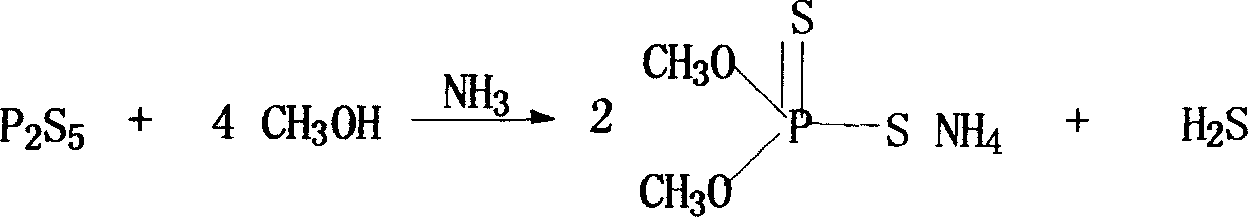
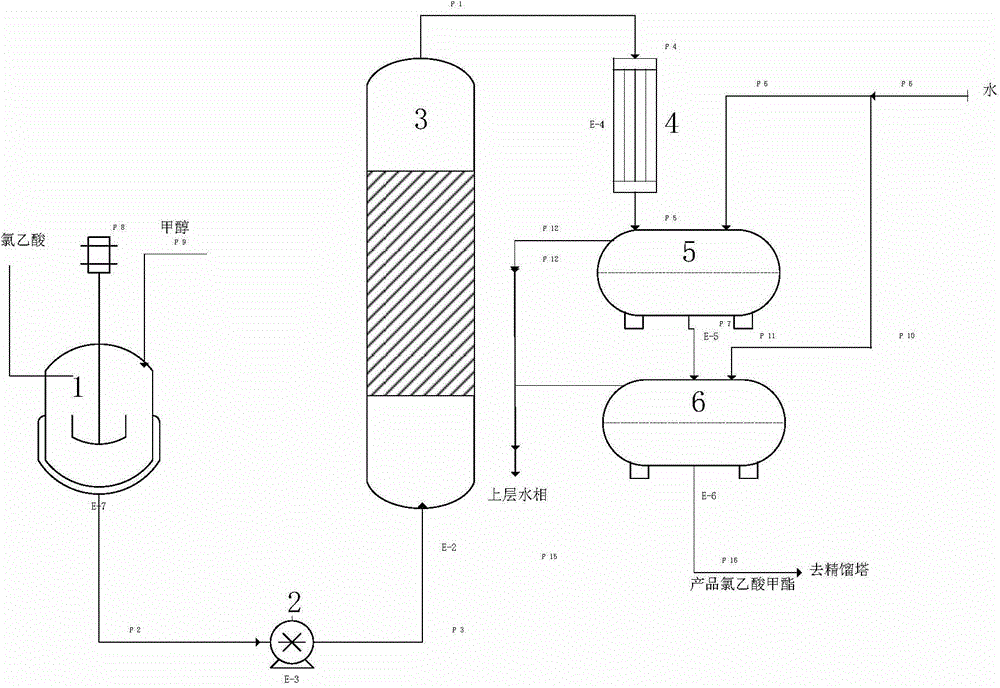
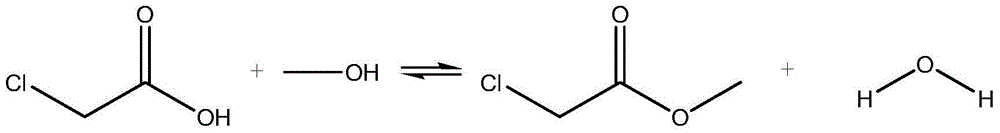
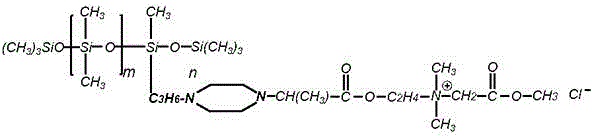
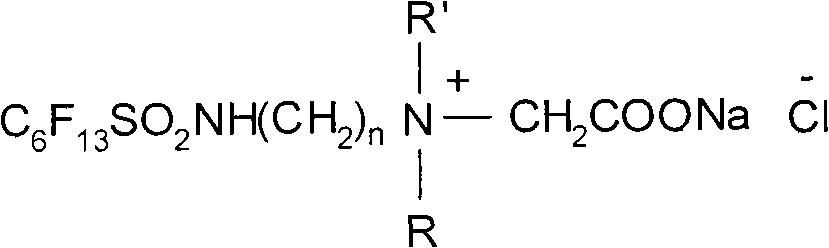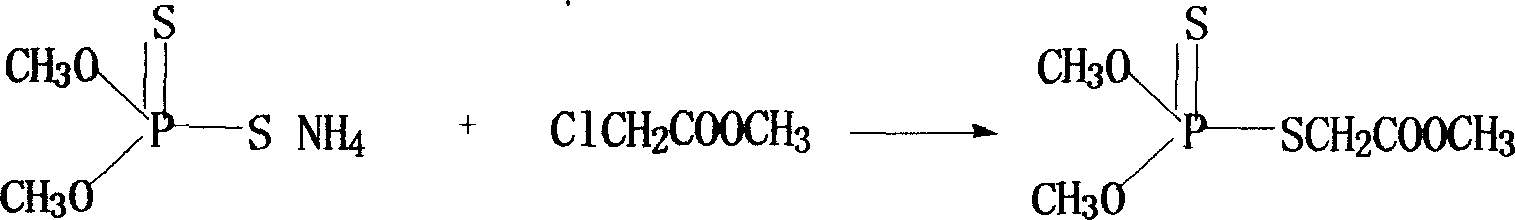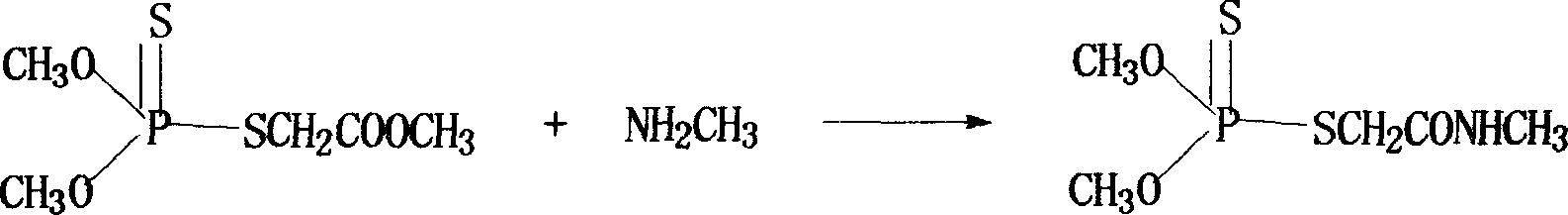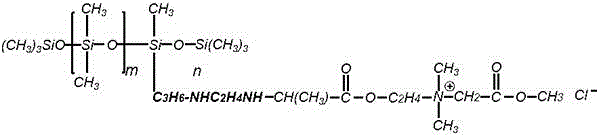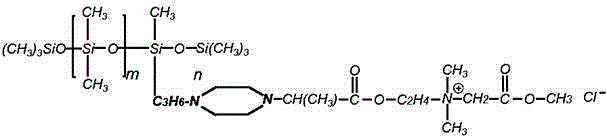Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
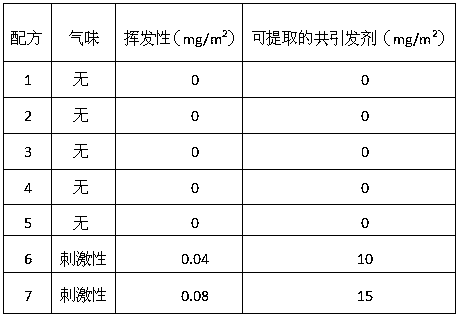
199 results about "Chloroacetates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
ACETIC ACID or acetic acid esters substituted with one or more CHLORINE atoms.
