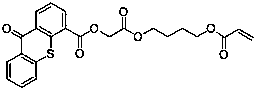
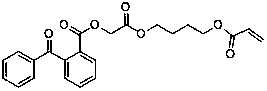
Polymerizable free radical II type photoinitiators and preparation method thereof
A photoinitiator and free radical technology, applied in the field of polymerizable free radical type II photoinitiator and its preparation, can solve the problems of difficult promotion, poor storage stability, complex synthesis process, etc., and achieve strong operability and strong adaptability , The preparation process is simple and controllable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: methyl thioxanthone-4-formyloxyacetate
[0051] In a 500ml four-neck flask equipped with mechanical stirring, add 50.0g thioxanthone-4-carboxylic acid, 250 ml tetrahydrofuran, 23.6g triethylamine, 25.6 g methyl chloroacetate, and heat to 50~60°C for 8 hours , suction filtration, the filtrate was cooled to 0~5°C, stirred and crystallized for 2 hours, and then the crude product was obtained by suction filtration. The crude product was recrystallized with toluene, and after drying, 53.7g of yellow flaky crystals were obtained. The yield was 84%, and the content was ≥98.0%. 1 H NMR (DMSO) δ: 8.25 (m, 1H), 8.16 (m, 1H), 8.01 (m, 1H), 7.83 (d,1H), 7.66 (m, 2H), 7.42 (m, 1H), 5.17 (s, 2H), 3.55 (s, 3H).
Embodiment 2
[0052] Embodiment 2: methyl thioxanthone-4-formyloxyacetate
[0053] In a 500ml four-neck flask equipped with mechanical stirring, add 50.0g thioxanthone-4-carboxylic acid, 250 ml tetrahydrofuran, 35.9g diisopropylethylamine, 28.9g methyl chloroacetate, and heat to reflux for 8 hours. Cool down to 40~50°C and filter with suction, cool the filtrate to 0~5°C, stir and crystallize for 2 hours, and then filter with suction to obtain the crude product. The crude product is recrystallized with toluene, and after drying, 48.1g of yellow flaky crystals are obtained. The yield is 75%, and the content is ≥ 98.0%.
Embodiment 3
[0054] Embodiment 3: Methyl benzophenone-2-formyloxyacetate
[0055] Add 22.6g of benzophenone-2-carboxylic acid, 120ml of tetrahydrofuran, 15.1g of triethylamine, and 11.8g of methyl chloroacetate into a 250ml four-necked flask equipped with mechanical stirring, and heat to 50~60°C for 8 hour, suction filtration, the filtrate was cooled to 0~5°C, stirred and crystallized for 2 hours, and the crude product was obtained by suction filtration. The crude product was recrystallized with toluene, and after drying, 27.1g of white crystals were obtained. The yield was 91%, and the content was ≥95.0%. 1 H NMR (DMSO) δ: 8.30 (t, 1H), 8.12 (d, 1H), 7.88 (m, 2H), 7.73 (d,1H), 7.51~7.63 (m, 4H), 5.20 (s, 2H) , 3.58 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com