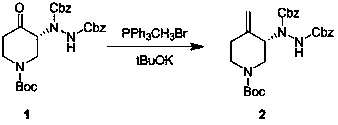Preparation method of (3S, 4R)-3-amido-4-methyl piperidine-1-tertiary butyl carboxylate
A technology of tert-butyl carboxylate and methylpiperidine, which is applied in the new preparation field of compound-3-amino-4-methylpiperidine-1-tert-butyl carboxylate, can solve the problem of long synthetic route and three-dimensional Poor selectivity and other problems, to achieve the effect of long synthetic route, poor stereoselectivity and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 : Operation steps: In a 100 ml single-necked bottle, triphenylmethylphosphonium bromide (1.3 g, 3.6 mmol) was dissolved in 20 ml of chloroform, and potassium hydroxide (0.37 g, 6.63 mg Moore). (R)-1-(1-tert-butoxycarbonyl)-4-oxo-3-piperidinylhydrazine-1,2-dicarboxylate dibenzyl ester was dissolved in (1.5 g, 3.01 mmol) in 15 mL of Slowly add chloroform into the reaction flask, stir at room temperature (20-30°C) for 20 hours, pour the reaction solution into ice water to quench, add dichloromethane for extraction, wash the organic phase with saturated brine, and dry over anhydrous sodium sulfate , purified by column chromatography, the eluent was petroleum ether: ethyl acetate = 5:1, and 0.25 g of a white solid product was obtained with a yield of 16%.
Embodiment 2
[0029] Example 2 : Operation steps: In a 100-ml single-necked bottle, triphenylmethylphosphonium bromide (1.3 g, 3.6 mmol) was dissolved in 20 ml of chloroform, and sodium methoxide (0.36 g, 6.63 mg Moore). (R)-1-(1-tert-butoxycarbonyl)-4-oxo-3-piperidinylhydrazine-1,2-dicarboxylate dibenzyl ester was dissolved in (1.5 g, 3.01 mmol) in 15 mL of Slowly add chloroform into the reaction flask, stir at room temperature (20-30°C) for 20 hours, pour the reaction liquid into ice water to quench, add ethyl acetate for extraction, wash the organic phase with saturated brine, and dry over anhydrous sodium sulfate , purified by column chromatography, the eluent was petroleum ether: ethyl acetate = 5:1, and 0.15 g of a white solid product was obtained with a yield of 10.6%.
Embodiment 3
[0030] Example 3 : Operation steps: In a 100 ml three-necked flask, triphenylmethylphosphonium bromide (2.6 g, 7.2 mmol) was dissolved in 36 ml of tetrahydrofuran, and sodium hydrogen (0.26 g, 6.63 mmol) was added at zero degrees Celsius. Dibenzyl (R)-1-(1-tert-butoxycarbonyl)-4-oxo-3-piperidinylhydrazine-1, 2-dicarboxylate was dissolved in 50 ml of THF and slowly added to the reaction flask , stirred at room temperature (20-30°C) for 4 hours, the reaction solution was quenched by pouring into ice water, extracted with ethyl acetate, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, purified by column chromatography, and the eluent was petroleum Ether: ethyl acetate = 5:1, 0.5 g of white solid product was obtained, yield 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com