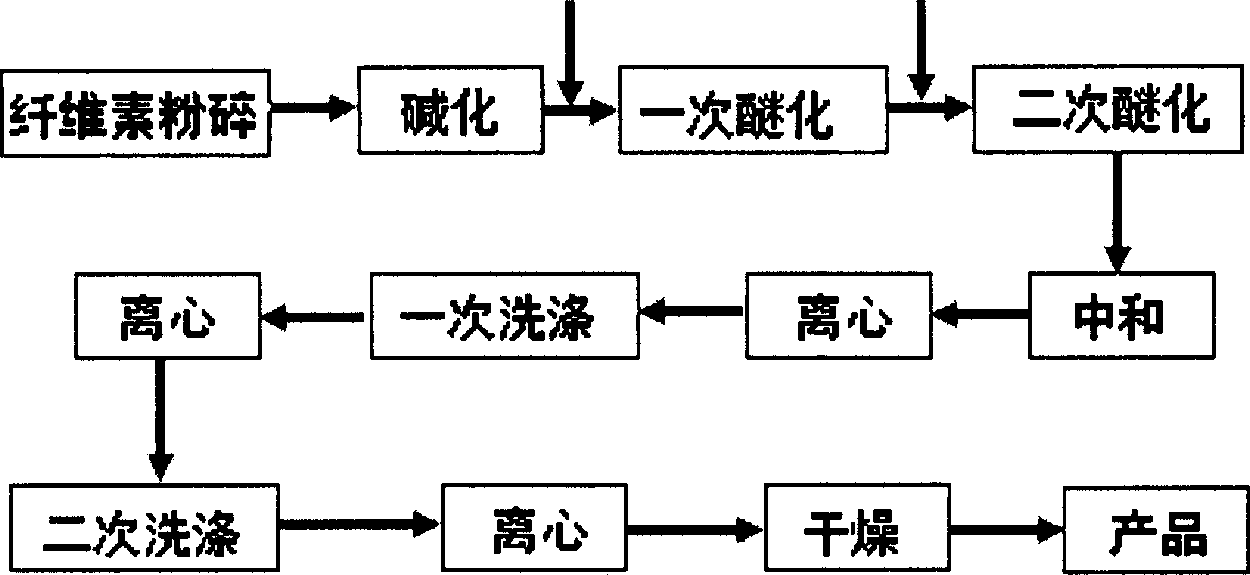
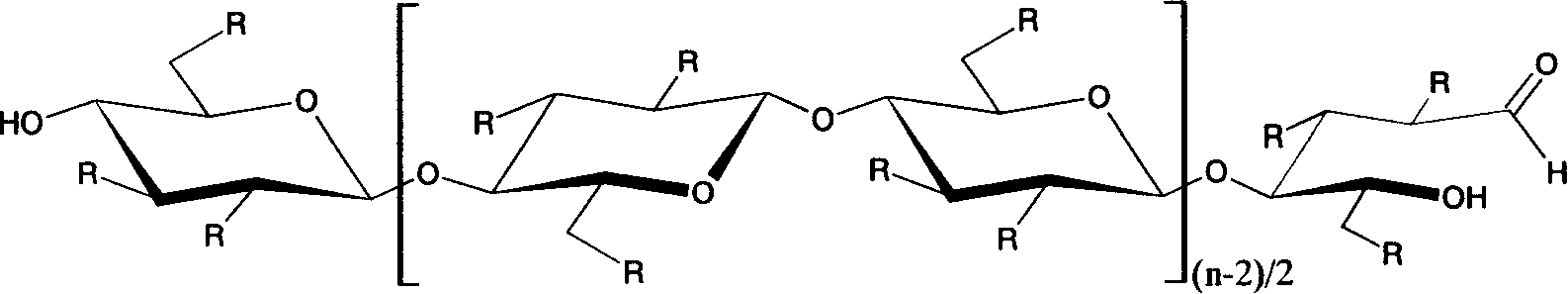
Method for preparing carboxymethyl cellulose in high degree of substitution
InactiveCN1916027AHigh degree of substitutionHigh degree of etherificationCarboxymethyl celluloseOrganic solvent
This invention provides a method for preparing carboxymethyl cellulose with ultrahigh substituent degree. The method comprises: (1) treating cotton and lignocellulose with 18-50% NaOH aqueous solution; (2) adding chloroacetate, chloroacetic acid or its sodium salt, and etherifying under stirring in an organic solvent under inert gas protection; (3) adding an alkali, heating for second etherification, neutralizing, washing and drying to obtain carboxymethyl cellulose with carboxymethyl mol substituent degree not lower than 2.0.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Polyoxyethylene alkylphenol ether ester cationic gemini surfactant and preparation method thereof
InactiveCN102126972ARich sourcesReduce surface tensionOrganic compound preparationTransportation and packagingGlycineAlkylphenol
The invention discloses a polyoxyethylene alkylphenol ether (n) ester cationic gemini surfactant using glycine ester group as linker and a preparation method thereof. The preparation method comprises the following steps: adding solvent, polyoxyethylene alkylphenol ether (n) chloroacetate and tetramethylethylenediamine in a reactor, stirring, heating and refluxing to react for serveral hours and obtain the surfactant solution; and further removing reaction solvent, purifying with mixed solvent, performing vacuum drying to obtain the polyoxyethylene alkylphenol ether (n) ester cationic gemini surfactant which uses glycine ester group as linker and has higher purity. The surfactant of the invention has higher surface activity and better emulsifying ability. The preparation method is simple, the conditions are easy to control, the product is easy to separate and the sources of raw materials are rich.
Owner:QIQIHAR UNIVERSITY
Polymerizable free radical II type photoinitiators and preparation method thereof
ActiveCN107629151AOrganic compound preparationCarboxylic acid esters preparationAcetic acidTransesterification
The invention discloses polymerizable free radical II type photoinitiators shown as formulae I and II in the description and a preparation method of the photoinitiators. Carboxylic acid compounds containing type II photo-initiation functional groups are taken as raw materials and subjected to a substitution reaction with methyl chloroacetate under an alkaline condition, then a product is subjectedto transesterification with hydroxy compounds containing polymerizable groups, and the polymerizable photoinitiators are generated. The photoinitiators structurally contain the polymerizable groups,so that the compatibility of the photoinitiators with a photopolymerization system is improved, and surface mobility is reduced greatly in the photocuring process; the photoinitiators can also serve as monomers for synthesis of macromolecular photoinitiators, thereby having broad application prospect in the field of photocuring.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
Alcohol ether gasoline used in new energy vehicles
InactiveCN101538491AReduce consumptionReduce pollutionLiquid carbonaceous fuelsNew energyLiquid fuel
The invention relates to a liquid fuel, and particularly a liquid carbonaceous fuel of the mixture of hydrocarbons and alcohol ether. The alcohol ether gasoline used in new energy vehicles comprises 25 to 85 percent of gasoline constituents and 15 to 75 percent of alcohol ether-major other chemical raw materials by weight percentage, wherein the components of the alcohol ether-major other chemical raw materials and the weight proportions thereof in the alcohol ether gasoline used in new energy vehicles are as follows: industrial methanol: 10 to 60 percent, n-hexane: 0 to 3 percent, isopropyl chloride: 0 to 1 percent, trichloroethylene: 0 to 1 percent, epichlorohydrin: 0.2 to 3 percent, bromoethanol: 0.2 to 2 percent, isodibutyl ether: 0.5 to 2 percent, ethylene glycol monobutyl ether: 0 to 1 percent, lauric acid : 0.4 to 2 percent, methyl chloroacetate: 0 to 4 percent, isopropyl nitrate: 0.2 to 4 percent, trimethyl phosphate: 0 to 3 percent, nitromethane: 0.1 to 1 percent, nitroethane: 0 to 1 percent, nitroguanidine: 0 to 3 percent, cyclopentadiene: 0 to 4 percent, and n-butylamine: 0 to 1 percent.
