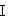Synthesis method of dye intermediate
The technology of a dye intermediate and a synthesis method is applied in the field of preparation of the dye intermediate, and can solve the problems of easy decomposition of methyl chloroacetate recovery, large proportion of methyl chloroacetate, difficulty in recovery and reuse, and the like, so as to eliminate the need for distillation and recovery. Process, production environment improvement, avoid the effect of storage perishable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
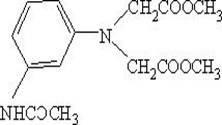
[0031] Add 162.8 g (1.50 mol) of methyl chloroacetate and 100.0 g of toluene into the flask, add 75.0 g (0.50 mol) of m-aminoacetanilide, 66.5 g of sodium carbonate, and 7.7 g of sodium bromide while stirring, and beat for 30 minutes. Slowly raise the temperature to 110~115°C, reflux and divide water, react for 6 hours to the end, and the main content is >96%. Cool to 40°C, add 450g of glacial acetic acid to obtain about 600g of esterification solution of dye intermediate with structural formula (I-1), and the yield is about 92.5% (calculated based on the coupling value). Collect the oil-water mixture separated by reflux, remove the water layer, and the oil layer will be used in the next batch.
[0032] (I-1)
[0033] Apply mechanically: add 135.6g (1.25mol) of methyl chloroacetate to the flask and separate the oil layer from the previous batch (less than 100g, add toluene to 100g), add m-aminoacetanilide 75.0g (0.50mol), sodium carbonate 66.5 g, sodium bromide 7.7g, beati...
Embodiment 2
[0037] Add 162.8g (1.50mol) of methyl chloroacetate and 100g of benzene into the flask, and add 90.0g (0.50mol) of 2-amino-4-acetamidoanisole, 105.4g of sodium bicarbonate and 3.3g of sodium bromide under stirring , beating for 30 minutes. Slowly raise the temperature to 110~115°C, reflux and divide water, react for 8 hours to the end, and the main content is >95%. Cool to 30°C, add 250g of glacial acetic acid to obtain about 420g of esterification solution of dye intermediate with structural formula (I-2), and the yield is about 90% (calculated based on the coupling value). Collect the oil-water mixture separated by reflux, remove the water layer, and the oil layer will be used in the next batch.
[0038] (I-2)
[0039]Apply mechanically: Add 135.6g (1.25mol) of methyl chloroacetate to the flask and separate the oil layer from the previous batch (less than 100g, add benzene to 100g), add 90.0g of 2-amino-4-acetamidoanisole ( 0.50mol), sodium bicarbonate 105.4g, sodium ...
Embodiment 3
[0042] Add 81.4 g (0.75 mol) of methyl chloroacetate and 120 g of n-propanol in the flask, and add 133.0 g (0.50 mol) of 3-(N-methoxyisopropionyl) amino-4-methoxyacetanilide, hydrogen Magnesium oxide 19.0g, sodium bromide 3.9g, beating for 30min. Slowly raise the temperature to 100~105°C, reflux and divide water, react for 8 hours to the end, and the main content is >94%. Cool to 40°C, add 270g of glacial acetic acid to obtain about 450g of esterification solution of dye intermediate with structural formula (I-3), and the yield is about 90% (calculated based on the coupling value). Collect the oil-water mixture separated by reflux, remove the water layer, and the oil layer will be used in the next batch.
[0043] (I-3)
[0044] Apply mechanically: Add 67.8g (0.625mol) of methyl chloroacetate to the flask and separate the oil layer from the previous batch (less than 120g, add n-propanol to 120g), add 3-(N-methoxyisopropionyl)amino under stirring - 133.0 g (0.50 mol) of 4-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com