase gene (the amino acid sequence is shown in SEQ ID No.2, the nucleotide sequence is shown in SEQ ID No.1) was constructed as an expression vector pET28b-CvFAP, transformed into Escherichia coli, and the starting strain E.coli BL21 (DE3 ) / pET28b-CvFAP.
[0042] The fatty acid photodecarboxylase mutant library was prepared by three rounds of site-directed saturation mutagenesis, and the primers were designed as shown in Table 1.
[0043] In the first round, the vector pET28b-CvFAP was used as a template, and the site-directed saturation mutation primers G402NYT-F and G402NYT-R in Table 1 were used as primers, and the fatty acid photodecarboxylase amino acid sequence shown in SEQ ID No.2 was subjected to saturation mutation PCR. The 402th threonine is mutated into one of alanine, isoleucine, phenylalanine, proline, serine, threonine and valine, and transf...
Embodiment 2
[0062] Example 2 Fatty acid photodecarboxylase mutant CvFAP-G402F-T370R-S513G strain catalyzes the decarboxylation of D-glufosinate-ammonium in racemic glufosinate-ammonium to prepare L-glufosinate-ammonium
[0063] Use the CvFAP-G402F / T370R / S513G mutant to produce optically pure L-PPT in grams at 25 °C in a total volume of 50 mL pH 6, 100 mM phosphate buffer, 10 μL 100 mM alkane molecule solution, 25 mL CvFAP crude enzyme solution (0.25 g wet cells in 10 mL pH 8.5, 100 mM phosphate buffer) and their solution for photobiocatalytic decarboxylation by D / L-PPT reaction. Add 100 mg D / L-PPT to a clear glass beaker (total volume 100 mL). Under gentle magnetic stirring, the beaker was exposed to blue LED light. After 12 h, an aliquot was taken for determination of conversion and e.e.
[0064] 0.1 g of D / L-PPT was dissolved in pH 6 phosphate buffer (100 mM) and decarboxylated under blue light. After 12 hours, the conversion rate was 50 and the e.e value was 96%. The product has bee...
Embodiment 3
[0065] Example 3 Fatty Acid Photodecarboxylase Mutant CvFAP-G402F-T370R-S513G Strain Whole Cell Catalyzed Decarboxylation of D-Glufosinate in Racemic Glufosinate-ammonium to Prepare L-glufosinate-ammonium
[0066] Use the CvFAP-G402F / T370R / S513G mutant to produce optically pure L-PPT in grams at 25 °C in a total volume of 50 mL pH 7, 100 mM phosphate buffer, 10 μL 100 mM alkane molecule solution, 25 mL CvFAP crude enzyme solution (0.75 g wet cells in 10 mL pH 7, 100 mM phosphate buffer) and their solution for photobiocatalytic decarboxylation by D / L-PPT reaction. Add 500 mg D / L-PPT to a clear glass beaker (total volume 100 mL). Under gentle magnetic stirring, the beaker was exposed to blue LED light. After 16 h, an aliquot was taken for determination of conversion and e.e.
[0067] 0.5g D / L-PPT was dissolved in pH 7 phosphate buffer (100mM) and decarboxylated under blue light. After 16h, the conversion rate was 50 and the e.e value was 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com