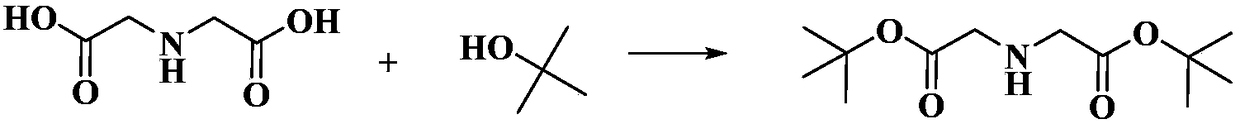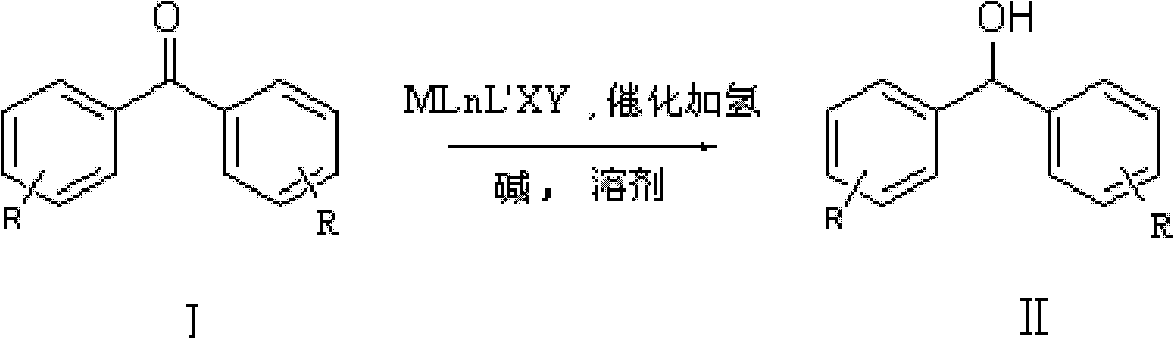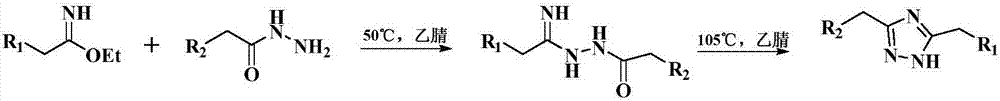Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about How to "Simple post-reaction handling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing polyol by using bio-oil and application
ActiveCN104341297AWide variety of sourcesPromote degradationOrganic compound preparationPolyureas/polyurethane adhesivesPtru catalystPotassium fluoride
The invention discloses a method for preparing polyol by using bio-oil. The method comprises the following steps: firstly performing methyl esterification, namely, performing ester exchange on biolipid and methanol under the catalysis of potassium fluoride loaded magnesium oxide solid alkali, converting the obtained product into fatty acid methyl ester with small molecular weight and byproduct glycerol, filtering to recycle the catalyst, and separating lower-layer glycerol; performing epoxidation on upper-layer fatty acid methyl ester in 30% of hydrogen peroxide under the catalysis of ionic liquid so as to form epoxidized fatty acid methyl ester.; then adding the glycerol in the methyl esterification process, continuously performing alkoxide ring-opening under the catalysis of ionic liquid, introducing hydroxyl, and finally separating liquid to recycle the ionic liquid catalyst, reducing pressure and distilling the upper-layer to remove water so as to obtain low-viscosity bio-oil-based polyol. The raw material is easily available, recyclable and good in biodegradability, the preparation process is environmental friendly, the industrial three-waste emission is small, the product structure and a hydroxyl value are adjustable, the application range is wide, and the environment influence level is low.
Owner:ZHEJIANG HENGFENG NEW MATERIAL
Method for preparing diphenyl carbinol and derivatives thereof
ActiveCN102241566ALess quantitySmooth responseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenation reactionSolvent
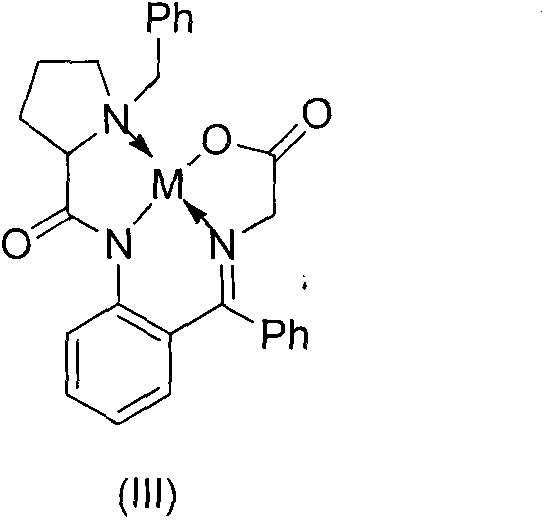
