Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
424 results about "1,2,4-Triazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1,2,4-Triazole (as ligand in coordination compounds, Htrz abbreviation is sometimes used) is one of a pair of isomeric chemical compounds with molecular formula C₂H₃N₃, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,4-Triazole is a basic aromatic heterocycle. 1,2,4-Triazole derivatives find use in a wide variety of applications, most notably as antifungals such as fluconazole and itraconazole. 1,2,4-Triazoles can be prepared using the Einhorn–Brunner reaction or the Pellizzari reaction.
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
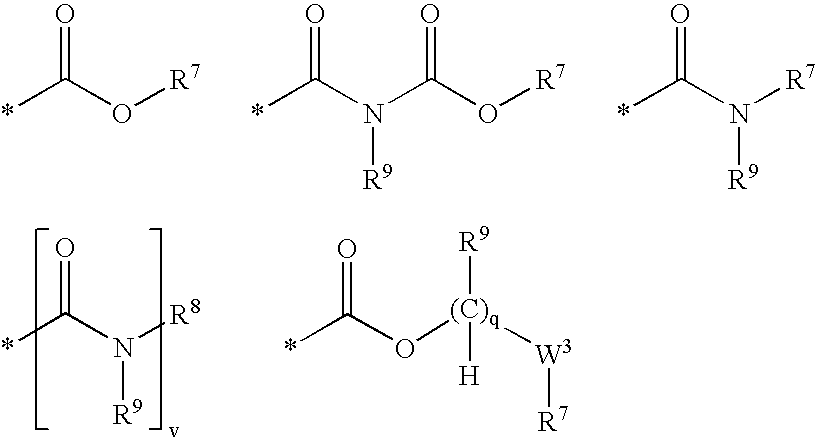
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Stabilized hydrotreated and hydrowaxed lubricant compositions
InactiveUS6410490B1Meet the requirementsLiquid carbonaceous fuelsAdditivesSarcosinePhenolic antioxidant
The instant invention relates to a lubricant composition stabilized against the deleterious effects of heat and oxygen. The composition comprises