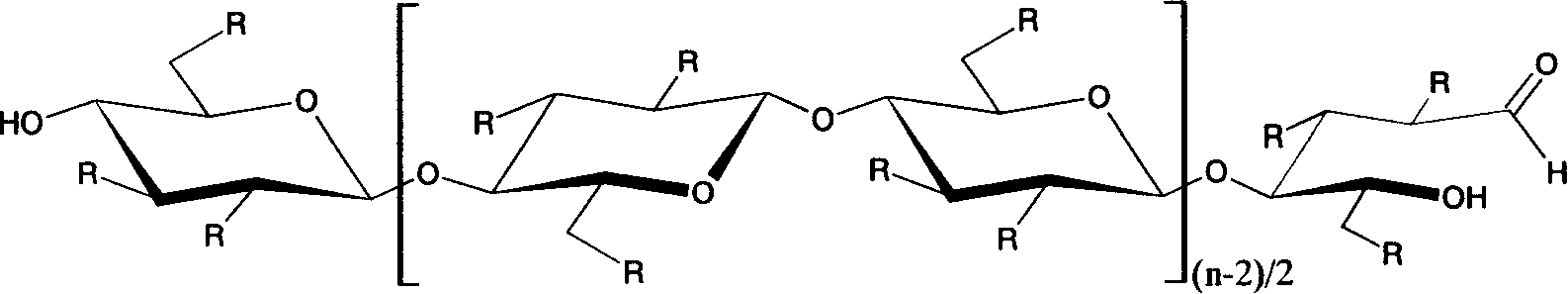
Method for preparing carboxymethyl cellulose in high degree of substitution
A technology of carboxymethyl cellulose and natural cellulose, applied in the field of polymer chemistry, can solve the problems of product polymerization degree and viscosity decrease, high process cost and high energy consumption, and achieve the effect of high etherification degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
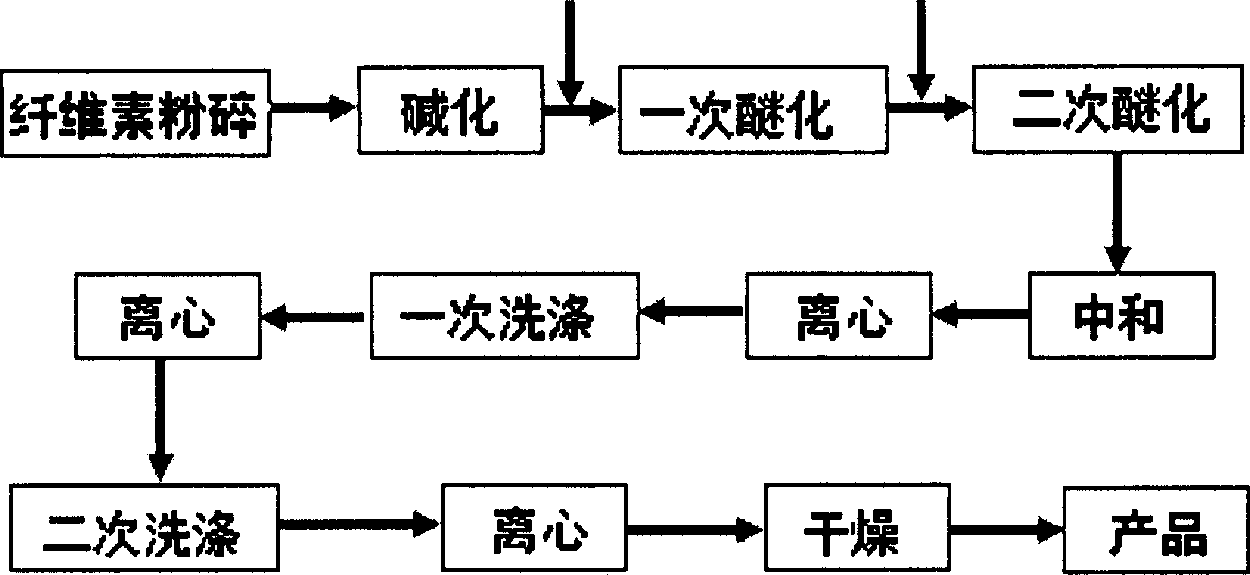
[0023] Under the reaction condition of 20°C, 450 parts of 40% NaOH aqueous solution and 1850 parts of isopropanol / toluene (85 / 15) were made into 2300 parts of mixed solution, 200 parts of cellulose were added and alkalized for 45 minutes; in 1 hour Slowly add 1050 parts of 65% chloroacetic acid-isopropanol / toluene solution, stir for 30 minutes, then add 690 parts of 40% lye, so far the reaction temperature can never exceed 40°C; heat up to 60°C and keep the temperature for reaction 1.5 hours; 425 parts of 40% NaOH aqueous solution was added, the temperature was raised to 72-74° C., and the temperature was maintained for 1.5 hours. Then use glacial acetic acid to neutralize to PH=7, wash the filter cake once with 72% and 75% ethanol aqueous solution after centrifugation, and dry in a vacuum oven at 80°C for 4 hours to obtain the product.
Embodiment 2
[0025] Under the reaction condition of 35°C, 500 parts of 35% NaOH aqueous solution and 2050 parts of n-butanol were formulated as 2550 parts of mixed solution, 200 parts of cellulose were added and alkalized for 1 hour; 70% chlorine was slowly added within 70 minutes 950 parts of acetic acid-n-butanol solution, stirred for 20 minutes, and then 190 parts of NaOH solid was added, so far the reaction temperature could not exceed 40°C; the temperature was raised to 65°C and kept for 2 hours; 400 parts of 45% NaOH aqueous solution was added, The temperature was raised to 75° C., and the reaction temperature was kept for 1 hour. Then use glacial acetic acid to neutralize to PH=7, wash the filter cake once with 79% and 82% methanol aqueous solution after centrifugation, and dry in a vacuum oven at 70°C for 6 hours to obtain the product.
Embodiment 3
[0027] Under the reaction condition of 10°C, 350 parts of 40% KOH aqueous solution and 1650 parts of isopropanol / ethanol (86 / 14) were made into 2000 parts of mixed solution, 200 parts of cellulose were added and alkalized for 30 minutes; in 50 minutes Slowly add 995 parts of 60% chloroacetic acid-propanol / ethanol solution, stir for 40 minutes, then add 750 parts of 45% lye, so far the reaction temperature cannot exceed 40°C; heat up to 55°C and keep the temperature for 3 hours; then add 180 parts of KOH solid, raise the temperature to 70°C, and keep the temperature for reaction for 2.5 hours. Then use glacial acetic acid to neutralize to PH=7, wash the filter cake once with 70% and 78% acetone aqueous solution after centrifugation, and dry in a vacuum oven at 85°C for 2 hours to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com