The invention discloses a method for preparing diphenyl carbinol and derivatives thereof. In an alkali and solvent environment, diphenyl ketone and derivatives thereof are subjected to a hydrogenation reaction in the presence of a transition metal complex serving as a catalyst to form the diphenyl carbinol and derivatives thereof, wherein the general formula of the transition metal complex is MLnL'XY and is a transitional metal pnictide formed by a ligand having a NH2-N(SP2) or NH2-NH2 structural characteristic and a transition metal. When the method for preparing the diphenyl carbinol and derivatives thereof is used, a small amount of catalyst is used, the reaction process is stable, the conversion rate is over 98 percent, the very few by-products are produced in the reaction, the treatment after reaction is simple, the whole process period is short, the cost is low, and the large-scale production can be realized easily.
Owner:ENANTIOTECH CORP
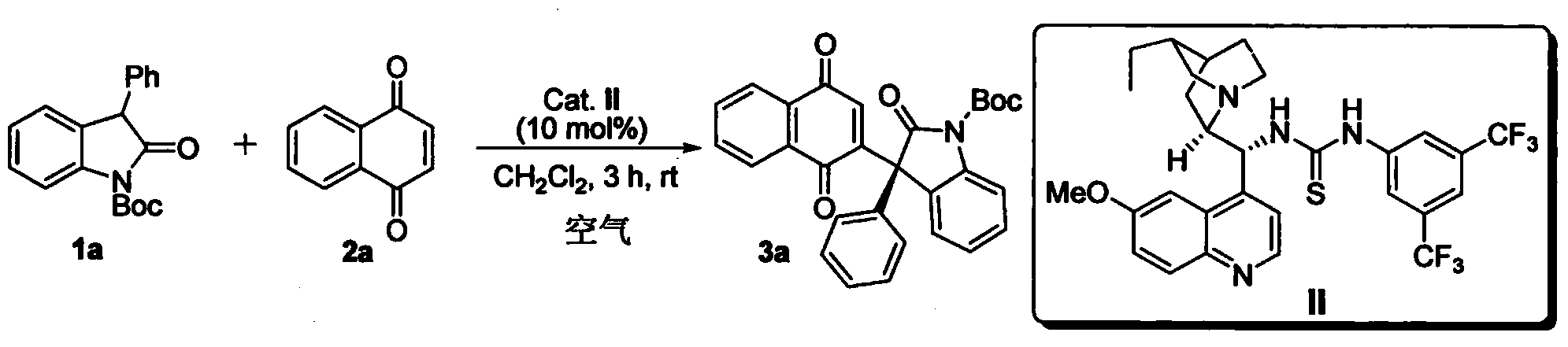
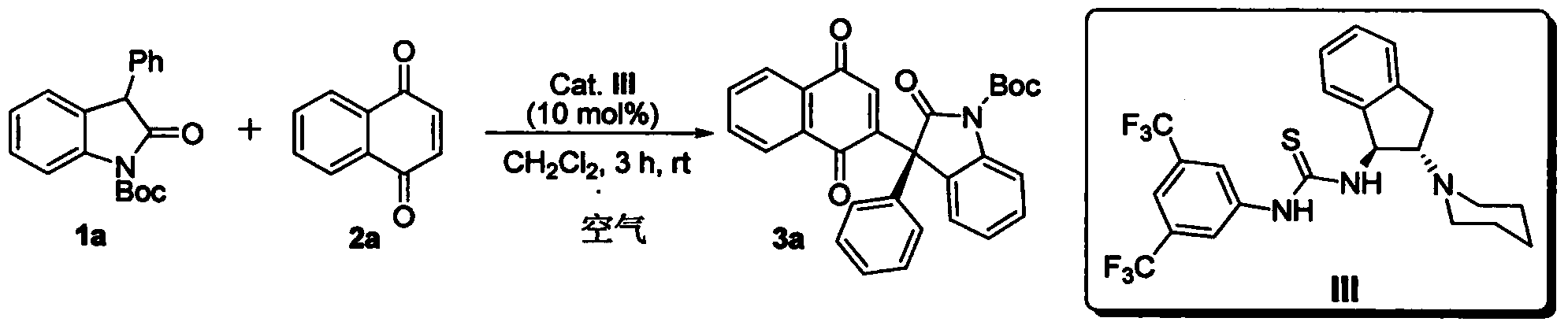
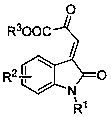
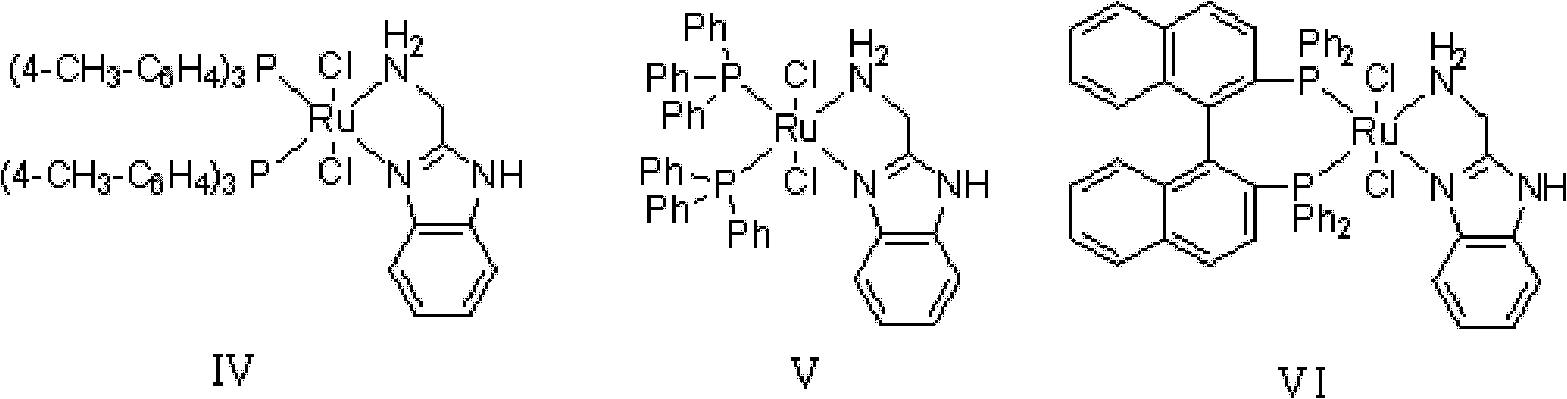
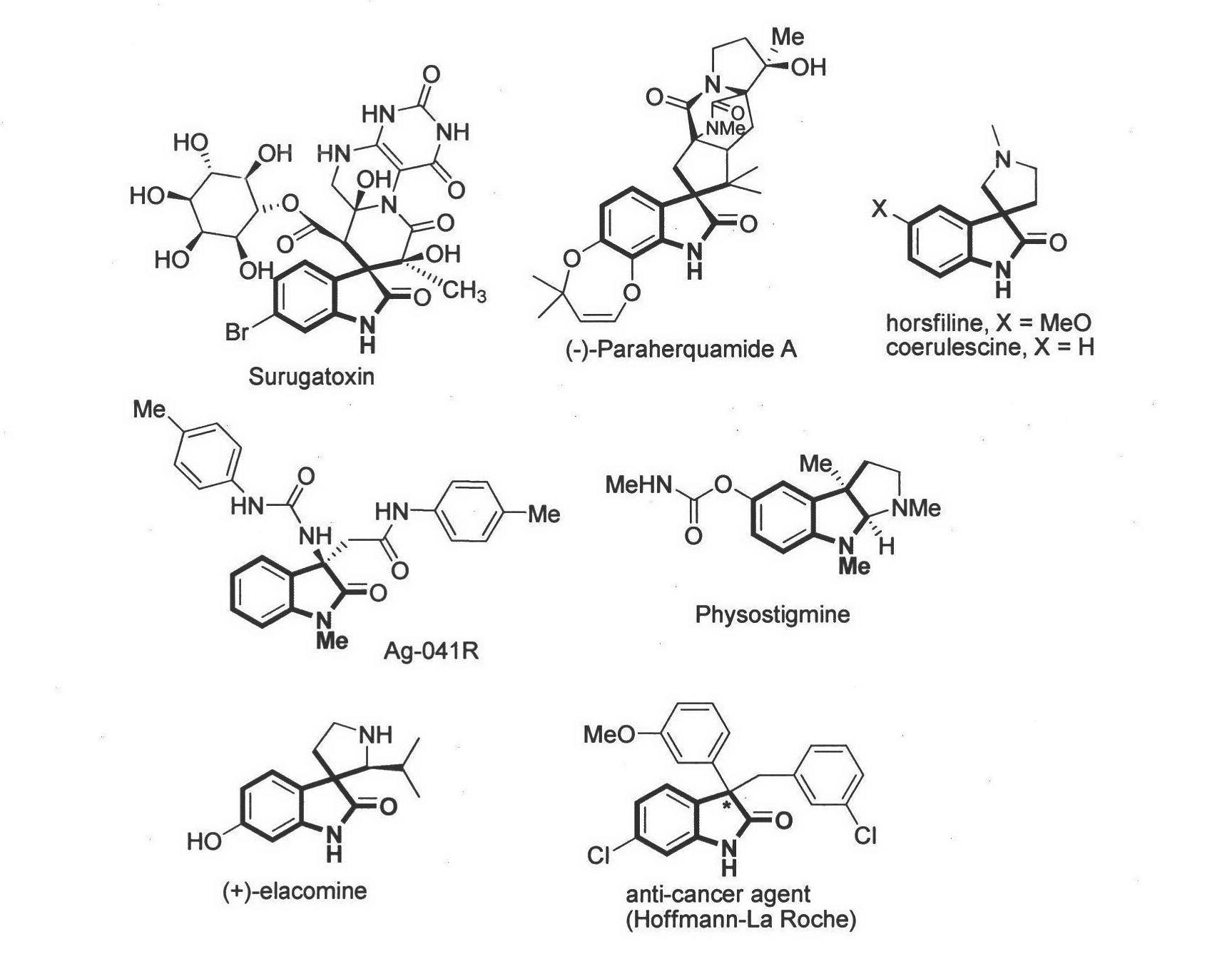
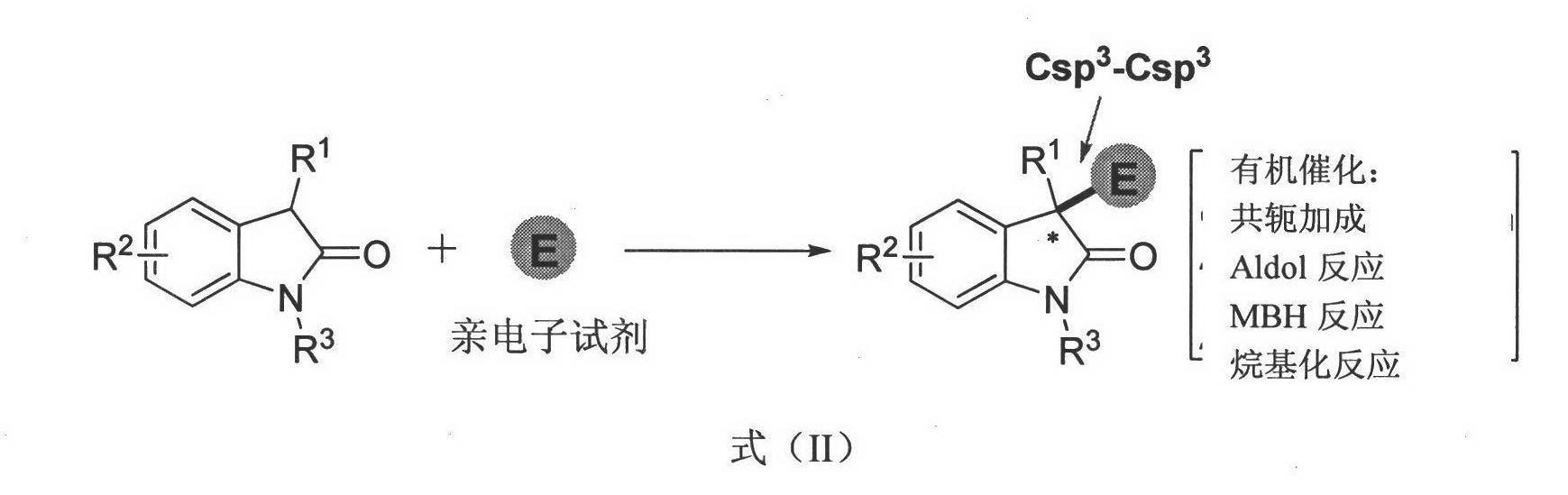
Method for asymmetric synthesis of 3,3-disubstituted-2-oxindole compound
InactiveCN102659494AHigh ee valueSimple post-reaction handlingAsymmetric synthesesNatural productEnantioselective synthesis
The invention discloses a method for asymmetric synthesis of a 3,3-disubstituted-2-oxindole compound. The method is characterized in that a 3-monosubstituted-2-oxindole compound and a 1,4-naphthoquinone compound as reaction raw materials undergo a reaction in the presence of chiral organic catalysts in air to produce the 3,3-disubstituted-2-oxindole compound. The method has mild reaction conditions and adopts easily available raw materials. The 3,3-disubstituted-2-oxindole compound obtained by the method has a very high ee value, provides a key skeleton structure for the synthesis of many natural products and drugs, and can be widely used for large-scale industrial production.