Owner:陈斌
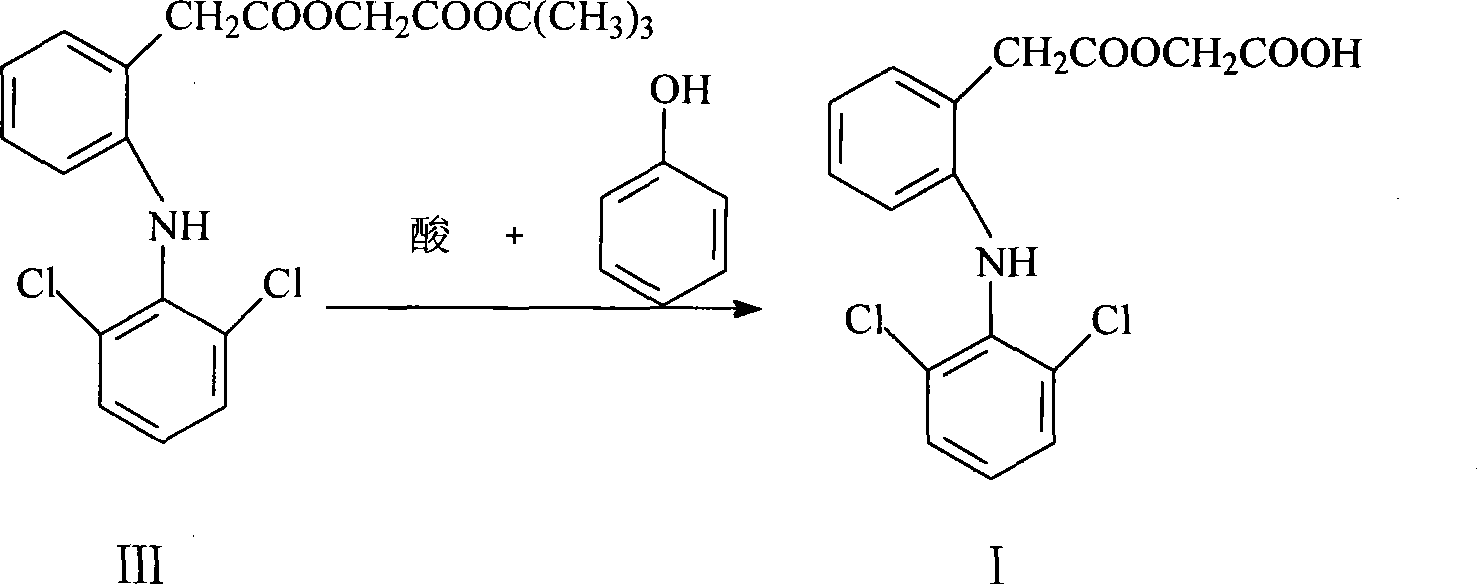
Improved method for preparing aceclofenac
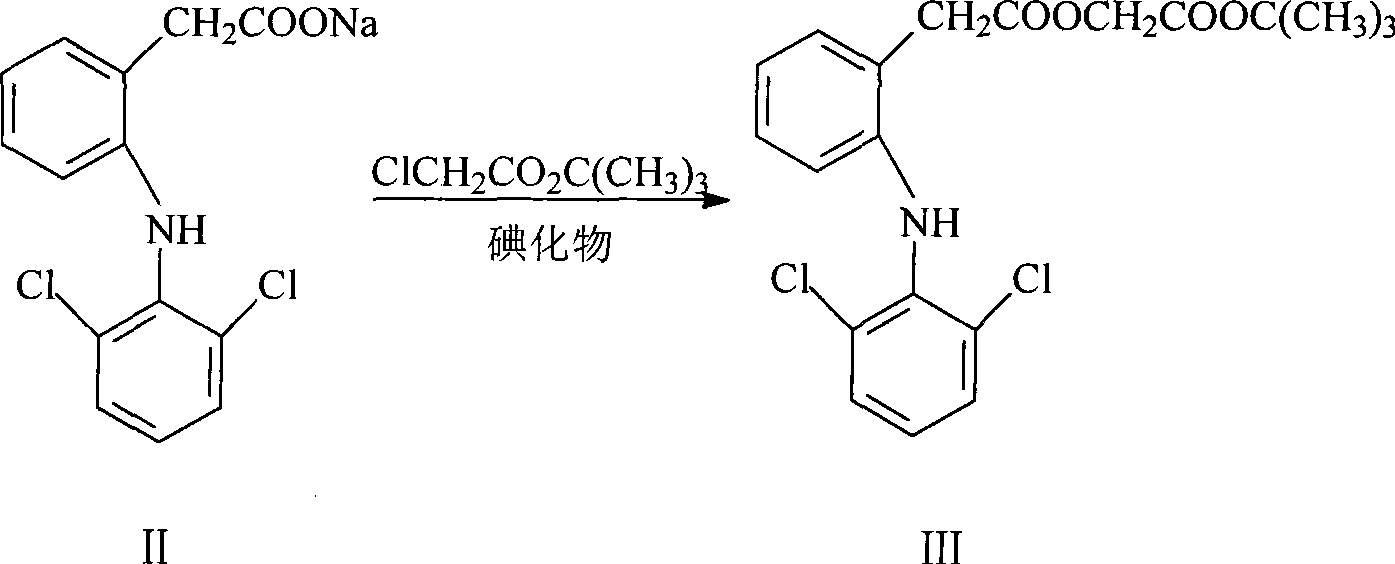
ActiveCN101531607AEasy to recycleShort reaction timeAntipyreticOrganic compound preparationIodideDiclofenac Sodium
The invention provides an improved method used for preparing aceclofenac. The improved method is characterized in that (1) the method takes diclofenac sodium and tert-butyl chloroacetate as raw materials and iodide as a catalyst, and heats the substances to carry out condensation reaction; (2) the method takes tert-butyl aceclofenac as a raw material to carry out acidolysis reaction under the action of phenol and acid, and obtains aceclofenac crystal after post treatment and fine purification; and the total yield of both steps is above 88 percent and the content is over 99.2 percent (detected by HPLC). The improved method increases the yield and the content, and has short reaction time, simple and convenient operation and mild reaction conditions; and a reaction reagent is easy to recycle, so the improved method reaches the effects of lowering cost and reducing environment pollution.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing perfluorohexane surfactant serving as main agent of aqueous film-forming extinguishing agent directly
The invention discloses a method for preparing a perfluorohexane surfactant serving as a main agent of an aqueous film-forming extinguishing agent directly. The method comprises the following steps of: performing ammonolysis reaction of perfluorohexane sulfuryl fluoride and diamine which serve as raw materials under the action of a catalyst to synthesize an amino sulfonamide compound without separation, and performing quaterisation reaction of the amino sulfonamide compound and chloroacetate to prepare a perfluorohexane betaine amphoteric surfactant which can be used for the aqueous film-forming extinguishing agent directly. The method has the characteristics of simple requirement on equipment, stable and convenient process, low energy consumption and no pollution; and the aqueous film-forming extinguishing agent prepared from the perfluorohexane betaine amphoteric surfactant serving as the main agent is short in fire control time and high in fire extinguishing efficiency, and all indexes reach the national standards of the industry.
Owner:徐衡
Method for synthesizing aliphatic alcohol polyethenoxy ether carboxylate
InactiveCN101497564AImprove conversion rateSimple processOrganic compound preparationTransportation and packagingEfficient catalystFatty alcohol
The invention provides a method for synthesizing fatty alcohol-polyoxyethyleneether carboxylate, which belongs to the technical field of synthesis of an anion surfactant in organic chemistry. The method uses fatty alcohol-polyoxyethyleneether and chloracetate as raw materials, leads the fatty alcohol-polyoxyethyleneether to perform a carboxy methylation reaction by a new catalyst under protection of inert gas, adds an ethanol solution of an alkali metal hydroxide after reaction so as to adjust the range of pH, and filters the pure fatty alcohol-polyoxyethyleneether carboxylate obtained after inorganic salt is dried in the ethanol, wherein the new catalyst can be sodium alcoholate and / or kalium alcoholate; and a ratio of the catalyst to the pure fatty alcohol-polyoxyethyleneether by weight can be 1:1.0-2.0. The method adopts the new high-efficiency catalyst with high conversion rate of the product, and can obtain the pure fatty alcohol-polyoxyethyleneether carboxylate. Compared with the prior method, the method has a more advanced and reasonable process, and saves equipment investment.
