Method for preparing diphenyl carbinol and derivatives thereof
A technology for benzyl alcohol and derivatives, applied in the field of preparation of benzyl alcohol and derivatives thereof, can solve the problems of environmentally unfriendly metal waste liquid, cumbersome post-processing, long time, etc., and achieves simple post-reaction treatment and process cycle time. Short, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
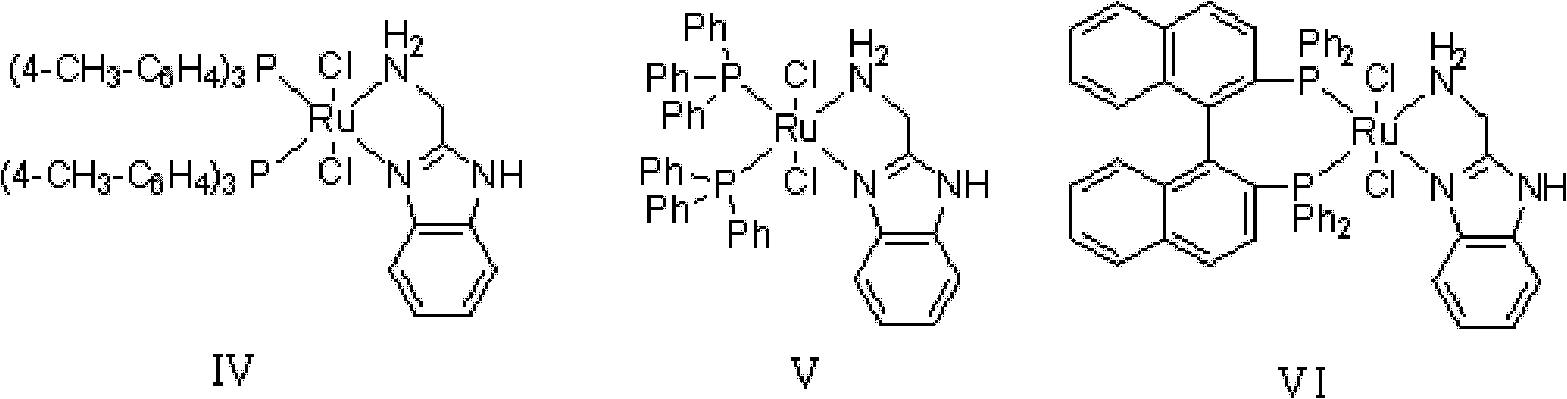
[0035] In 100L stainless steel reaction kettle, add benzophenone, toluene, in N 2 Atmosphere, add catalyst trans-[RuCl 2 (PPh 3 ) 2 {(1H-benzo[alpha]imidazol-2-yl)methanamine}] and potassium tert-butoxide; filled with H 2 To 5 atm, 100 ℃ of stirring reaction, when the hydrogen pressure is constant (about 4 hours), stop stirring, the H in the reactor 2 Empty, sample the reaction solution, carry out conventional post-processing (filtration, centrifugation, filtration, extraction, concentration, drying, etc.) to obtain a white or off-white solid product, which is detected by gas chromatography. The conversion rate of the reaction is 99.2%.
Embodiment 2
[0037]
[0038]
[0039] In 100L stainless steel reaction kettle, add benzophenone, tetrahydrofuran, in N 2 Atmosphere, add catalyst trans-[RuCl 2 (PPh 3 ) 2 {(1H-benzo[alpha]imidazol-2-yl)methanamine}] and triethylamine; filled with H 2 To 10atm, 30 ℃ of stirring reaction, when the hydrogen pressure is constant (about 8 hours), stop stirring, the H in the reactor 2 Vent, sample the reaction solution, and perform conventional post-treatment to obtain a white or off-white solid product, which is detected by gas chromatography, and the conversion rate of the reaction is 99%.
Embodiment 3
[0041]
[0042] In the 100L stainless steel reaction kettle, add benzophenone, dichloromethane, in N 2 Atmosphere, add catalyst trans-[RuCl 2 {P(C 6 h 4 -4-CH 3 ) 3} 2 {(1H-benzo[alpha]imidazol-2-yl)methanamine}] and sodium tert-butoxide; filled with H 2 To 60atm, 25 ℃ stirring reaction, when the hydrogen pressure is constant (about 18 hours), stop stirring, the H in the reactor 2 Vent, sample the reaction solution, and perform conventional post-treatment to obtain a white or off-white solid product, which is detected by gas chromatography, and the conversion rate of the reaction is 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com