Owner:EAST CHINA NORMAL UNIV +1
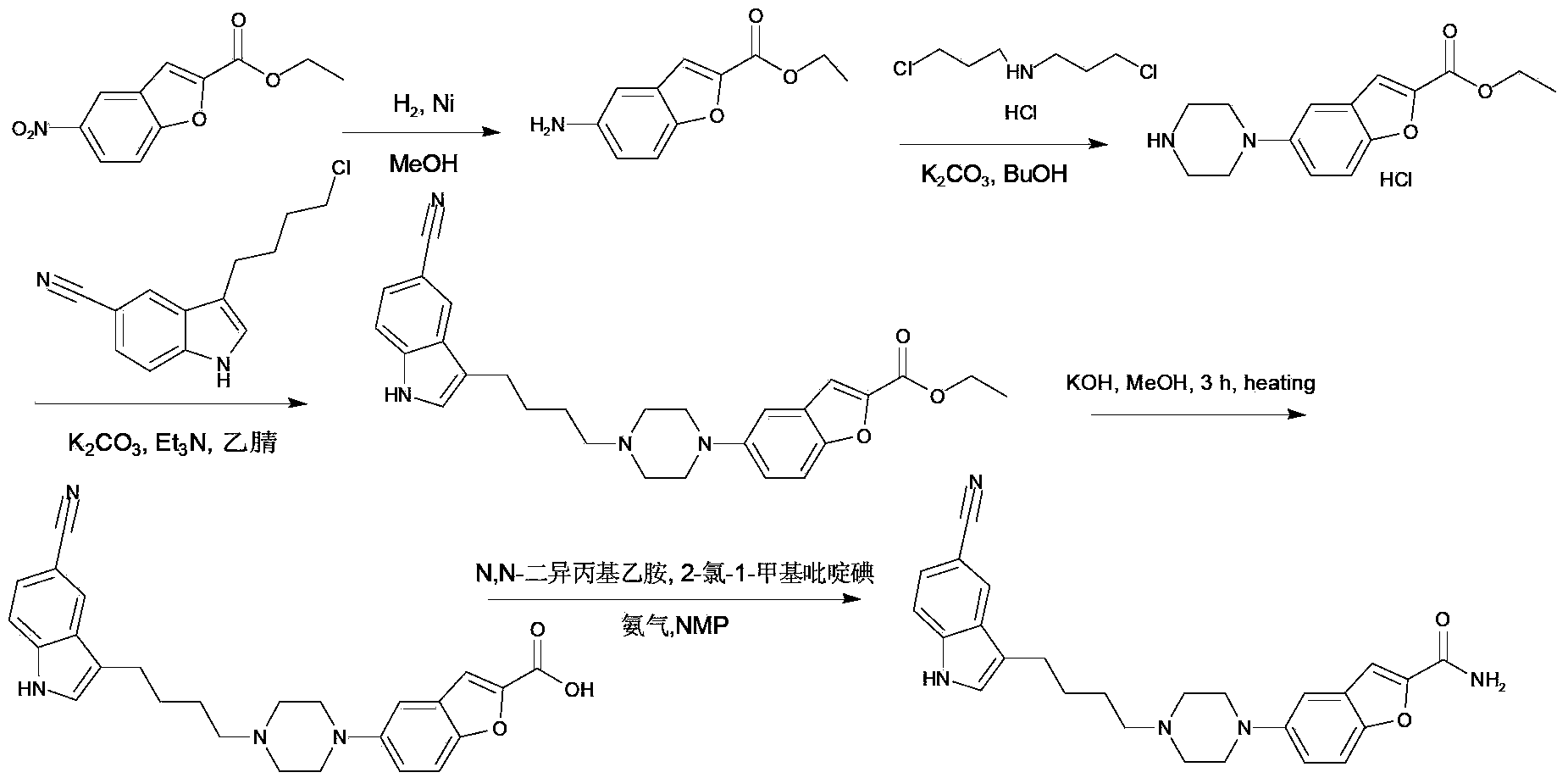
Synthesis method of vilazodone and salt thereof
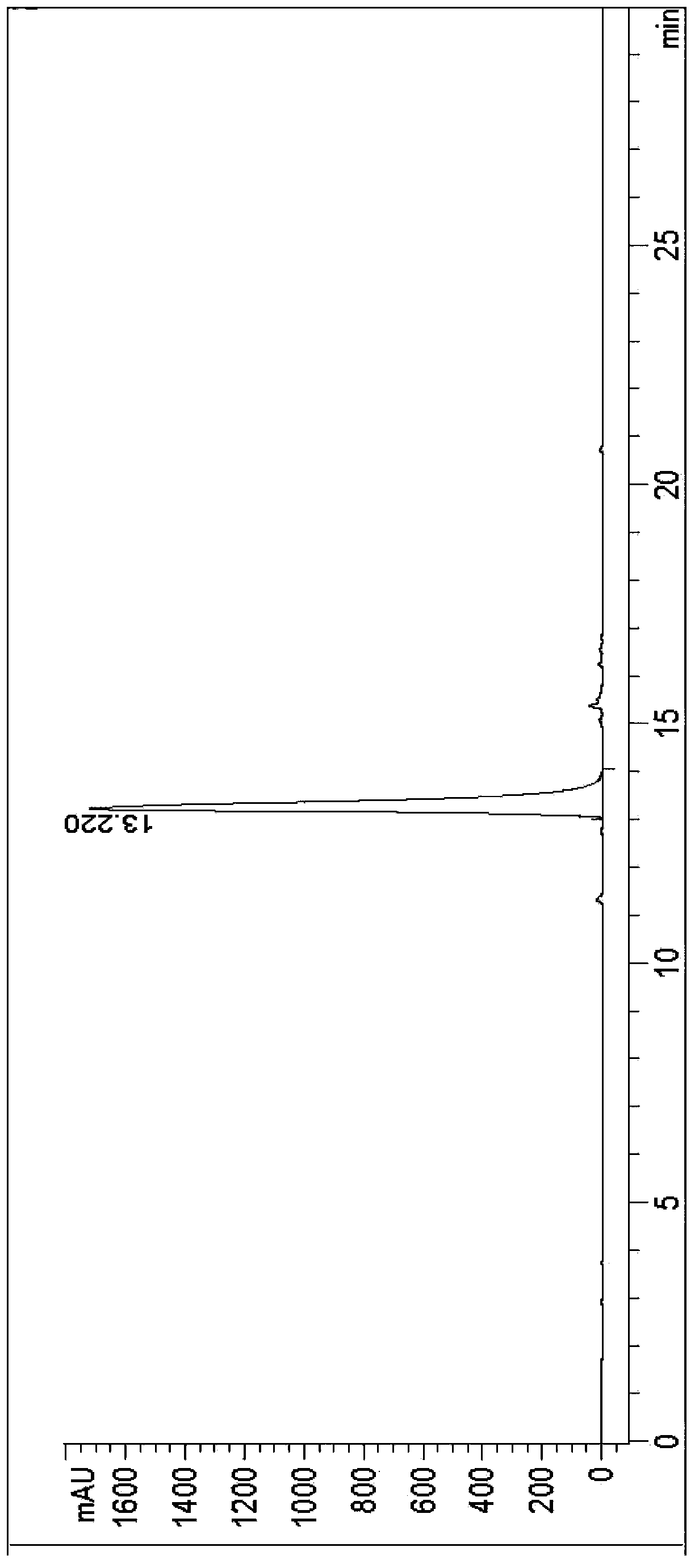
ActiveCN103360374AFew reaction stepsSimple post-reaction handlingOrganic chemistryDrugs synthesisAqueous solution
The invention relates to a synthesis method of vilazodone and a salt thereof, belonging to the technical field of drug synthesis. The synthesis method comprises the following steps of: with 5-fluorin-2-hydroxybenzaldehyde as a raw material, subjecting the 5-fluorin-2-hydroxybenzaldehyde and acetobromamide to reaction under the action of an acid binding agent to obtain a compound as shown in the formula (I); subjecting piperazine and the compound (I) as shown in the formula (I) to reaction at the temperature of 100-140 DEG C under the action of alkaline to obtain a compound as shown in the formula (II); and subjecting 3-(4-chlorobutyl)-5-cyanoindole and the compound as shown in the formula (II) to reflux reaction for 14-18h under the actions of a catalyst and alkaline, and then, adding the product into an aqueous alkaline solution until a solid is separated out to obtain a compound as shown in the formula (III), namely the vilazodone, wherein the compounds as shown in the formulas (I), (II) and (III) respectively have the structural formulas in the specification. The synthesis method is low in production cost, environment-friendly, high in conversion ratio, few in byproducts, high in product yield and purity, good in quality and suitable for large-scale industrial production.
