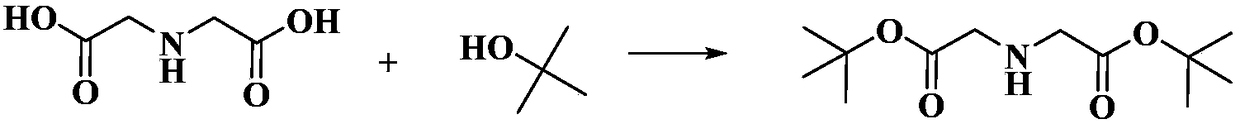Preparation method of di-tert-butyl iminodiacetate
A technology of iminodiacetic acid and tert-butyl chloroacetate is applied in the preparation of organic compounds, chemical instruments and methods, preparation of cyanide reaction, etc., and can solve the problems of complex reaction process, low catalyst and low yield, and achieves a high yield. The effect of high rate, low price and low industrial energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, a kind of preparation method of di-tert-butyl iminodiacetate, carries out following steps successively:
[0041] In a 250mL three-necked flask equipped with a thermometer, a condenser and a stirrer, add 120mL of methanol, 120.0g of tert-butyl chloroacetate (0.8mol) and heat up to 65°C under mechanical stirring, and slowly introduce 5.1g of ammonia gas (0.3 mol), 2h pass through, insulation reaction 5h. Cool to room temperature after the reaction is completed, remove the white solid salt ammonium chloride produced by the reaction by filtration, and recover 110 mL of methanol by rotary evaporation of the filtrate. 41°C, yield 95.0%.
Embodiment 2
[0042] Embodiment 2, a kind of preparation method of di-tert-butyl iminodiacetate, carries out following steps successively:
[0043] In a 250mL three-necked flask equipped with a thermometer, a condenser and a stirrer, add 120mL of ethanol, 120.0g of tert-butyl chloroacetate (0.8mol) and heat up to 65°C under mechanical stirring, and slowly introduce 5.1g of ammonia gas (0.3 mol), 2h pass through, insulation reaction 5h. After the reaction, cool to room temperature, remove the white solid salt ammonium chloride produced by the reaction by filtration, and recover 112mL of ethanol by rotary evaporation of the filtrate. 41°C, yield 93.9%.
Embodiment 3
[0044] Embodiment 3, a kind of preparation method of di-tert-butyl iminodiacetate, carries out following steps successively:
[0045] In a 250mL three-necked flask equipped with a thermometer, a condenser and a stirrer, add 120mL of tert-butanol, 120.0g of tert-butyl chloroacetate (0.8mol) and heat up to 65°C under mechanical stirring, and slowly introduce 5.1g of ammonia gas (0.3mol), after 2h pass through, keep warm for 5h. After the reaction, cool to room temperature, remove the white solid salt ammonium chloride by filtration, and recover 108 mL of tert-butanol by rotary evaporation of the filtrate, cool the remaining liquid to 0°C, and filter the precipitated solid to obtain 22.6 g of di-tert-butyl iminodiacetate, melting point 41°C, yield 92.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com