Owner:ZHEJIANG HUANGMA TECH
Preparation method of Bola type betaine surfactant
ActiveCN104447380AImprove conversion rateIncrease profitOrganic compound preparationCarboxylic acid amides preparationAcetic acidBetaine
The invention discloses a preparation method of a Bola type betaine surfactant. The preparation method comprises the following steps: (1) a preparation step of N, N-bis(N, N-dimethylamino propyl) alkyl amide; (2) a quaternization preparation step. In the preparation method disclosed by the invention, the N, N-bis(N, N-dimethylamino propyl) alkyl amide is prepared through a two-step process, so that the conversation ratio of the alkyl acid can be increased to be more than 99.0% to the greatest extent, the raw material utilization rate is high and the industrial production requirements are achieved; in the quaternization preparation step of the Bola type betaine surfactant, the conversation ratio of the N, N-bis(N, N-dimethylamino propyl) alkyl amide is increased to be more than 99.9% and the consumption of chloroacetate is lowered by controlling the molar ratio, the temperature and the pH of the materials and the addition amount of an alkaline catalyst; the water is used as a solvent to avoid using an organic solvent, so that the preparation cost is lowered and the industrial production requirements are achieved.
Owner:GUANGZHOU TINCI MATERIALS TECH
Reactive distillation method for preparing methyl chloroacetate
InactiveCN104592021AReduce dosageNo secondary pollutionOrganic compound preparationChemical industryTriflic acidReaction temperature
The invention relates to a new method for preparing methyl chloroacetate. The method is characterized in that chloroacetic acid and methanol are used as raw materials, a reactive distillation tower is adopted, and a pyridine trifluoromethanesulfonic acid polymeric ion liquid catalyst is used as mixed filler in a reaction section to perform gas-liquid-solid three-phase reaction. The reaction can be finished by adopting a one-section reactive distillation reactor, a water carrying agent does not need to be added in the process, the reaction temperature is low, the flow is simple, the investment cost is reduced, the energy consumption is low, the conversion rate is more than 99.5%, and the selectivity is 100%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Wine yeast with low yield of higher alcohol
InactiveCN103627646AReduce contentHigh health valueFungiMicroorganism based processesDecompositionGrape wine
The invention relates to wine yeast with low yield of higher alcohol. The wine yeast is characterized in that a certain amount of isoamyl chloroacetate is added in a flat plate for cultivating yeast, the isoamyl chloroacetate generates chloroacetic acid under the catalysis of saccharomycetes isoamyl acetate hydrolase; the chloroacetic acid has a strong restraining effect on the growth of saccharomycetes, if the activity of the ester decomposition enzyme of a mutant strain is high (the higher the activity of the ester decomposition enzyme is, the more higher alcohol produced by the strain is), more chloroacetic acid is produced by decomposition, so that a bacterial colony grows slowly under the inhibition effect of the chloroacetic acid. The wine yeast can be utilized to accurately, conveniently and quickly screen out saccharomycetes with low yield of the higher alcohol. When the saccharomycetes with low yield of the higher alcohol, which is bred through the wine yeast, is used for producing grape wine, the content of the higher alcohol in the grape wine can be lowered by about 10% to 15%; when the saccharomycetes with low yield of the higher alcohol, which is bred through the wine yeast, is used for alcohol fermentation, the content of the higher alcohol in grape wine can be lowered effectively, and the health-care values of the grape wine can be improved.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Process for synthesizing dimethoate
InactiveCN1702074AHigh yieldReduce pollutionBiocideGroup 5/15 element organic compoundsPhosphatePotassium
The invention discloses a method for synthesizing dimethoate, which takes microcosmic salt and methyl chloroacetate as material to synthesize intermediate phosphate ester. The invention chooses phosphate ester and aminomethane as material to synthesize dimethoate. The reaction reacts in homogeneous system of organic solvent for 80-160 minutes in temperature of 0-60 DEG C. The invention synthesizes dimethoate in temperature of -10-5 DEG C for 60-150 minutes. Said microcosmic salt comprises ammonium salt, sodium salt, potassium salt and lithium salt. Said solvent comprises carbinol, alcohol, acetone, alcohol isopropylicum and tetrahydrofuran. The invention possesses carry out homogeneous phase reaction system to produce dimethoate with gross yield of up to 30% and pureness of 90% under the condition of existing device and material.
Owner:姚文刚
Method for preparing methyl chloroacetate
InactiveCN104151164AReduce dosageNo accumulationOrganic compound preparationCarboxylic acid esters preparationAcetic acidReaction temperature
The invention relates to a novel method for preparing methyl chloroacetate. The method is characterized in that resin modified by acidic ionic liquid is taken as a catalyst and a section of fixed bed reactor is just adopted for completing the method; no aqueous solution needs to be added in the preparation process; the method is low in reaction temperature, simple in flow, low in investment cost and low in energy consumption; the conversion rate of the methyl chloroacetate is above 99.5% and the selectivity of the methyl chloroacetate is 100%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
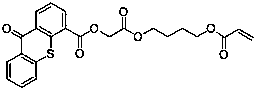
Hydrogen self-supply type photoinitiator and preparation method thereof
ActiveCN106349213ASolve pollutionMild reaction conditionsOrganic chemistryInksCarboxylic acidPhotochemistry
The invention relates to a hydrogen self-supply type photoinitiator and a preparation method of the hydrogen self-supply type photoinitiator. The hydrogen self-supply type photoinitiator is a compound having a structure of Formula (I) as below, wherein L represents core framework structure of n function, and is independently selected from hydroxyl-containing small molecular compound or residual base of polyether; m and n represent integers 1-6 independently, and n is not more than m; PI is photosensitive radical group, and selected from thioxathone or substituted thioxathone radial group; R is selected from H, linear chain or branch chain C1-C18 alkyl, phenyl, substituted phenyl, benzyl or substituted benzyl as the (III), (IV), (V), (VI), (VII) structures of the Formula below: FORMULA. The photoinitiator can be the hydrogen self-supply type, and can efficiently initiate light polymerization reaction under the condition of not adding initiator, and avoid the shortcomings of volatile, migration and odor of assistant initiator. The preparation method directly introduces the ester exchange radical group through the direct reaction between methyl chloroacetate and thioxathone carboxylic acid, and overcomes the environment pollution caused by using thioxathone acyl chloride in ordinary method; the synthetic process is green and environment-friendly, and the reaction condition is relatively gentle. The FORMULA (I) is as shown in the description.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
Quaternary ammonium type hydrophilic amino-modified silicone oil emulsion and preparation method thereof
InactiveCN106589386ASignificant deepening effectSoft touchGrip property fibresPolydimethyl siloxaneSelf emulsifying
The invention discloses a preparation method of a quaternary ammonium type hydrophilic amino-modified silicone oil emulsion. The preparation method includes the following preparation steps that firstly, amino-modified silicone oil is prepared from alpha,omega-hydroxy polydimethyl siloxane (linear organosilicon), an amino coupling agent, a sealing agent and a basic catalyst, then the amino-modified silicone oil and dimethylaminoethyl methacrylate react, acrylate-modified amino-modified silicone oil is obtained, and finally quaternization is carried out through methyl chloroacetate. The quaternary ammonium type hydrophilic amino-modified silicone oil emulsion is simple in preparation process, has self-emulsifying performance, and is used for after-finishing of fabrics, and the fabrics are made to have excellent soft and slip hand feeling, and are low in yellowing, small in color alteration and good in hydrophilicity.
Owner:WACKER DYMATIC SILICONES SHUNDE CO LTD FOSHAN
Selenium-containing surfactant as well as preparation method and application thereof
ActiveCN105498622AImprove surface activityEasy to prepareCosmetic preparationsHair cosmeticsChemical industryAcute toxicity testing
The invention discloses a selenium-containing surfactant as well as a preparation method and application thereof and belongs to the technical field of a daily chemical industry. The surfactant is long-chain alkyl selenium base acetate, and a structural character of the surfactant is shown as a formula (1) as shown in the specification; in the formula (1), n is an integer from 11 to 15; M<+> is one of a sodium ion, a potassium ion or an ammonium ion. The preparation method of the selenium-containing surfactant is simple, and has moderate synthesis conditions, no special requirements on equipment and relatively low energy consumption; raw materials are easy to obtain and the product does not contain hydroxyl acetate and chloroacetate; the selenium-containing surfactant has relatively high surface activity, excellent foaming and foam stabilization performance, unique hard water resistance and oxidation resistance and free radical removing capability, and relatively low cytotoxicity, aquatic organism acute toxicity and skin irritation.