Owner:SUZHOU UUGENE BIOPHARMA
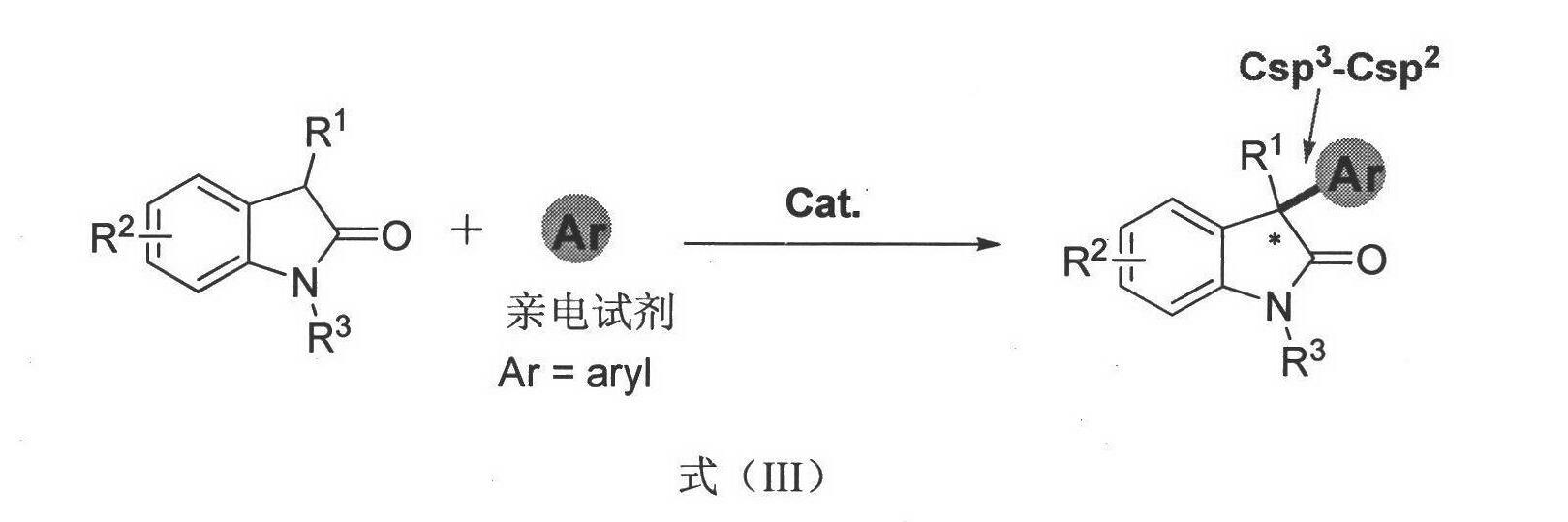
Chiral bridge ring skeleton oxindole piperidine compound and synthesis method of compound
InactiveCN107602577ASimple and efficient operationGood yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSolventImide
The invention discloses a chiral nitrogen oxygen bridge ring skeleton and spiro oxindole compound and a synthesis method of the compound. The method specifically comprises the following steps: takinga 3-pyrrolyl oxindole and beta,gamma-unsaturated alpha-keto ester derived from salicylaldehyde as a reactant, and in the presence of a chiral thiourea compound and bis(trifluoromethanesulfonyl)imide,performing an asymmetric tandem cyclization reaction in a methyl tert-butyl ether and toluene solvent to obtain a product. The method disclosed by the invention has simple and easily available raw materials, mild reaction conditions, simple and convenient post-treatment, wide application range, good yield, and high enantioselectivity and non-enantioselectivity; and therefore, the synthesized product has a potential medicinal value.
Owner:SUZHOU UNIV
Preparation method of hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative
InactiveCN102070638AMild conditionsSimple post-reaction handlingOrganic chemistrySulfonyl chlorideSynthesis methods
The invention relates to a preparation method of a hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative, which mainly solves the technical problems of long route, low yield, difficult purification and difficult repetition and amplification of reaction of the conventional synthesis method. The preparation method comprises the following steps of: firstly, performing a 1,3-dipolar cycloaddition on monoethyl maleate and N-methoxy-methyl-N-(trimethylsilyl methyl) benzyl amine which serve as raw materials to obtain a benzyl-protected pyrrole ring so as to finish a basic skeleton of an intermediate; secondly, reducing carboxylic acid into hydroxyl groups by using borane, converting the hydroxyl groups into activated ester by using methane sulfonyl chloride, and alkylating 'N'; and finally, reducing azido ester groups under a hydrogenation condition, and closing the ring to obtain the hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative. The hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative prepared by the method is a useful intermediate or product of the synthesis of a number of medicaments.
Owner:上海药明康德新药开发有限公司 +1
Synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and salt thereof
InactiveCN103193658AReduce pollutionSuitable for industrialized mass productionOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsPhenethyl alcohol
The invention provides a synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and salt thereof, belongs to the technical field of medicine synthesis and solves the problems that in the prior art the cost is high, the product yield is low, the synthesis method is not applicable to large-scale industrial production, and the like. The synthesis method comprises the following steps of: under the action of an oxidant, oxidizing hydroxyl on p-nitrobenzene ethanol into an aldehyde group so as to obtain p-nitrobenzene acetaldehyde; under the action of a reducing agent, carrying out dehydration, condensation and reduction on amino on (R)-2-amino-1-phenethyl alcohol and the aldehyde group on p-nitrobenzene acetaldehyde so as to obtain (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol; and mixing a rough product of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol with an acid so as to generate precipitate or stirring till solid (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol salt is separated out. The synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and the salt thereof is low cost and high in product yield, and is applicable to large-scale industrial production.
Owner:SUZHOU UUGENE BIOPHARMA
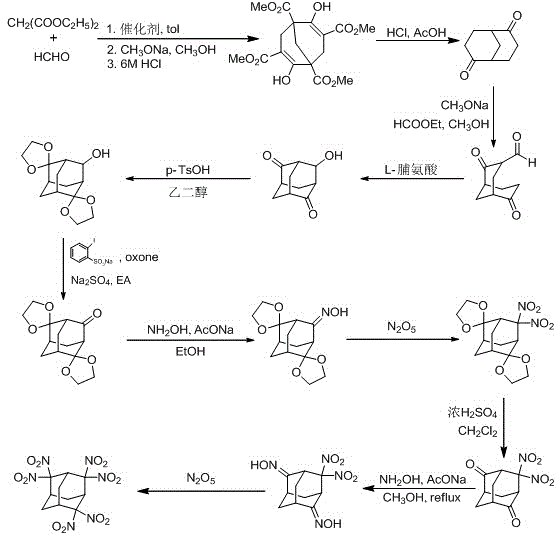
Energetic material 4, 4, 8, 8-tetranitroadamantane-2, 6-dinitrate and preparation method thereof
InactiveCN103864621AHigh purityAvoid the disadvantages of using excess nitrating agentNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsNitro compound preparationNitrateHigh density
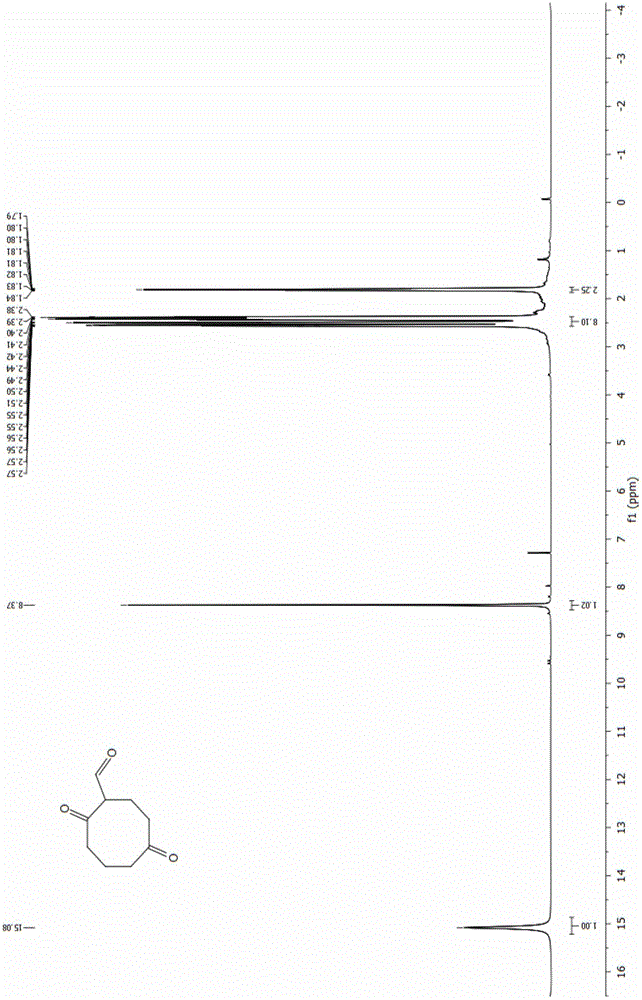
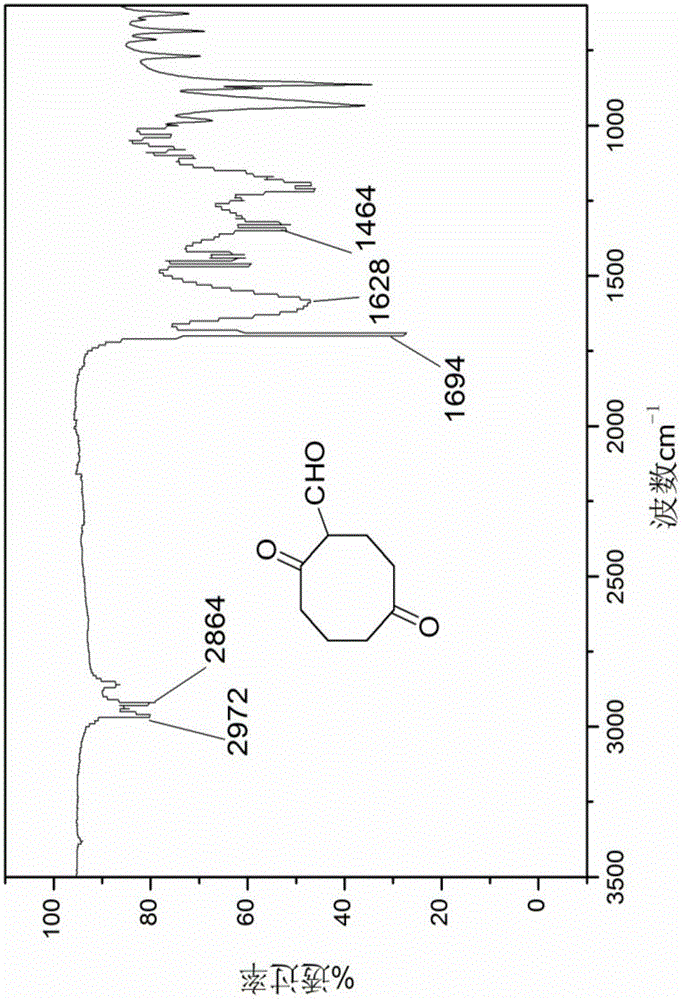
The invention discloses an energetic material 4, 4, 8, 8-tetranitroadamantane-2, 6-dinitrate and a preparation method thereof. According to the preparation method, cyclooctadiene as the raw material undergoes the steps of hydroboration-oxidation, oxidation, formylation, cyclization, nitric acid esterification, oximation, germinal nitration and the like to be finally synthesized into 4, 4, 8, 8-tetranitroadamantane-2, 6-dinitrate. Polynitroadamantane and adamantine nitrate have the advantages of symmetrical structure, high density, excellent explosion property, low sensitivity and the like, and can be widely used in the fields of explosives, propellants, pyrotechnic compositions, fuels and the like.
