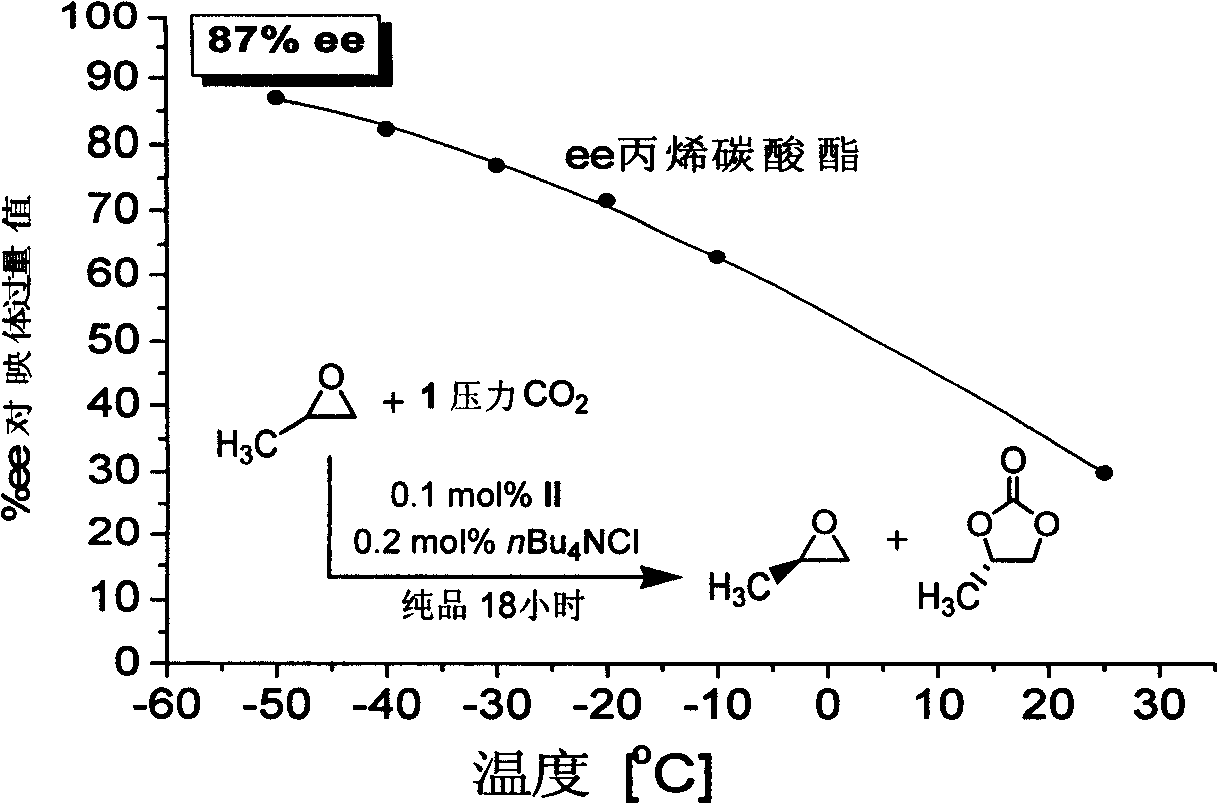
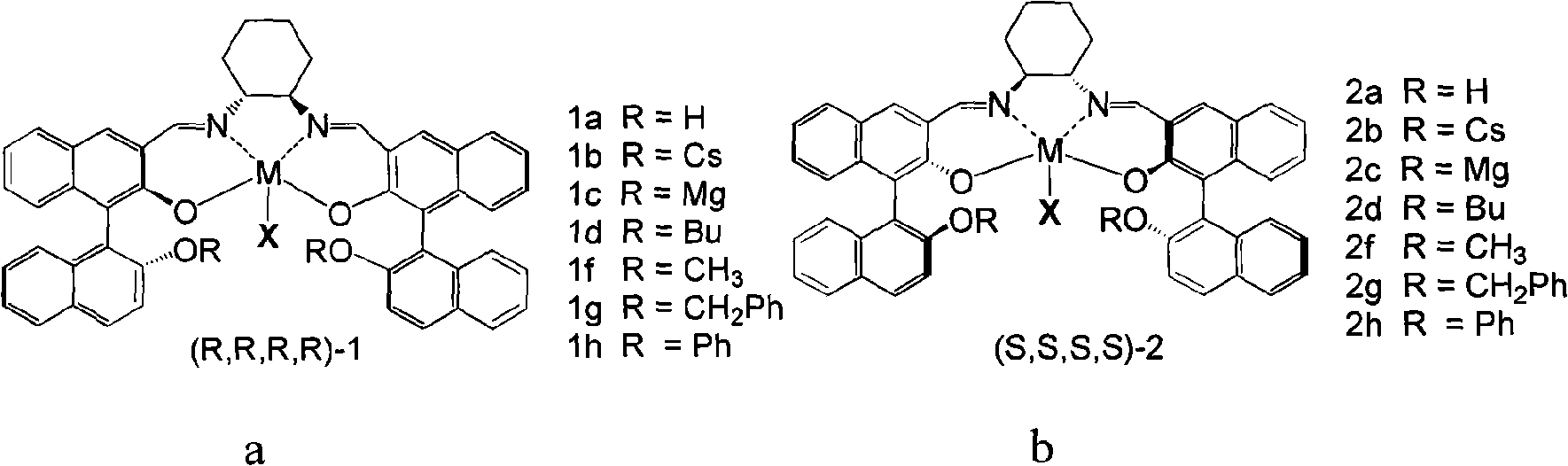
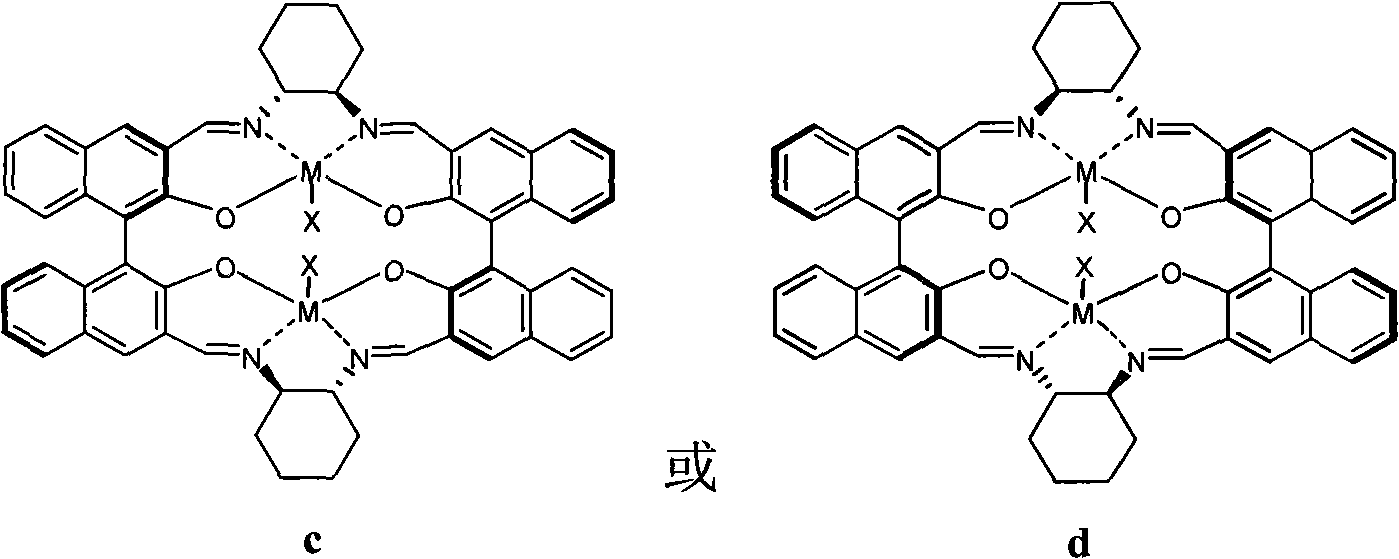
Preparation of multi-chiral catalyst, preparation and application of cyclic carbonates with optical activity
A cyclocarbonate and optically active technology, which is applied in the field of preparation of highly optically active cyclocarbonate, can solve the problems of exaggerated catalyst performance and achieve the effect of easy acquisition, simple process and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In this embodiment, the catalyst 1aCo(OAc) is taken as an example. 1aCo(OAc) refers to the catalyst in the a structure described in formula 3, R=H, M=Co, X=OAc, referred to as 1a. The following 1b refers to the catalyst prepared with R=Cs in the structure of formula 3a, 1c refers to the catalyst prepared with R=Mg in the structure of formula 3a, ..., 2h refers to the catalyst prepared with R=Ph in the b structure of formula 3 Catalysts, 3 and 4 respectively refer to the catalysts prepared with the c structure and d structure in formula 4.
[0044] 1) (+)-(R)-3-formyl-2,2'-dihydroxy-1,1'-binaphthyl (0.314g, 1mmol), dissolved in 4ml ethanol by heating, cooled to room temperature, ( -)-(1R,2R)-Tartrate (0.5mmol) of cyclohexanediamine was dissolved in 0.5ml of water, and 0.5ml of ethanol was added. At this time, a solid appeared, and the supernatant was added to (+)- In (R)-3-formyl-2,2'-dihydroxy-1,1'-binaphthyl, a pink solid appeared, stirred at room temperature for 24 ...
Embodiment 2
[0051] In a stainless steel autoclave with an effective volume of 100ml, add successively: 0.1mmol 1dCo (OAc), 0.1mmol phenyltrimethylammonium tribromide (PTAT), 29 grams (500mmol) propylene oxide (in this embodiment, use Represent racemic propylene oxide, hereinafter the same), then pass into 0.8Mpa carbon dioxide gas. Control the temperature at 25°C, react under magnetic stirring for 24 hours, slowly let off the unreacted carbon dioxide in the autoclave, distill 11 grams of propylene carbonate under reduced pressure, the yield is 21.6%, and modify β by γ-butyl - Cyclodextrin gas chromatography column (30m x 0.25mm x 0.25mm) measured on a Varian CP-3800 chromatogram with an e.e. value of 60.0%. In the reaction, the ratio of co-catalyst to main catalyst is 5-1:; the ratio of main catalyst to alkylene oxide is 0.05%-1%; the pressure of carbon dioxide is 0.1-2Mpa.
Embodiment 3
[0053] In the same equipment used in Example 1, under the same conditions, just using 1fCo(OAc) as a catalyst, after reacting at 25°C for 48 hours, 15 grams of propylene carbonate were obtained, with an e.e. value of 55.3%, yield was 29.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com