Owner:JIANGNAN UNIV
Synthesis method of dye intermediate
InactiveCN102633666AReduce the ratioReduce consumptionOrganic compound preparationCarboxylic acid amides preparationAcetic acidAlkyl transfer
The invention relates to the technical field of dye. In order to solve the problem that the methyl chloroacetate N-alkylation reaction in the existing preparation process of -CH2COOCH3-containing substituted aniline dye intermediate is large in required methyl chloroacetate proportion, hard to recycle, easy to deteriorate during storing and the like, the invention provides a synthesis method of dye intermediate, i.e. a synthesis method of dye intermediate shown in the general formula (I), comprising the following steps of: taking substituted aniline shown in the general formula (II) as a raw material, adding water aqua, methyl chloroacetate, catalyst sodium bromide and acid-binding agent, pulping and heating to reflow and divide water, and carrying out the N-alkylation reaction, so that the dye intermediate is prepared. After the method in the invention is adopted, the consumption of methyl chloroacetate can be greatly reduced, the methyl chloroacetate distillation recovery working procedure can be eliminated, and the problem that the recovered methyl chloroacetate is easy to decompose can be avoided.
Owner:HANGZHOU JIHUA JIANGDONG CHEMICAL CO LTD
Preparation method of valnemulin hydrochloride
InactiveCN102225905AReduce pollutionMild reaction temperatureSulfide preparationReaction temperatureSolvent
The invention relates to a preparation method of valnemulin hydrochloride. The preparation method comprises the following steps: reacting pleuromutilin with p-toluenesulfonyl chloride, substituting with 1-amino-2-methyl propyl-2-mercaptan hydrochloride so as to obtain [(2-amino-1,1-dimethyl S, 6S, 8R,9R, 9aR, 10R)-6-vinyl decahydro-5-hydroxyl-4,6,9,10-tertamethyl-1-oxo-3a, 9-propanol-3aH-cyclopentacyclooctene-8-yl ester for later use; reacting D-valine with methyl acetoacetate; synthesizing the product with isobutyl chloroacetate so as to generate anhydride; and reacting anhydride with [(2-amino-1,1-dimethyl ethyl) sulfenyl] acetic acid (3aS, 4R, 5, 6S, 8R,9R, 9aR, 10R)-6-vinyl decahydro-5-hydroxyl-4,6,9,10-tertamethyl-1-oxo-3a, 9-propanol-3aH-cyclopentacyclooctene-8-yl ester so as to generate amide, and then carrying out deprotection with hydrochloric acid so as to prepare valnemulin hydrochloride. The method has the advantages that (1) reaction temperature is mild, thereby being applicable to large-scale production; (2) post-treatment is simple, thereby directly obtaining the product; (3) used solvents are less, thereby reducing environmental pollution; and (4) the valnemulin hydrochloride content and yield of the obtained product are high.
Owner:HUBEI SHENZHOU CHEM
Synthetic method of 2-thiopheneacetic acid
ActiveCN103467441AShort process routeRaw materials are cheap and easy to buyOrganic chemistryAluminium chlorideIce water
The invention relates to a synthetic method of 2-thiopheneacetic acid, and the synthetic method comprises the following steps: adding thiophene, methylbenzene and aluminium chloride anhydrous into a reaction container, dropping methyl chloroacetate while heating to 50-60 DEG C, and performing heat preservation after dropping; after cooling a reaction liquid to room temperature, pouring proper ice water and hydrochloric acid, layering, extracting the water layer by using proper methylbenzene, drying the organic layer by using anhydrous sodium sulphate, filtering, and concentrating a filtrate and alkaline liquor to be dry so as to obtain 2-thiophene methyl acetate; adding a sodium hydroxide aqueous solution and 2-thiophene methyl acetate into the reaction container, heating to 70-80 DEG C, performing heat preservation for 2-3 hours, cooling to room temperature after performing heat preservation, adjusting ph to 0.5 by using concentrated hydrochloric acid, cooling to 0-10 DEG C, performing heat preservation for 1-2 hours, filtering, and drying the filter cake to obtain 2-thiopheneacetic acid. The synthetic method of 2-thiopheneacetic acid disclosed by the invention has the advantages of being short in process route, cheap and easily available in raw materials, high in yield, good in product quality and suitable for large-scale production.
Owner:LIANYUNGANG DIPU CHEM
Low-density polyethylene-modified polyvinyl chloride (PVC) synthetic leather and manufacturing method thereof
InactiveCN102995447AGood flexibilityHas a natural and comfortable feelSynthetic resin layered productsLaminationLow-density polyethylenePolyester
The invention discloses low-density polyethylene-modified polyvinyl chloride (PVC) synthetic leather. The low-density polyethylene-modified PVC synthetic leather comprises a surface layer, a foamed layer, a bonding layer and a brushing fabric, and is characterized in that the surface layer is prepared by mixing the following raw materials in parts by weight to form slurry: 30 to 40 parts of SG-1 type PVC resin, 70 to 80 parts of SG-6 type PVC resin, 45 to 50 parts of propanediol sebacate polyester, 20 to 25 parts of n-butyl methacrylate, 5 to 6 parts of PVC color paste, 1 to 2 parts of di-hydrosulfo isooctyl chloroacetate di-n-octyl tin, 1 to 2 parts of zinc stearate, 5 to 10 parts of light calcium carbonate, 2 to 3 parts of 4,4'-oxo bi-benzenesulfonyl hydrazide, 40 to 45 parts of nano- barite powder and 50 to 60 parts of modified bentonite. Through the improvements of the formula and the process, the flexibility of the synthetic leather is improved, and the synthetic leather has a natural comfortable hand feeling similar to that of natural leather and is difficult to damage or tear after being used for 3 to 5 years.