Owner:NANJING UNIV OF SCI & TECH
(3R)-2-iodo-4-benzyloxy-3-methyl-1-ene compound as well as preparation method and application thereof
InactiveCN110041160ANovel structureEasy to prepareOrganic compound preparationOrganic chemistry methodsIodineGrubbs' catalyst
The invention provides a (3R)-2-iodo-4-benzyloxy-3-methyl-1-ene compound as well as a preparation method and an application thereof, more particularly relates to a novel compound ERP-2 as well as a preparation method and an application thereof, also provides a novel preparation method of ERP ((3R)-2,4-diiodo-3-N-methyl butylamine-1-ene) and relates to use of the novel compound ERP-2. According tothe method, compared with the prior art, the yield and the quality of ERP-1 and ERP are notably increased when ERP-1 and ERP are prepared through the compound ERP-2, hazardous articles such as dimethylzinc or dimethyl aluminum are not required in the reaction process, expensive Hoveyda-Grubbs catalysts, Amano lipase PS-8000 and the like are not required, aftertreatment of a reaction is simple, theproduction cost is greatly saved, the production safety is improved, and the method is more suitable for industrial production.
Owner:NANTONG NUOTAI BIOLOGICAL PHARMA CO LTD
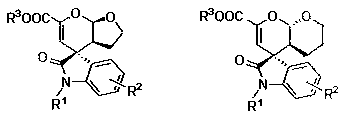
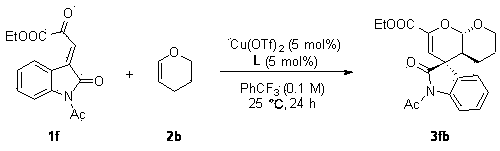
Chiral spiro oxindole dihydropyran derivative and synthesizing method thereof
ActiveCN107176959ASimple and fast operationHigh yieldOrganic chemistry methodsSolventEnantio selectivity
The invention discloses a synthesizing method of a chiral spiro oxindole dihydropyran derivative. The synthesizing method comprises the following steps of using dihydropyran (pyran) and isatin-derived beta,gamma-unsaturated alpha-keto ester as reaction matters; under the existence of a chiral (Box) / Cu(II) complex, synthesizing in a benzotrifluoride solvent to obtain a product. The synthesizing method disclosed by the invention has the advantages that the raw materials are simple, the obtaining is easy, the reaction condition is mild, the post-treatment is simple and convenient, the applicable substrate range is broad, the yield is high, and the enantioselectivity and non-enantioselectivity are high; the synthesized product has potential medical value.
Owner:SUZHOU UNIV
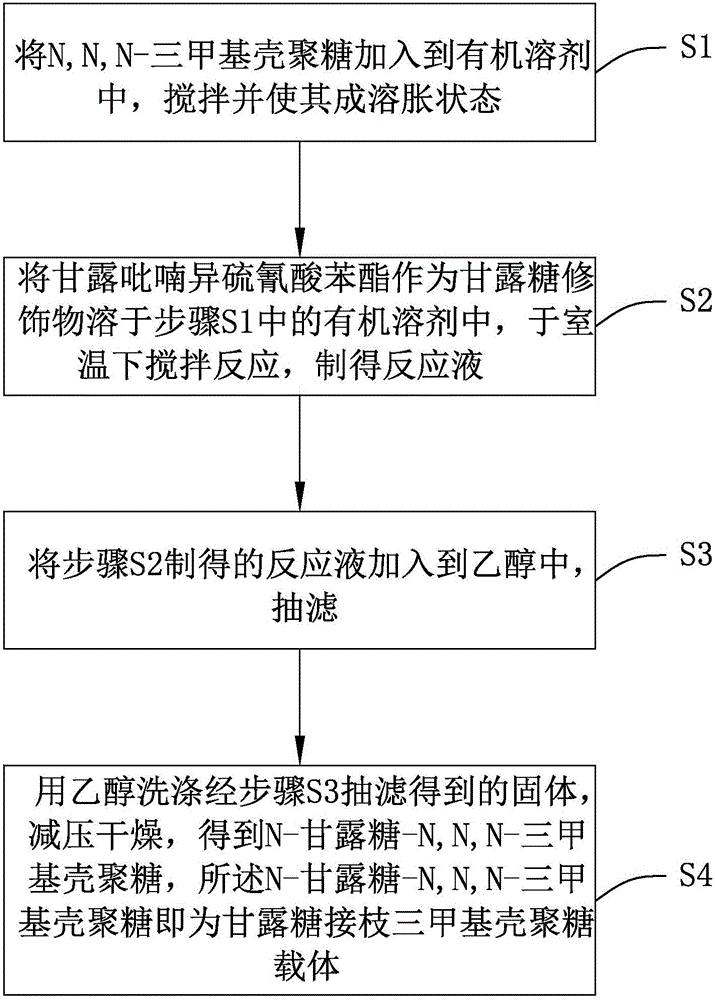
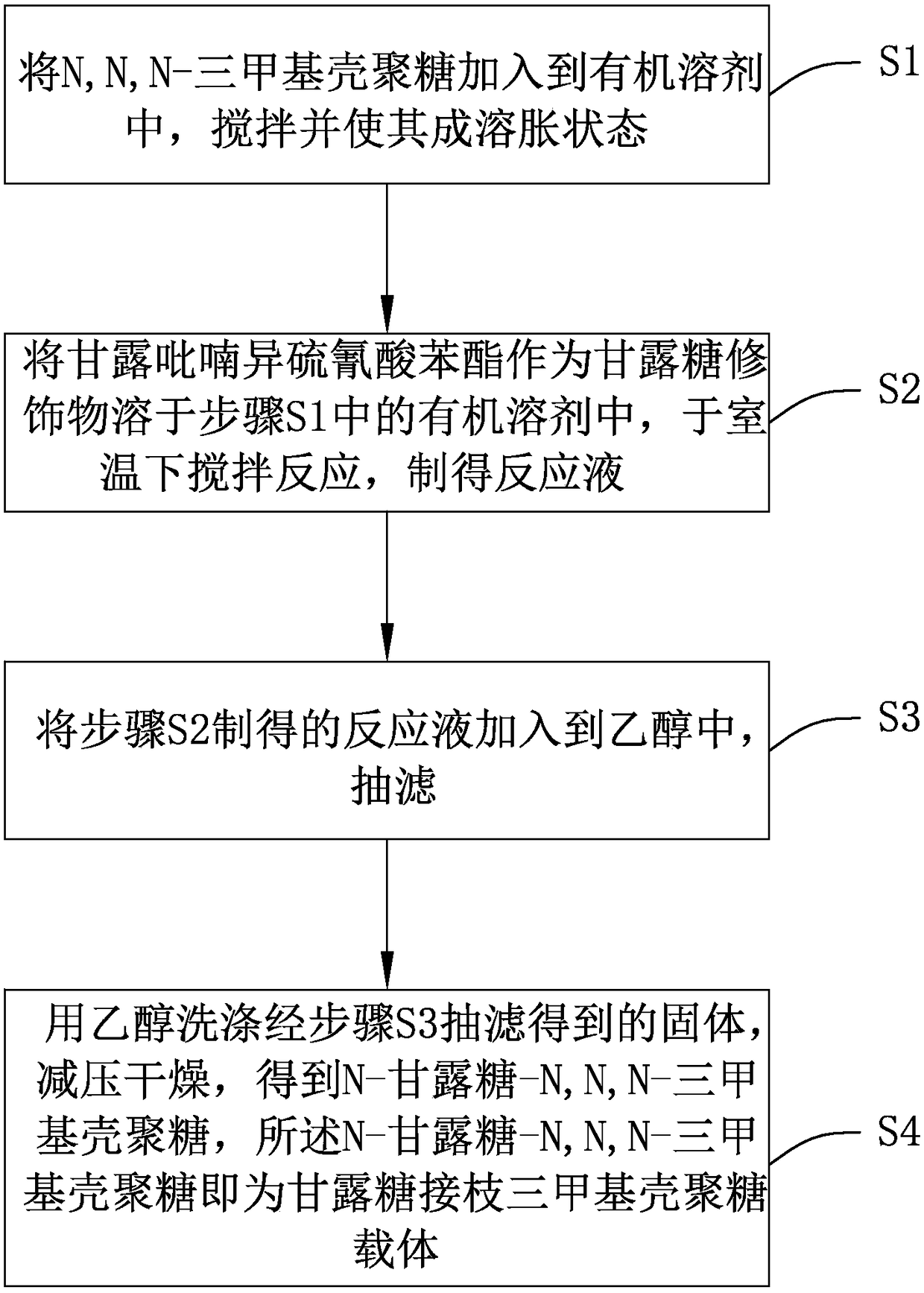
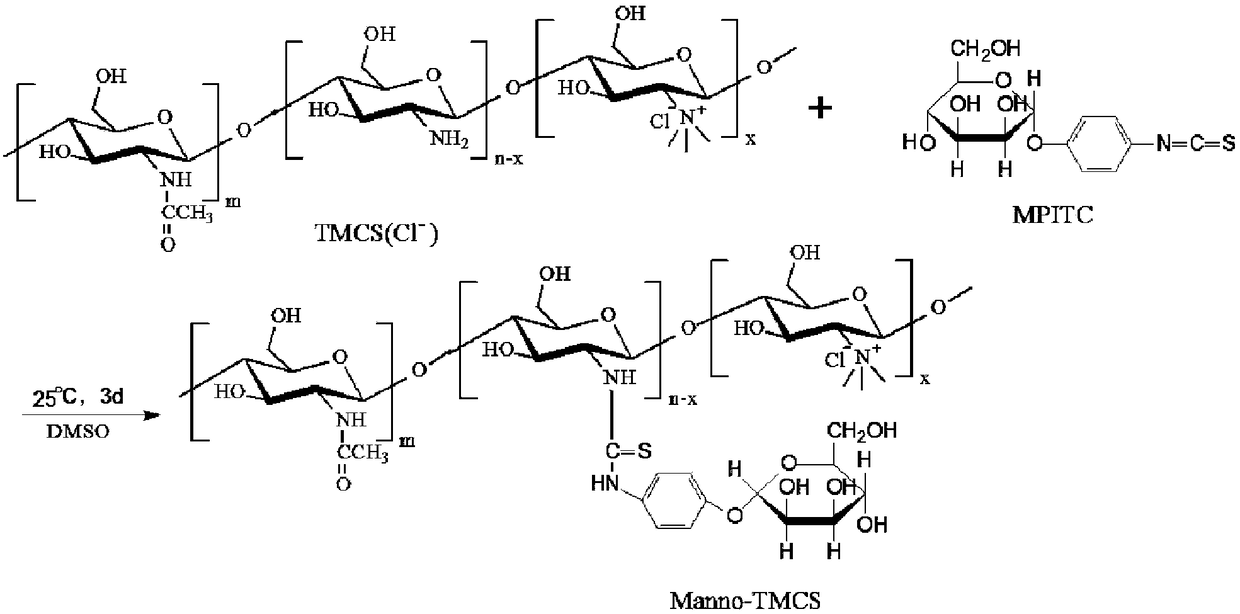
Preparation method of mannose-grafted trimethyl chitosan and application of preparation method
ActiveCN105754017AImprove reaction efficiencyEasy to handlePharmaceutical non-active ingredientsGranular deliveryMannoseChemistry
The invention relates to a preparation method and application of mannose-grafted trimethyl chitosan.The preparation method comprises: adding N,N,N-trimethyl chitosan into an organic solvent so that the organic solvent wells, adding mannopyran phenyl isothiocyanate for full reacting, and preparing N-mannose-N,N,N-trimethyl chitosan by ethanol washing, suction filtering and vacuum drying.The invention also provides application of the preparation method of the mannose-grafted trimethyl chitosan in the preparation of drug-carrying microspheres.The preparation method of a mannose-grafted trimethyl chitosan carrier has mild conditions, and a prepared mannose-grafted trimethyl chitosan drug-carrying microsphere preparation can target dendritic cells, overcoming mucosal barriers.
Owner:ZHEJIANG PHARMA COLLEGE
Oxo-bi(N-Methylphthalimide) synthesis method
An oxo-bi(N-Methylphthalimide) synthesis method, which solves problems existing in prior art, such as long reaction time, incomplete reaction and low yield. The method comprises the following steps: adding N-methyl-4-nitrophthalimide, sodium carbonate, potash, N- methyl pyrrolidone, toluene and alkyl guanidine haloid into a three-neck flask; then heating for refluxing; cooling to room temperatureand pouring into water; filtering solid; washing and drying to finish. The method of the invention has moderate reaction conditions without requiring a waterless condition or an inert gas protection,a short reaction time, a complete reaction, and a simple post treatment. The product, which is easy to separate, has a yield higher than 85%, a melting point of 269-271 DEG C, and a high purity. In addition, the method with a reduced production costs is environment-friendly and suitable for large scale production.
Owner:INST OF PETROCHEM HEILONGJIANG ACADEMY OF SCI
Preparation method of alpha arylglycine
