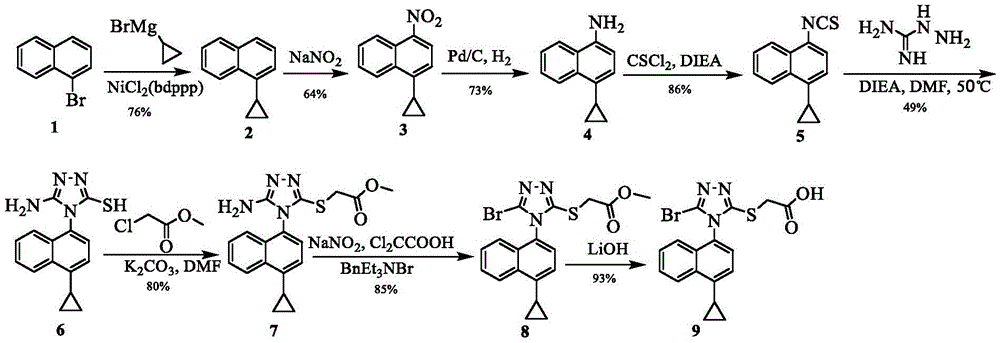
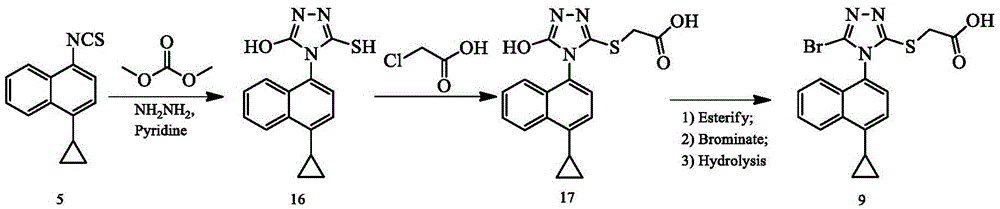
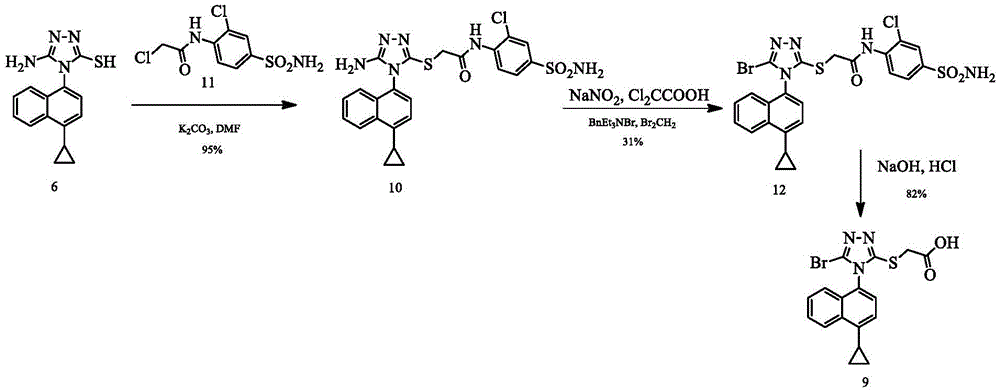
Preparing method of triazole thioglycolic acid compound for curing metabolic arthritis
A compound, the technology of methyl chloroacetate, which is applied in the field of preparation of triazole thioglycolic acid compounds, can solve the problems of low reaction yield, long reaction time, and harsh reaction conditions, so as to shorten the reaction time, reduce energy consumption, The effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Synthesis of compound 4-cyclopropyl-1-naphthylamine (IV)
[0061]
[0062] 4-Bromo-1-naphthylamine II (90mmol, 20.0g), cyclopropylboronic acid III (116mmol, 10.0g), potassium phosphate (300mmol, 64.0g) and tetrakistriphenylphosphine palladium (6mmol, 7.0g) Add it to a mixed solvent of 100mL toluene and 4mL water, and react at 100°C for 12h under the protection of nitrogen. When the reaction solution is cooled to room temperature, add 100mL of aqueous solution to the reaction solution, extract it three times with ethyl acetate, and then add sodium sulfate to dry it. . Half an hour later, it was filtered, and 13.8 g of intermediate compound IV was obtained by distillation under reduced pressure, with a yield of 83.6%. 1 HNMR (400MHz, DMSO) δ8.25 (d, J = 7.9Hz, 1H, Naph-H), 8.07 (d, J = 8.2Hz, 1H, Naph-H), 7.49 (ddd, J = 8.2, 6.8, 1.1Hz, 1H, Naph-H), 7.39(ddd, J=8.1, 6.8, 1.2Hz, 1H, Naph-H), 7.00(d, J=7.6Hz, 1H, Naph-H), 6.59(d, J=7.7Hz, 1H, Naph-H), 5.54(s, 2H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com