Owner:HEFEI ANSHAN COATING FABRICS
Preparing method of triazole thioglycolic acid compound for curing metabolic arthritis
ActiveCN105566237AReduce consumptionHigh selectivityOrganic chemistryArthritisNucleophilic substitution
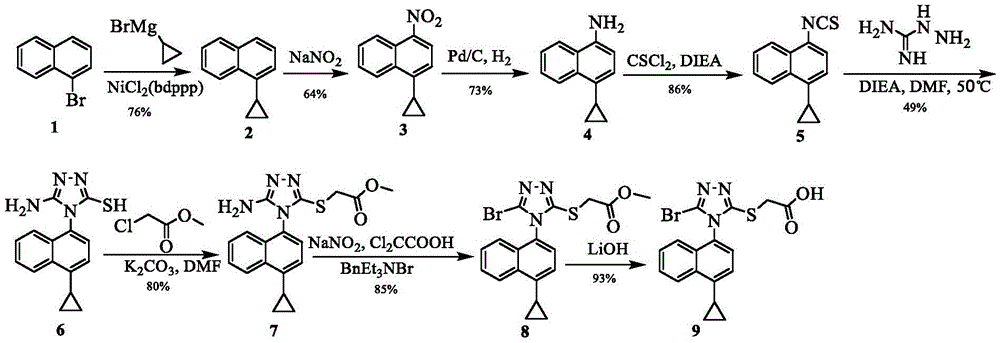
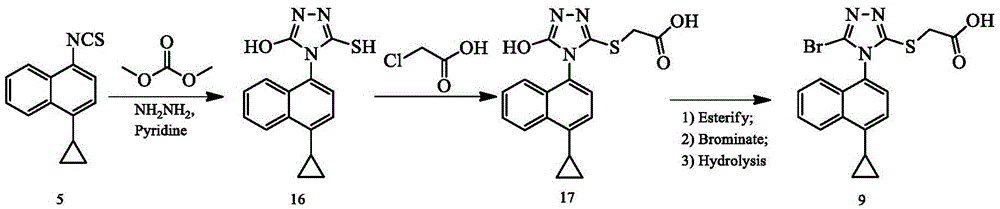
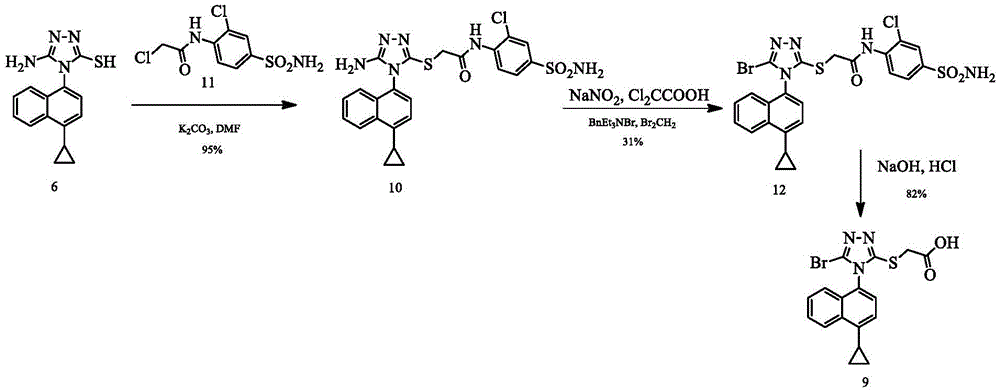
The invention discloses a preparing method of a triazole thioglycolic acid compound for curing metabolic arthritis. According to the method, compound II and compound III are utilized as initial materials and are generated into intermediate compound IV through Suzuki reaction; then nucleophilic substitution happens between the intermediate compound IV and 1,1minute-thiocarbonyl polypyridobisinudazole to generate intermediate compound V; the compound V generates intermediate compound VI through cyclization reaction, and then nucleophilic substitution happens between the compound VI and methyl chloroacetate to generate intermediate compound VII; the intermediate compound VII generates intermediate compound VIII through bromination reaction; and a target product 2-((5-bromine-4-(4-cyclopropyl naphthalene-1-yl)-4H-1,2,4-triazole-3-yl)sulfenyl) acetic acid (I) is generated through hydrolysis reaction. The preparing method is high in selectivity and simple to operate and avoids poisonous reagents and rigorous reacting conditions. Compared with an original synthetic method, the reacting time of the preparing method is shortened, the energy consumption is reduced, and the reaction yield is improved.
Owner:SHANDONG HAIYA PHARMA TECH CO LTD
Environmental-protection preparation method of betaine hydrochloride
InactiveCN101863785ALow costSeparation is simple and sufficientOrganic compound preparationAmino-carboxyl compound preparationChloroacetic acidsBetaine Hydrochloride
The invention belongs to the technical field of fine chemical engineering, in particular to an environmental-protection preparation method of betaine hydrochloride, which is characterized by comprising the following steps: firstly, carrying out neutralization reaction by taking calcium hydroxide or calcium oxide, chloroacetic acid, trimethylamine and sulfuric acid as raw materials to generate calcium chloroacetate; carrying out quaternization reaction with the trimethylamine; and then acidizing with the sulfuric acid to prepare the betaine hydrochloride. The invention does not need expensive alkali and salt, and therefore, the cost is 15-20 percent lower than that of the traditional process; the product has simple and sufficient separation, and three wastes can not be generated, and therefore, the betaine hydrochloride has higher economy and environment protection.
Owner:山东齐鲁中牧生物科技有限公司 +1
Fluroxypyr sec-octyl ester synthesizing method
A fluroxypyr sec-octyl ester synthesizing method comprises the following steps that (1) 4-amino-3,5-dichloro-6-fluorine-2-pyridine potassium phenoxide is dissolved in N-methyl pyrrolidone, an excessive amount of methyl chloroacetate is added into the solution, and stirring and temperature rising are conducted for reaction, wherein the reaction time is 5-5.5 hours and the reaction temperature is 52 DEG C-55 DEG C; (2) an excessive amount of sec-octyl ester and 4-amino-3,5-dichloro-6-fluorine-2 pyridine oxygen methyl acetate are mixed and stirred and o.5%-1% of butyl titanate is added, wherein the alcohol and ester molar ratio is 2-2.3:1, the reaction temperature is 115 DEG C and the reaction time is 4-4.5 hours; after reaction ends, fluroxypyr sec-octyl ester is obtained.
Owner:JIANGSU INST OF ECOMONES
Method for producing cloquintocet-mexyl
The invention discloses a method for producing cloquintocet-mexyl, and belongs to the field of herbicides. By taking 5-chlorine-8-hydroxyquinoline, methyl chloroacetate, 2-heptanol, a solvent 1, a solvent 2, a solvent 3, a catalyst 1, a catalyst 2 and a catalyst 3 as raw materials, the method comprises the following steps: 1) synthesizing an intermediate 5-chlorine-8-quinoline oxygroup methyl acetate; and 2) synthesizing the cloquintocet-mexyl, thereby obtaining a final product. The method has the advantages that the general-purpose equipment is adopted; the process is simple; and the reaction condition is moderate. By utilizing the synthesizing method of ester exchange, the synthesized cloquintocet-mexyl is less in impurity, high in purity, high in yield and good in quality. The cloquintocet-mexyl is mainly utilized to be compounded with clodinafop-propargyl at a ratio of 1:4 so as to form the herbicide.
Owner:新沂市永诚化工有限公司
Cold-resistant acrylate rubber
The invention relates to cold-resistant acrylate rubber. The main chain of the rubber is a saturated carbon chain, and the rubber is synthesized by an emulsion polymerization method. The scheme comprises the following steps of: fully emulsifying 40 to 90 mass percent of butyl acrylate, 30 to 60 mass percent of ethyl acrylate and 1 to 10 mass percent of vinyl chloroacetate with 1 to 10 percent emulsifier aqueous solution in a reaction kettle, performing polymerization reaction under the initiation of 0.0001 to 0.1 mass percent of initiator, demulsifying reaction liquid in 2 to 20 percent electrolyte salt solution, coagulating to form a white granular elastomer, and washing and drying to obtain a product. The invention has the advantages that: the cold-resistant acrylate rubber has high physical and mechanical properties and postprocessing property and can meet the requirements of automobiles, motorcycles and the like on rubber materials; and the finished product has high cold resistance, heat resistance and oil resistance.
Owner:JIUJIANG SHILONG RUBBER
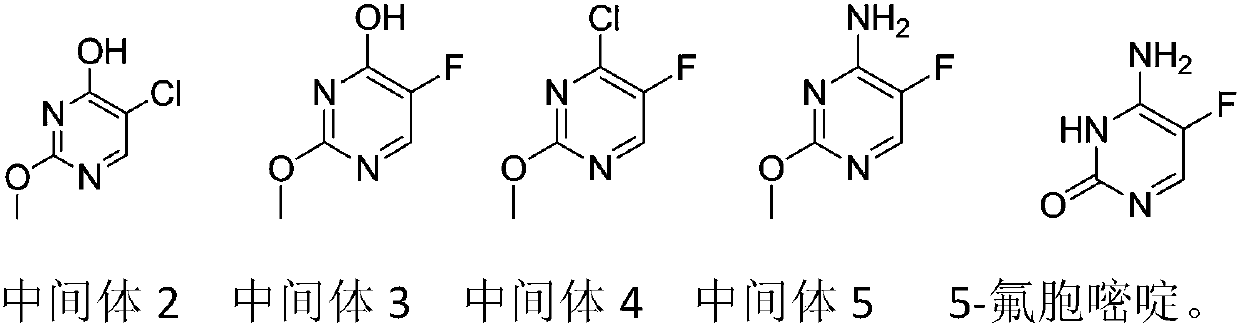
Preparation method of 5-flucytosine
ActiveCN108033917AReduce usageThe process is environmentally friendlyOrganic chemistryChemical synthesisPotassium fluoride
The invention belongs to the technical field of chemical synthesis of medicines and relates to a preparation method of 5-flucytosine. The preparation method comprises the following steps: utilizing ethyl formate and methyl chloroformate to synthesize 2-chloro-3-oxo methyl propionate, then utilizing oxymethylisourea to close rings to obtain pyrimidine rings, utilizing potassium fluoride to substitute chlorine on the pyrimidine rings, utilizing phosphorus oxytrichloride to substitute hydroxyl groups on the pyrimidine rings, then adding ammonia water to lead chloride to be substituted with aminogroups, and hydrolyzing under an acid condition to obtain a product, namely the 5-flucytosine. The preparation method has the beneficial effects that the methyl chloroacetate is adopted for substituting methyl fluoroacetate to be used as a synthetic raw material of the 5-flucytosine, so that the use of highly-toxic chemicals such as the methyl fluoroacetate is avoided; simultaneously, since the price of the methyl chloroacetate is much lower than the price of the methyl fluoroacetate, the production cost can be saved; by utilization of the synthetic route provided by the invention, the higher-purity 5-flucytosine can be prepared without need of complex aftertreatment steps; simultaneously, the preparation method has higher overall yield and obvious industrial value and is worthy of being promoted and used on a large scale.