InactiveCN105884636AHigh ligand recoverySimple post-reaction handlingCarboxylic acid nitrile preparationOrganic compound preparationMetalAryl
The invention relates to the field of pharmacy and concretely relates to a novel synthesis route of alpha arylglycine. The preparation method comprises contracting an arylated metal complex through a transition metal-catalyzed coupling reaction of a halogenated aryl compound and a metal complex and carrying out arylated metal complex dissociation to realize alpha arylglycine preparation. The preparation method is simple and economic and is suitable for synthesis of alpha arylglycine with a novel structure type.
Owner:CHINA PHARM UNIV
Synthesis method of drug Tafamidis
PendingCN113372290ASimple post-reaction handlingEsterification EconomyOrganic chemistryBenzoic acidCarboxyl radical
The invention relates to a synthesis method of a drug Tafamidis. According to the synthesis method, 3-hydroxy-4-amidobenzoic acid is used as a raw material, and the drug Tafamidis is prepared through esterification, amidation, cyclization and hydrolysis reaction. According to the method, concentrated sulfuric acid and methanol are used for replacing expensive and dangerous trimethylsilyl diazomethane to carry out methylation reaction of carboxyl; in the cyclization reaction, the dosage of p-toluenesulfonic acid is obviously reduced; reaction post-treatment is simplified, column chromatography for product purification is not needed in each step of reaction, the total yield of the reaction is remarkably increased, and the method is more suitable for industrial production.
Owner:ZUNYI MEDICAL UNIVERSITY
A kind of method and application of bio-oil for preparing polyol
ActiveCN104341297BWide variety of sourcesPromote degradationOrganic compound preparationPolyureas/polyurethane adhesivesPotassium fluorideGlycerol
The invention discloses a method for preparing polyol by using bio-oil. The method comprises the following steps: firstly performing methyl esterification, namely, performing ester exchange on biolipid and methanol under the catalysis of potassium fluoride loaded magnesium oxide solid alkali, converting the obtained product into fatty acid methyl ester with small molecular weight and byproduct glycerol, filtering to recycle the catalyst, and separating lower-layer glycerol; performing epoxidation on upper-layer fatty acid methyl ester in 30% of hydrogen peroxide under the catalysis of ionic liquid so as to form epoxidized fatty acid methyl ester.; then adding the glycerol in the methyl esterification process, continuously performing alkoxide ring-opening under the catalysis of ionic liquid, introducing hydroxyl, and finally separating liquid to recycle the ionic liquid catalyst, reducing pressure and distilling the upper-layer to remove water so as to obtain low-viscosity bio-oil-based polyol. The raw material is easily available, recyclable and good in biodegradability, the preparation process is environmental friendly, the industrial three-waste emission is small, the product structure and a hydroxyl value are adjustable, the application range is wide, and the environment influence level is low.
Owner:ZHEJIANG HENGFENG NEW MATERIAL
Synthetic method of peramivir intermediate
InactiveCN105198827AReduce manufacturing costHigh reaction yieldOrganic chemistryPtru catalystAmmonium compounds
The invention relates to a synthetic method of a peramivir intermediate, in particular to a synthetic method of a key intermediate by adopting an anti-influenza drug, namely peramivir, wherein the key intermediate is (3aR,4R,6S,6aS)-4-[[(1,1-dimethyl ethoxy) carbonyl] amino]-3-(1'-ethyl propyl)-3a,5,6,6a-tetralin-tetrahydro-4H-cyclopentano[d]isoxazole-6-carboxylic acid ammonium tertiary butyl (compound IV). According to the synthetic method of peramivir intermediate, provided by the invention, (1S,4R)-(-)-[[(1,1-dimethyl ethoxy)carbonyl]amino]cyclopentyl-2-alkenyl-1- carboxylic acid methyl ester (compound I) is taken as the raw material (preparation reference patent is CN101367750B) to be subjected to ring-closure reaction with butyric imine acyl chloride under catalyzation of a metal catalyst, so that the target compound is formed. According to the synthetic method of peramivir intermediate, provided by the invention, the low-cost, easily available and efficient metal catalyst is used, in addition, compared with the conventional technological process, the synthetic method is obviously increased in the yield, the technological process is simple, and industrial large-scale production is facilitated.
