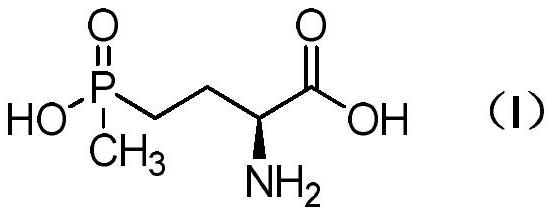
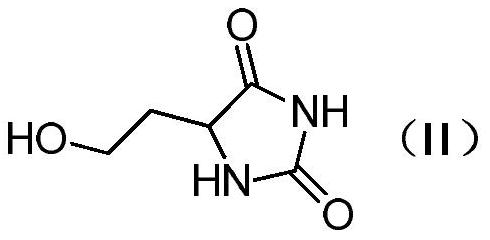
Method for preparing L-glufosinate-ammonium
A technology of glufosinate-ammonium and methylphosphine dichloride, applied in the field of preparation of L-glufosinate-ammonium, can solve the problems of difficult separation, low yield, poor substrate tolerance, etc., and achieve easy-to-obtain raw materials and simple steps , cost controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Synthesis of compound 2
[0041]
[0042] In a nitrogen atmosphere, add 27.1g of compound (1) (90% ee value), 38.1g of triethylamine and 100mL of trimethylbenzene into a three-necked flask, drop down to -20°C, add 10g of methylphosphine dichloride dropwise, and keep - The reaction was stirred at 20°C, and the reaction solution was monitored by Ms. After the raw materials were completely reacted, trimethylbenzene and triethylamine were distilled off under reduced pressure to obtain 28.1 g of crude product compound (2). The molar yield based on compound (1) was 90%. The crude product compound (2) was directly used in the next step without further purification.
[0043] (2) Synthesis of compound 3
[0044]
[0045] Under a nitrogen atmosphere, compound (2) was dissolved in trimethylbenzene (120 mL), and 1.2 g of granular iodine was added. The temperature was raised to 150° C., and the reaction was stirred until the raw materials were completely reacted. Trimet...
Embodiment 2
[0052]According to the method of Example 1, the base type, solvent type and reaction temperature in step (1) were changed, and the results are shown in Table 1 below.
[0053] The molar yield in the table is the molar yield of compound (2) based on compound (1).
[0054] Table 1
[0055]
Embodiment 3
[0057] According to the method of Example 1, the catalyst type, solvent type and reaction temperature in step (2) were changed, and the results are shown in Table 2 below.
[0058] The molar yield in the table is the molar yield of compound (3) based on compound (2).
[0059] Table 2
[0060]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com