Owner:ZHEJIANG XIANFENG TECH
Ionic liquid polymer containing imidazole in main chain and method for synthesizing same
The invention provides a synthetic method of ionic liquid polymer containing imidazole in main chain, comprising imidazole respectively reacting with 2-chlorohydrin and methyl chloroacetate to synthesize chloridized 1,3- bi(2-hydroxyethyl)imidazole ionic liquid and chloridized 1,3- bi(2-methoxy-2-oxyethyl)imidazole ionic liquid; the two ionic liquid being used as monomer to perform melt phase polycondensation in the present of catalyst SnCl2. The ionic liquid polymer has good conductivity property (conductivity: 5.4-7.2*10-5S / m.). Because the ionic liquid unit is induced, the new type polymer has application future in polyelectrolyte, gas separation film and so on.
Owner:NORTHWEST NORMAL UNIVERSITY
Method for producing glycine by electrodialysis membrane separation
InactiveCN109180508AReduce consumptionSolve pollutionOrganic compound preparationAmino-carboxyl compound preparationFiltrationSeparation technology
The invention provides a method for producing glycine by an electrodialysis membrane separation technology. The method reduces the consumption of a catalyst, and fundamentally solves the wastewater pollution problem of traditional aqueous phase process glycine technologies. Solid ammonium chloroacetate or solid chloroacetic acid undergoes an ammonia introduction reaction is the presence of a catalyst (urotropine or paraformaldehyde) in aqueous solution to synthesize glycine and ammonium chloride, and cooling crystallization and filtration are carried out to separate glycine and obtain a cyclereaction mother liquor; and ammonium chloride in the cycle reaction mother liquor is separated by an electrodialysis device, and the solid ammonium chloroacetate or chloroacetic acid is added and undergoes a cycle reaction to obtain glycine and a byproduct ammonium chloride.
Owner:刘长飞
N-imidazolyl acetate derivative, and preparation method and application thereof
ActiveCN102603641APromote environmental protectionGood corrosion inhibitionOrganic chemistryLiquid carbonaceous fuelsSolubilityDistillation
The invention discloses an N-imidazolyl acetate derivative, and a preparation method and application of the derivative, wherein the N-imidazolyl acetate derivative is composed of imidazole and acetate groups. The preparation method comprises the following steps: 1) adding chloroacetic acid and low-carbon alcohol into a reaction flask, slowly heating and refluxing for 6-8 hours; after the reaction is complete, performing after-treatment and reduced pressure distillation to obtain colorless chloroacetate liquid; 2) adding imidazole, acetone, polyethylene glycol 400 and potassium carbonate into the reaction flask, mixing uniformly, heating to 75-95 DEG C, gradually dropping chloroacetate, heating to 80-105 DEG C and refluxing for 5.5-6.5 hours after dropping is complete, performing after-treatment and reduced pressure distillation to obtain the N-imidazolyl acetate derivative after the reaction is complete. The N-imidazolyl acetate derivative disclosed by the invention can be used as a multi-effect methanol gasoline corrosion inhibitor and is friendly to the environment, low in toxicity, low in dosage and good in alcohol solubility, and has a multi-effect corrosion inhibition function; and the N-imidazolyl acetate derivative is applied to methanol gasoline corrosion inhibition in a single-component pattern.