Owner:GUANGZHOU NANXIN PHARMA
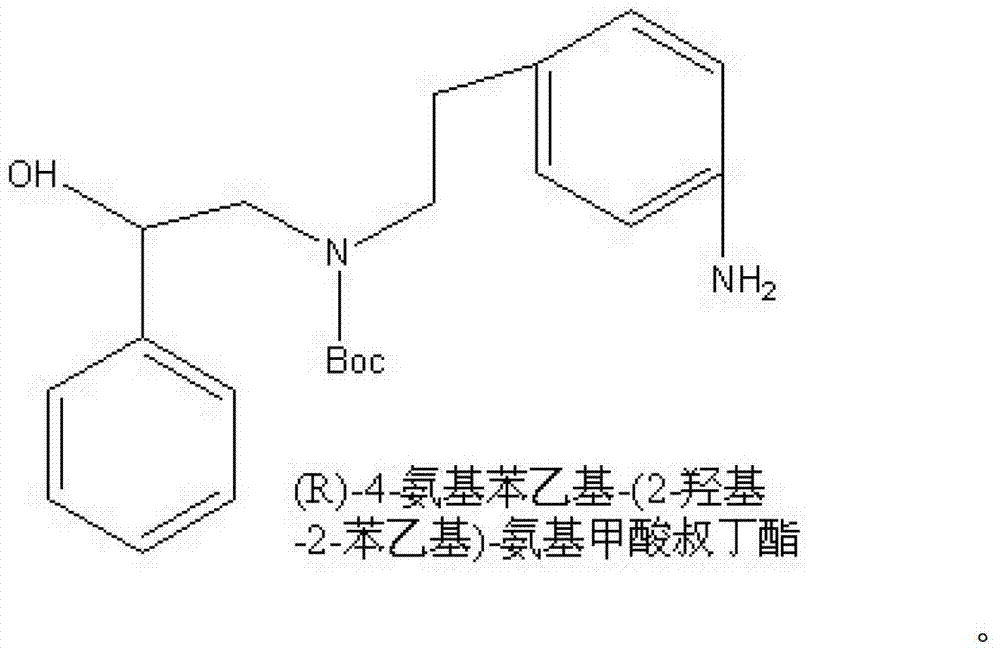
Synthesizing method of (R)-4-nitrophenethyl-(2-hydroxy-2-phenylethyl)-tert-butyl carbamate
ActiveCN103232368AReduce pollutionSuitable for industrialized mass productionCarbamic acid derivatives preparationOrganic compound preparationChromatographic separationCarbamate
The invention provides a synthesizing method of (R)-4-nitrophenethyl-(2-hydroxy-2-phenylethyl)-tert-butyl carbamate, and belongs to the technical field of medicine synthesis. The method assists in solving problems of low product yield, requirement on column chromatographic separation, and unsuitability for large-scale industrial productions of (R)-4-nitrophenethyl-(2-hydroxy-2-phenylethyl)-tert-butyl carbamate synthesizing methods in prior art. The method comprises the steps that: (1) hydroxyl group on p-aminophenylethanol is oxidized into aldehyde group, such that nitrobenzene acetaldehyde is obtained; (2) (R)-2-amino-1-phenylethanol is subjected to reductive amination, such that (R)-2-p-nitrobenzene ethylamine-1-phenylethanol is obtained; and (3) (Boc)2O is used for substituting the amino group on (R)-2-p-nitrobenzene ethylamine-1-phenylethanol, such that the final product is obtained. With the method, production cost is low, and finished product yield is high. The method is suitable for large-scale industrial productions.
Owner:SUZHOU UUGENE BIOPHARMA
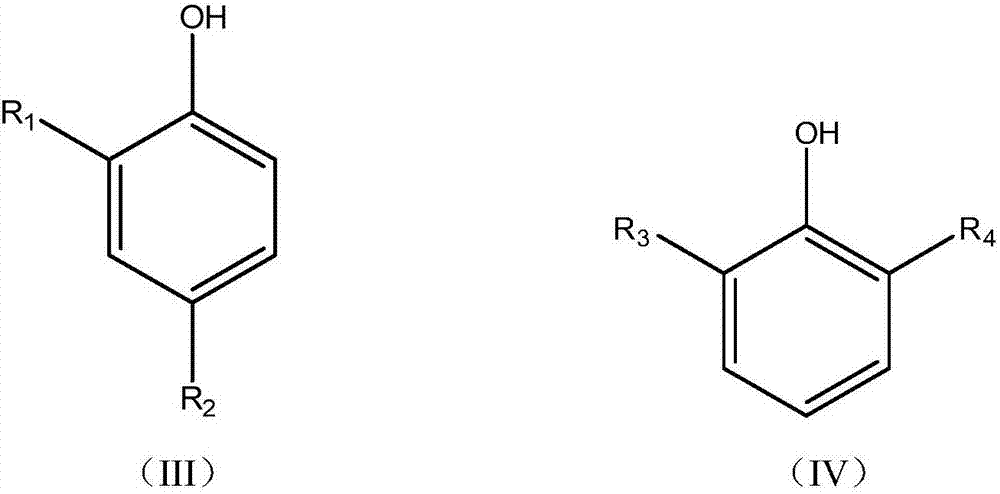
Preparation method and application of bisphenol antioxidant based on mixed base catalyst catalysis
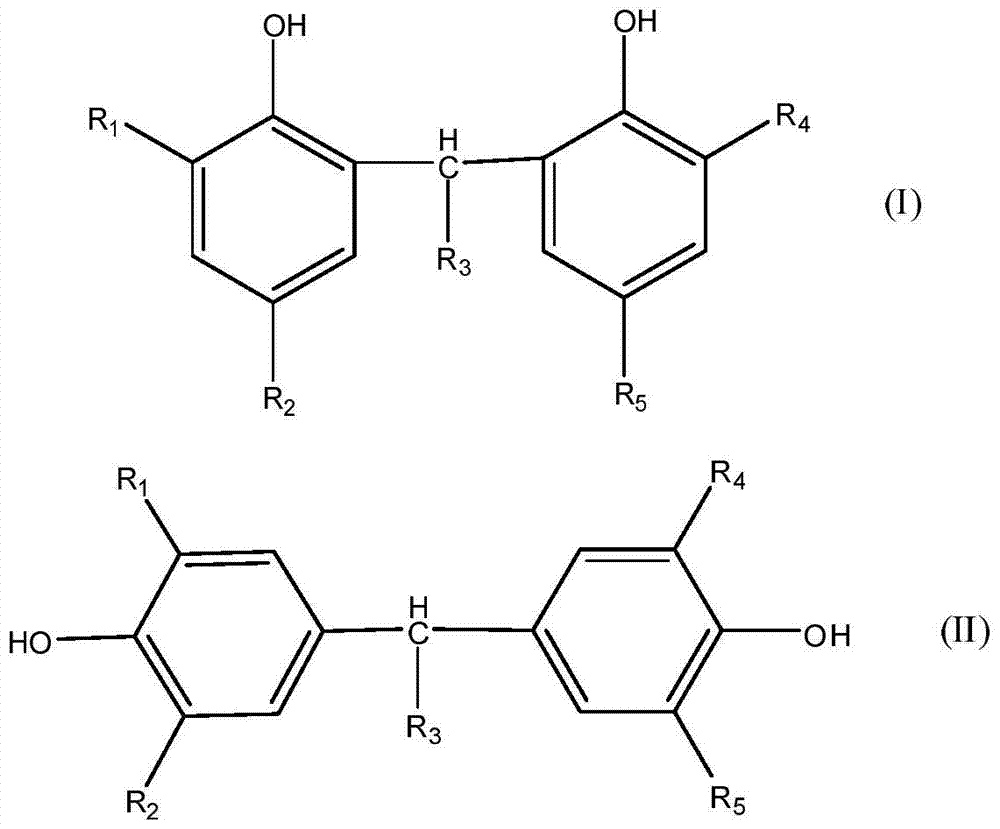
ActiveCN105503535BScientific and reasonable route designMild process conditionsOrganic chemistryOrganic compound preparationFuel oilPhenol
The invention discloses a mixed base catalyst catalysis-based bisphenol anti-oxidant preparation method and use. The preparation method comprises putting dialkyl-substituted phenol and an aldehyde compound into an enclosed reactor, carrying out a reaction process on the mixture in the presence of a mixed base catalyst under the condition of use or no use of an organic solvent and carrying treatment on the reaction product to obtain a bisphenol anti-oxidant after the reaction. The bisphenol anti-oxidant can be used for preparation of a lubricating oil composition, a fuel oil composition or an inhibitor of plastic and rubber. The preparation method of the bisphenol anti-oxidant has simple processes and mild conditions, utilizes cheap and easily available raw materials with a wide source, has a short reaction period, is convenient for control, has high technical safety, low production energy consumption, a high yield and high product purity, can effectively control and reduce pollutant discharge and is a novel technology with advantages of safety, energy saving and environmental friendliness.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Preparation method and application of mannose grafted trimethyl chitosan
ActiveCN105754017BSolve the problem of easy hydrolysis during the reactionEasy to removePharmaceutical non-active ingredientsGranular deliveryOrganic solventEpidermal Dendritic Cells
Owner:ZHEJIANG PHARMA COLLEGE
Preparation method of di-tert-butyl iminodiacetate
InactiveCN108191686ANo toxicityNo pollution in the processOrganic compound preparationAmino-carboxyl compound preparationReaction temperatureEthyl Chloride
The invention discloses a preparation method of di-tert-butyl iminodiacetate. The method is characterized in that ammonia and tert-butyl chloroacetate are used as raw materials. The method comprises the following steps: carrying out a reaction between ammonia and tert-butyl chloroacetate based on the molar ratio of 1:(1-6) in an organic solvent under a closed condition, wherein the reaction temperature is 10-65 DEG C, the reaction pressure is normal pressure, and the reaction lasts for 2-10h; after the reaction ends, cooling until the room temperature is reached; filtering; rotatably evaporating the filtrate to remove the organic solvent; then cooling to reach the temperature of 0 DEG C to obtain a solid which is di-tert-butyl iminodiacetate. According to the method, the solvent is nontoxic and free of pollution; the solvent is recycled; no catalyst or cocatalyst is used; the reaction separation method is simple; the di-tert-butyl iminodiacetate yield is high.