Owner:福建中源新能源股份有限公司
Alkylamine ether derived surfactants and preparation method thereof
ActiveCN106187789APrevents yellowingOrganic compound preparationGroup 5/15 element organic compoundsChemical structurePhosphate
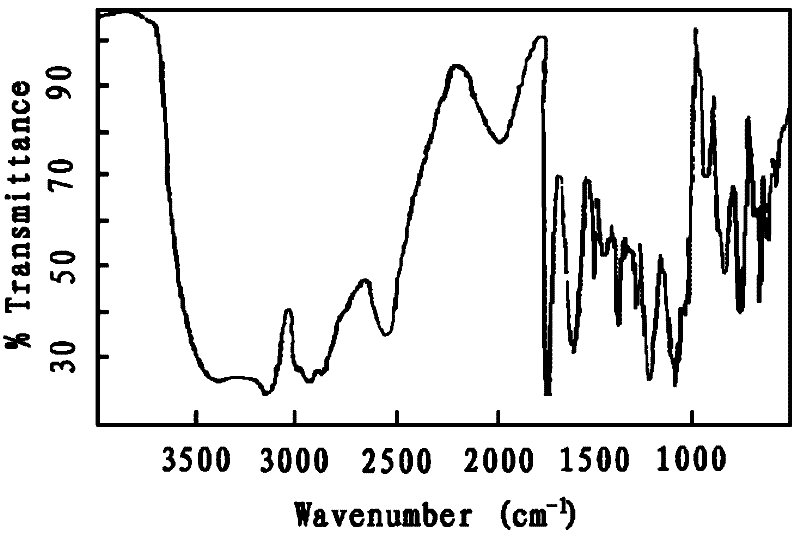
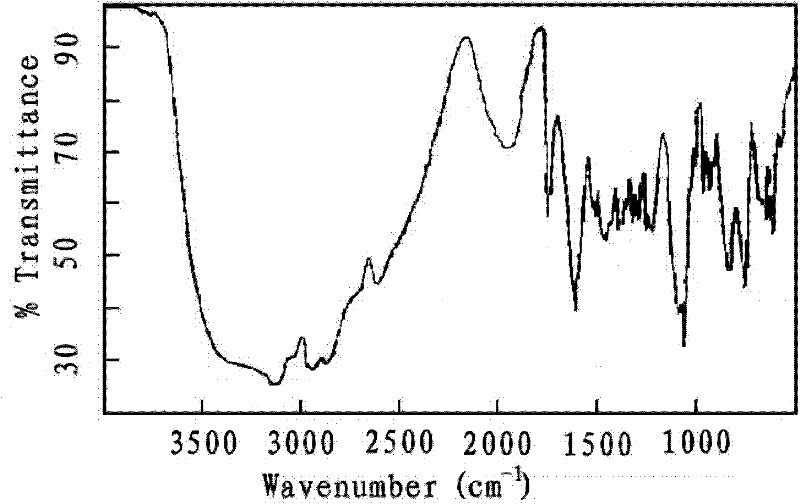
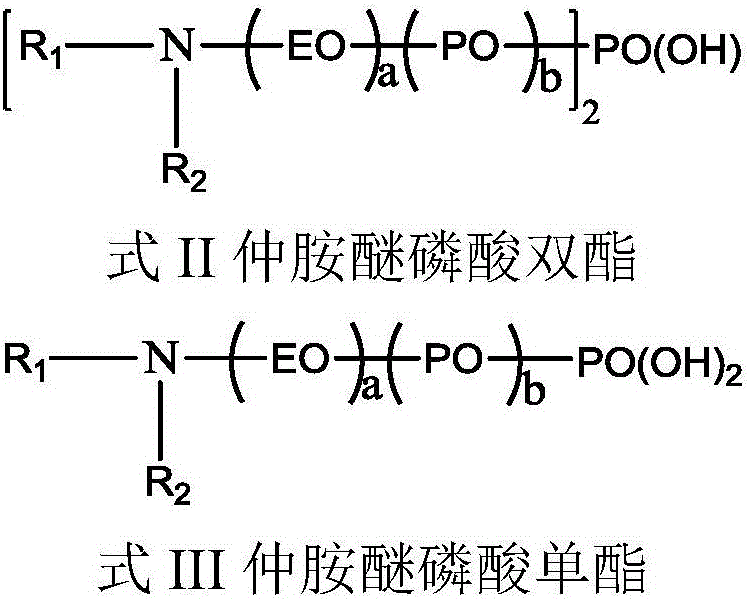
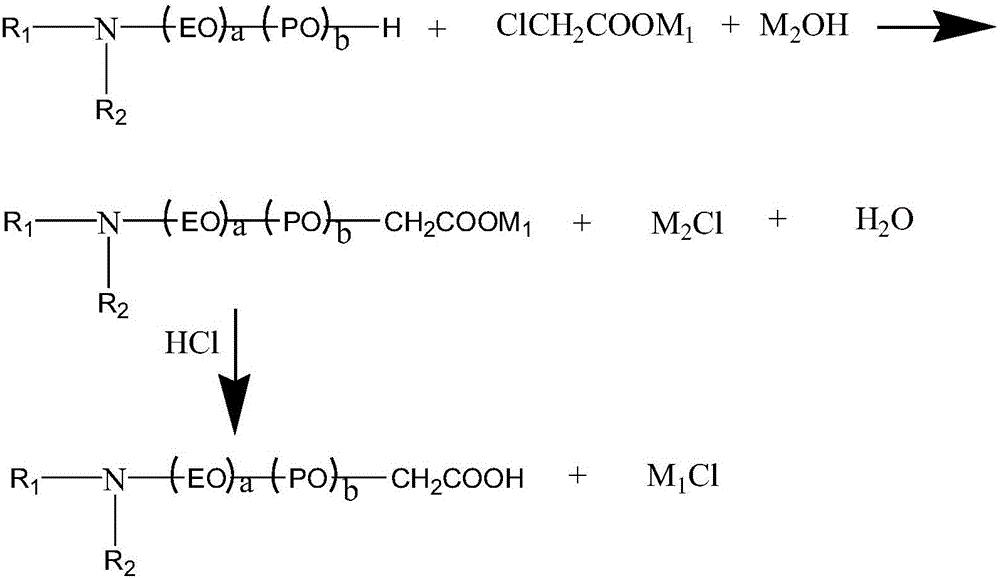
The invention relates to alkylamine ether derived surfactants with chemical structures as shown in a formula I, a formula II and a formula III in the specification. In the formulas, R1 and R2 are respectively selected from the group consisting of C4-C22 straight-chain or branched-chain saturated alkyl or unsaturated alkyl; EO and PO are described in the specification; R1 and R2 are the same or different; and a and b are integers in a range of 0 to 100 and are not 0 at the same time. The invention also relates to a method for preparing the alkylamine ether derived surfactants. The method comprises the following steps: subjecting secondary amine ether and chloroacetate to a reaction under hydroxide of a metal so as to generate secondary amine ether carboxylate; and acidifying the secondary amine ether carboxylate with hydrochloric acid, and separating the obtained product so as to obtain amine ether carboxylic acid, or subjecting secondary amine ether and phosphorus pentoxide to phosphate esterification so as to obtain monoester and diester of secondary amine ether. According to different adduct number of an amine ether alkyl group, PO and EO, performances of the finally-obtained surfactants are adjusted from a molecular structure. The synthetic method provided by the invention is simple and convenient, facilitates to operation and has little harm to the environment. According to the invention, the secondary amine ether carboxylic acid is described as the formula I in the specification; the secondary amine ether phosphate diester is described as the formula II in the specification; and the secondary amine ether phosphate monoester is described as the formula III in the specification.
Owner:山东转化科技有限公司
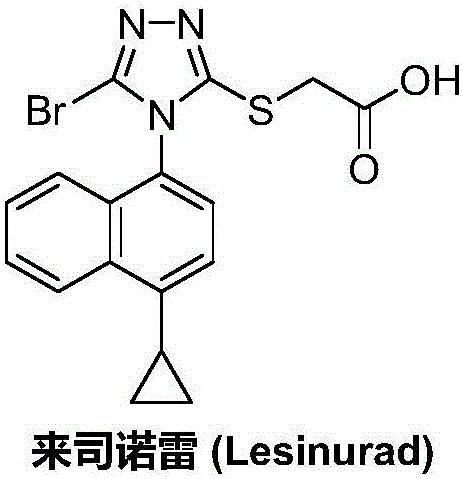
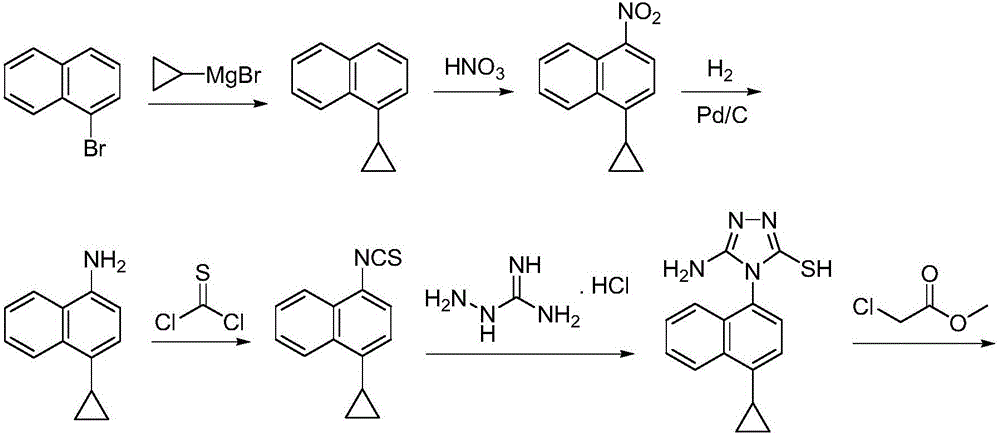
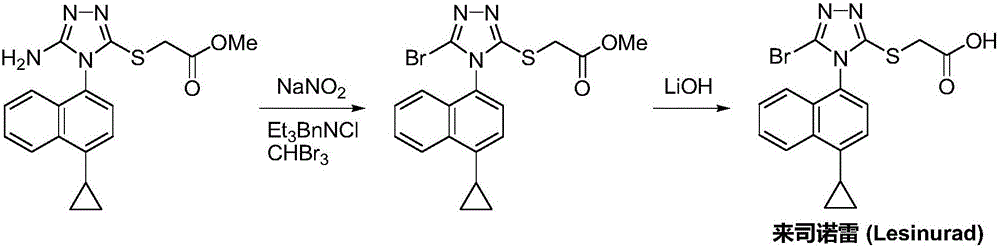
Preparation method of lesinurad
The invention discloses a preparation method of lesinurad. The method comprises: allowing 1-cyclopropylnaphthalene-4-yl isorhodanate and acethydrazide to undergo an addition reaction, allowing the obtained 4-acetyl-1-(4-cyclopropylnaphthalene-1-yl) thiosemicarbazide to undergo a cyclization reaction in the function of an alkali reagent, allowing the obtained 4-(4-cyclopropylnaphthalene-1-yl)-5-methyl-4H-1,2,3-triazole-3-mercaptan and methyl chloroacetate (or methyl bromoacetate) to undergo a condensation reaction, allowing the obtained 2-{[4-(4-cyclopropylnaphthalene-1-yl)-5-methyl-4H-1,2,4-triazole-3-yl]sulfo} methyl acetate to undergo an oxidation reaction, allowing the obtained 5-[(methoxycarbonyl)methylthio]-4-(4-cyclopropylnaphthalene-1-yl)-4H-1,2,4-triazole-3-carboxylic acid undergo a bromination reaction, and carrying out an esterolysis reaction to obtain lesinurad. The method is reasonable and simple in technical route, simplified in operation and low in cost. The method is green and eco-friendly and suitable for industrial production.
Owner:湖南欧亚药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com