Owner:ZHEJIANG UNIV
Method for synthesis of phenylacetic acid by carbonylation of benzyl chloride
InactiveCN103193619BThe synthesis process is simpleHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsCarboxylic preparation from carbon monoxide reactionPhenylacetic acidReaction temperature
Owner:NORTHWEST UNIV +1
Energetic material 4,4,8,8-tetranitroadamantane-2,6-dinitrate and preparation method thereof
InactiveCN103864621BHigh purityAvoid the disadvantages of using excess nitrating agentNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsNitro compound preparationOctocryleneRaw material
The invention discloses an energetic material 4, 4, 8, 8-tetranitroadamantane-2, 6-dinitrate and a preparation method thereof. According to the preparation method, cyclooctadiene as the raw material undergoes the steps of hydroboration-oxidation, oxidation, formylation, cyclization, nitric acid esterification, oximation, germinal nitration and the like to be finally synthesized into 4, 4, 8, 8-tetranitroadamantane-2, 6-dinitrate. Polynitroadamantane and adamantine nitrate have the advantages of symmetrical structure, high density, excellent explosion property, low sensitivity and the like, and can be widely used in the fields of explosives, propellants, pyrotechnic compositions, fuels and the like.
Owner:NANJING UNIV OF SCI & TECH
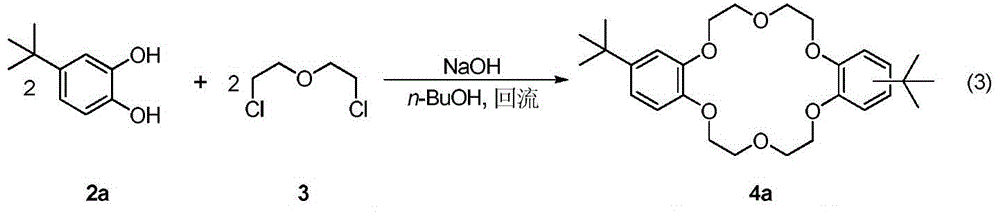
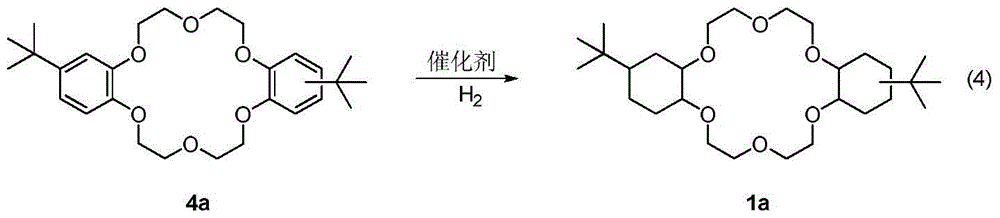
A kind of synthetic method of dicyclohexyl crown ether
The invention discloses a method for synthesizing dicyclohexyl crown ether compounds. Using catechol compounds and dichloroethyl ether as starting materials, through the Pedersen synthesis method, cyclization produces dibenzo-18-crown-6 ether compounds, and then undergoes catalytic hydrogenation to form dicyclohexyl- 18‑Crown‑6 ether compounds. The main feature of the present invention is that the heterogeneous metal catalyst is used to efficiently convert the dibenzocrown ether into the dicyclohexyl crown ether. After the reaction is completed, the product only needs simple treatment such as filtration and concentration, and the purity can reach more than 90%. The heterogeneous metal catalyst used is convenient to prepare, low in price, and can be recycled.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing diphenyl carbinol and derivatives thereof
ActiveCN102241566BLess quantitySmooth responseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenation reactionSolvent
The invention discloses a method for preparing diphenyl carbinol and derivatives thereof. In an alkali and solvent environment, diphenyl ketone and derivatives thereof are subjected to a hydrogenation reaction in the presence of a transition metal complex serving as a catalyst to form the diphenyl carbinol and derivatives thereof, wherein the general formula of the transition metal complex is MLnL'XY and is a transitional metal pnictide formed by a ligand having a NH2-N(SP2) or NH2-NH2 structural characteristic and a transition metal. When the method for preparing the diphenyl carbinol and derivatives thereof is used, a small amount of catalyst is used, the reaction process is stable, the conversion rate is over 98 percent, the very few by-products are produced in the reaction, the treatment after reaction is simple, the whole process period is short, the cost is low, and the large-scale production can be realized easily.
Owner:ENANTIOTECH CORP
Continuous synthesis process and device for 3-bromopyridine
InactiveCN112125839AShort reaction timeSimple post-reaction handlingOrganic chemistryChemical industryProcess engineeringPyridyne
The invention discloses a continuous synthesis process and device of 3bromopyridine, which comprises the following steps: respectively pumping pyridine, hydrobromic acid and hydrogen peroxide into a mixer according to a certain proportion, mixing by the mixer, and reacting in a first-stage tubular reactor and a second-stage tubular reactor; a reaction solution obtained after the reaction of the first-section tubular reactor and the second-section tubular reactor enters a first condenser to be cooled, the cooled reaction solution enters a continuous neutralization and liquid separation device,and meanwhile a sodium hydroxide solution is added into the continuous neutralization and liquid separation device; an upper-layer water phase of the continuous neutralization liquid separation deviceenters a wastewater storage tank, a lower-layer organic phase is evaporated and flashed by a negative-pressure film evaporator to remove low-boiling-point substances brought by separated liquid and then enters a negative-pressure rectifying tower to be rectified, the reflux ratio is controlled, a product at the top of the negative-pressure rectifying tower is collected by a product storage tank,and a bottom material at the bottom of the negative-pressure rectifying tower can be used as high-calorific-value fuel. The method can effectively solve the problems in 3bromopyridine synthesis, improves the production efficiency, reduces the energy consumption, and saves the production cost.
Owner:ANHUI COSTAR BIOCHEM CO LTD
A kind of synthetic method of (r)-4-aminophenethyl-(2-hydroxyl-2-phenethyl)-tert-butyl carbamate
ActiveCN103265457BReduce pollutionSuitable for industrialized mass productionCarbamic acid derivatives preparationOrganic compound preparationCarbamateSynthesis methods
The invention provides a (R)-4-amino phenethyl-(2-hydroxy-2-phenylethyl)-tert-butyl carbamate synthesis method, and belongs to the technical field of drug synthesis. With the present invention, problems of low product yield, complex treatment steps, heavy environment pollution and the like of the synthesis method in the prior art are solved. The synthesis method comprises the following steps that: hydroxyl on p-nitrophenylethanol is oxidized into aldehyde to obtain p-nitrophenylacetaldehyde; amino on (R)-2-amino-1-phenylethanol and the aldehyde on the p-nitrophenylacetaldehyde are subjected to dehydration condensation reduction to obtain (R)-2-p-nitrophenylethylamine-1-phenylethanol; (Boc)2O is adopted to replace amino on the (R)-2-p-nitrophenylethylamine-1-phenylethanol; and nitro on (R)-4-nitro phenethyl-(2-hydroxy-2-phenylethyl)-tert-butyl carbamate is reduced into amino so as to obtain the final product. The synthesis method has characteristics of low production cost, high product yield, and low environmental pollution.
Owner:SUZHOU UUGENE BIOPHARMA
Synthesis method for 2,2,4,4,6,6-hexanitro-adamantane
InactiveCN103435490BLow costHigh purityOrganic chemistryOrganic compound preparationSynthesis methodsNitration
The invention discloses a synthesis method for 2,2,4,4,6,6-hexanitro-adamantane. According to the method, diethyl malonate is used as a raw material, and the steps of cyclization, ozonization and the like with complex aftertreatment and low yield in the traditional route are avoided by a new route; and in the new route, N2O5 is used as a nitration agent in a nitration step, and the advantages of fast reaction speed, easily-controlled reaction temperature, easily-separated products, high product purity, capacity of effectively reducing waste acid, and the like are achieved. The method is lower in cost, less in reaction pollution and higher in yield compared with the traditional route, the reaction yield is increased to 14% from 0.5% of the original route, and possibility is provided for industrialized production for 2,2,4,4,6,6-hexanitroadamantane.
Owner:NANJING UNIV OF SCI & TECH
Preparation method for cyan coupler intermediate
InactiveCN106966995AImprove conversion rateSimple post-reaction handlingOrganic chemistryPropanoic acidRoom temperature
The invention discloses a preparation method for a cyan coupler intermediate. The preparation method comprises the following steps: with 4-t-butyl-benzoylhydrazine and ethyl 3-ethoxy-3-iminopropionate as raw materials, reacting 4-t-butyl-benzoylhydrazine with ethyl 3-ethoxy-3-iminopropionate in a lower-ester solvent at 50 to 100 DEG C, carrying out cooling to room temperature after completion of the reaction and then carrying out filtering so as to obtain ethyl 3-[2-(4-t-butyl-phenyl)-hydrazyl]-3-iminopropionate used as a transition intermediate; and subjecting ethyl 3-[2-(4-t-butyl-phenyl)-hydrazyl]-3-iminopropionate to reaction in an alkaline aqueous solution at 70 to 90 DEG C, carrying out cooling to room temperature after completion of the reaction, and successively carrying out neutralization via halogenated acid, filtering and drying so as to obtain the cyan coupler intermediate 2-[3-(4-t-butyl-phenyl)-1H-1,2,4-triazolyl-5]-propionic acid.
Owner:ZHEJIANG UNIV
Method for asymmetric synthesis of 3,3-disubstituted-2-oxindole compound
InactiveCN102659494BHigh ee valueSimple post-reaction handlingAsymmetric synthesesNatural productEnantioselective synthesis
Owner:EAST CHINA NORMAL UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Preparation method of hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative Preparation method of hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8eb5575a-5596-4a93-8576-66069183efe5/G2009102018598D00011.PNG)
![Preparation method of hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative Preparation method of hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8eb5575a-5596-4a93-8576-66069183efe5/G2009102018598D00021.PNG)
![Preparation method of hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative Preparation method of hexahydro-pyrrolo [3,4-c] pyrrole-1-ketone derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8eb5575a-5596-4a93-8576-66069183efe5/G2009102018598D00031.PNG)