a hydrotreated or hydrodewaxed oil and an effective antioxidant stabilizing amount of a mixture of a phenolic antioxidant; an N,N-disubstituted aminomethyl-1,2,4-triazole; an aromatic amine antioxidant; an alkyl phenoxy alkanoic acid; and an N-acyl sarcosine derivative. Optionally, further additives are added to the subject lubricant compositions.
Owner:CIBA SPECIALTY CHEM CORP
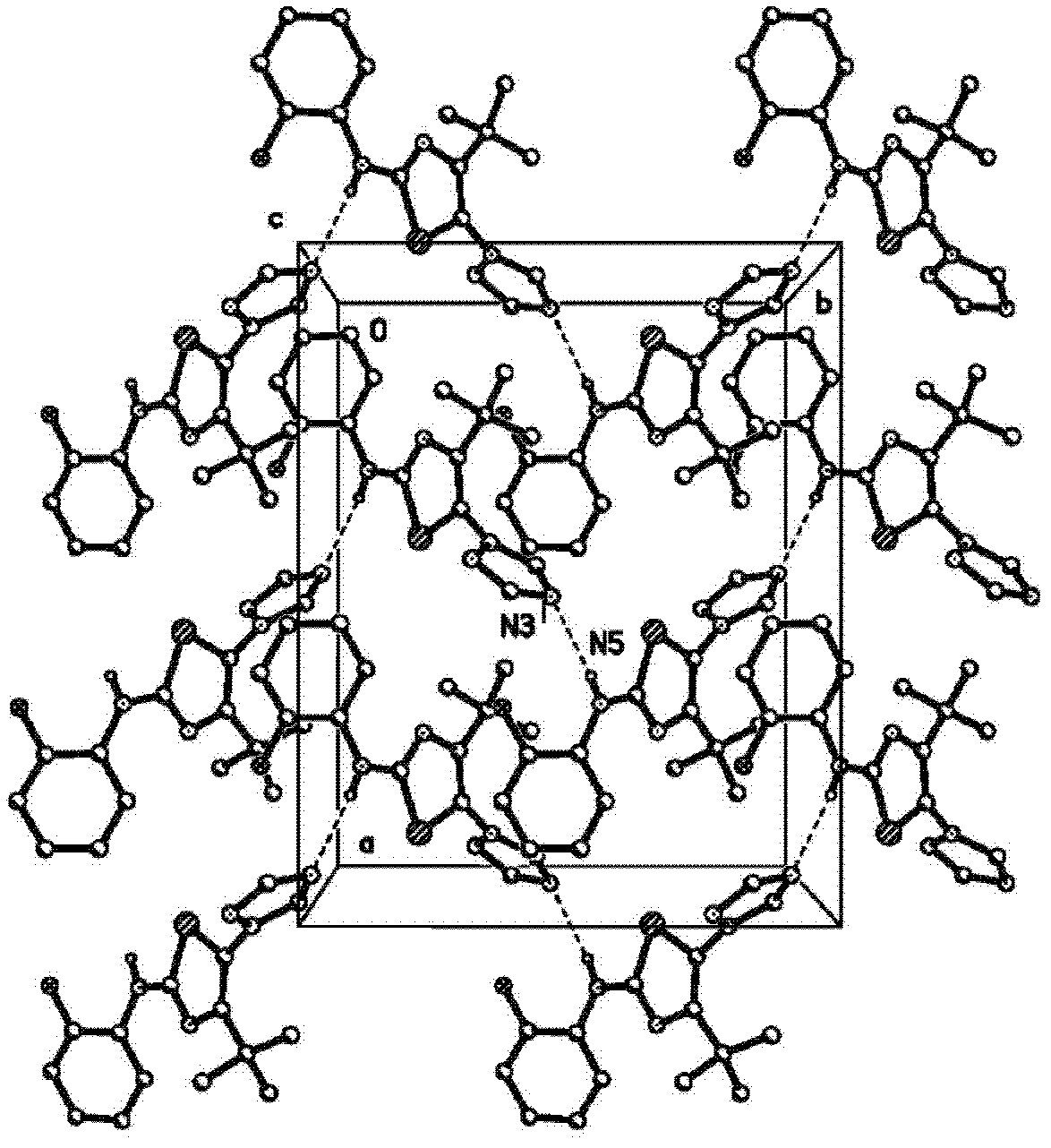
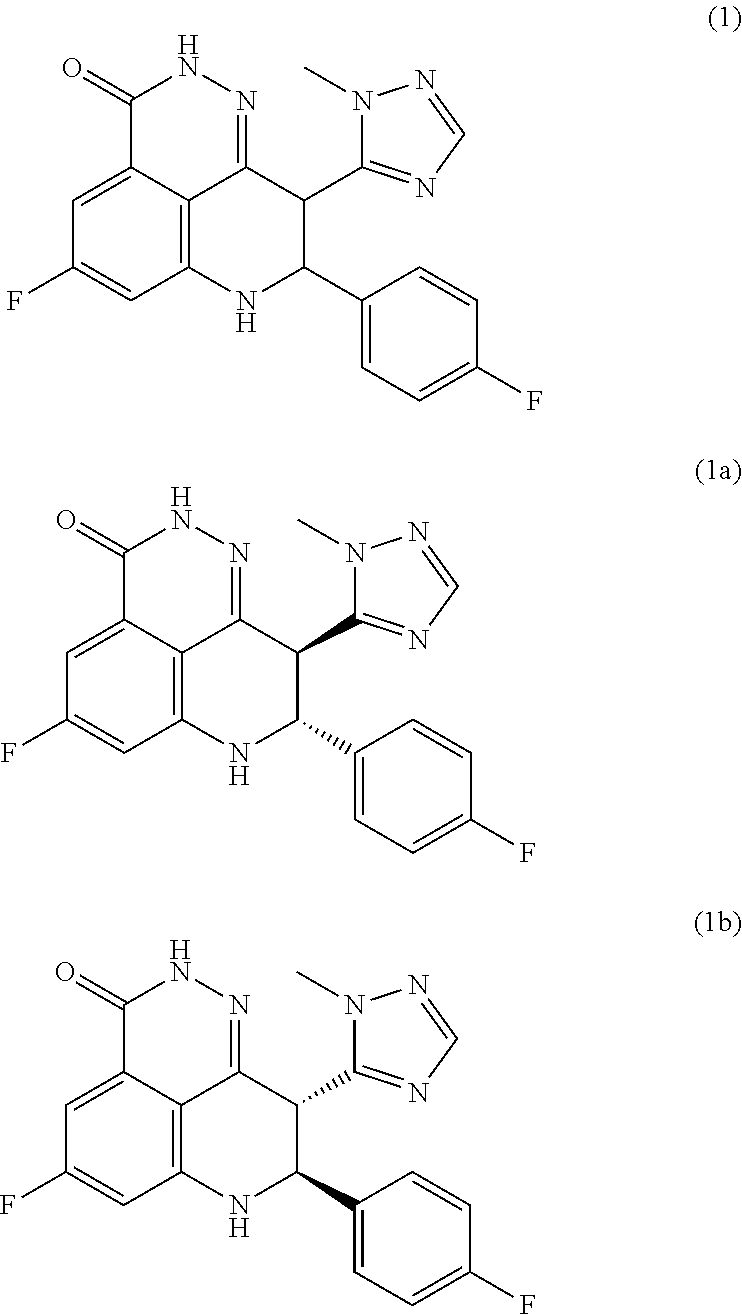
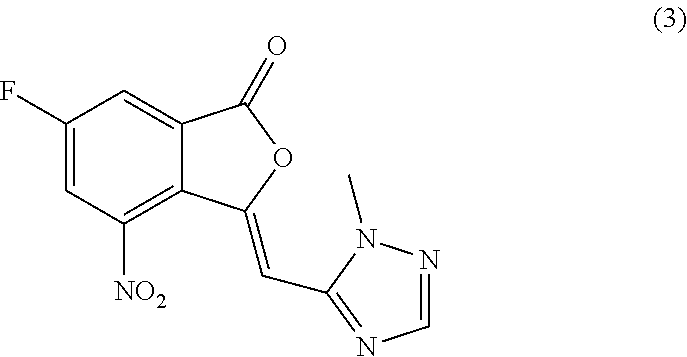
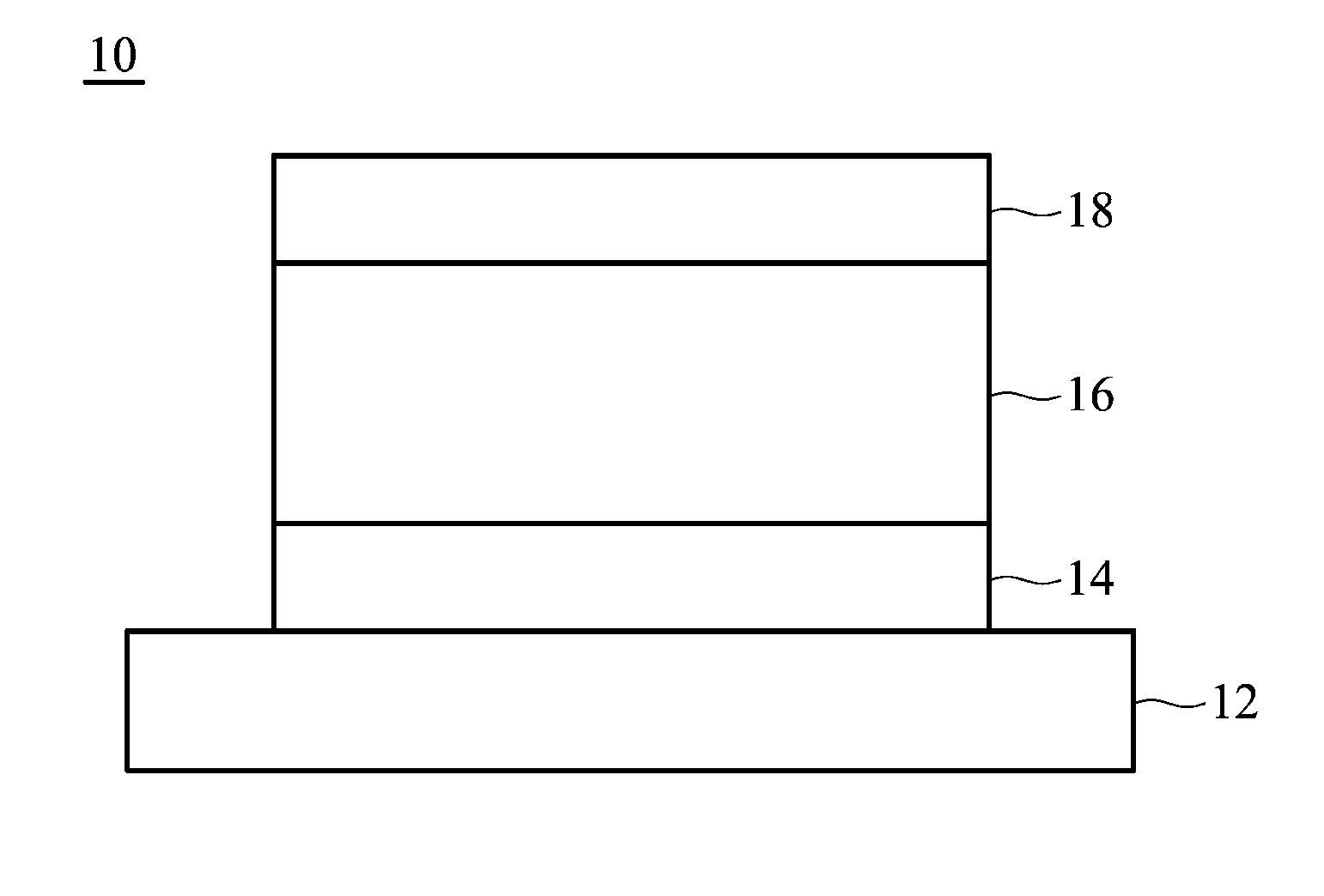
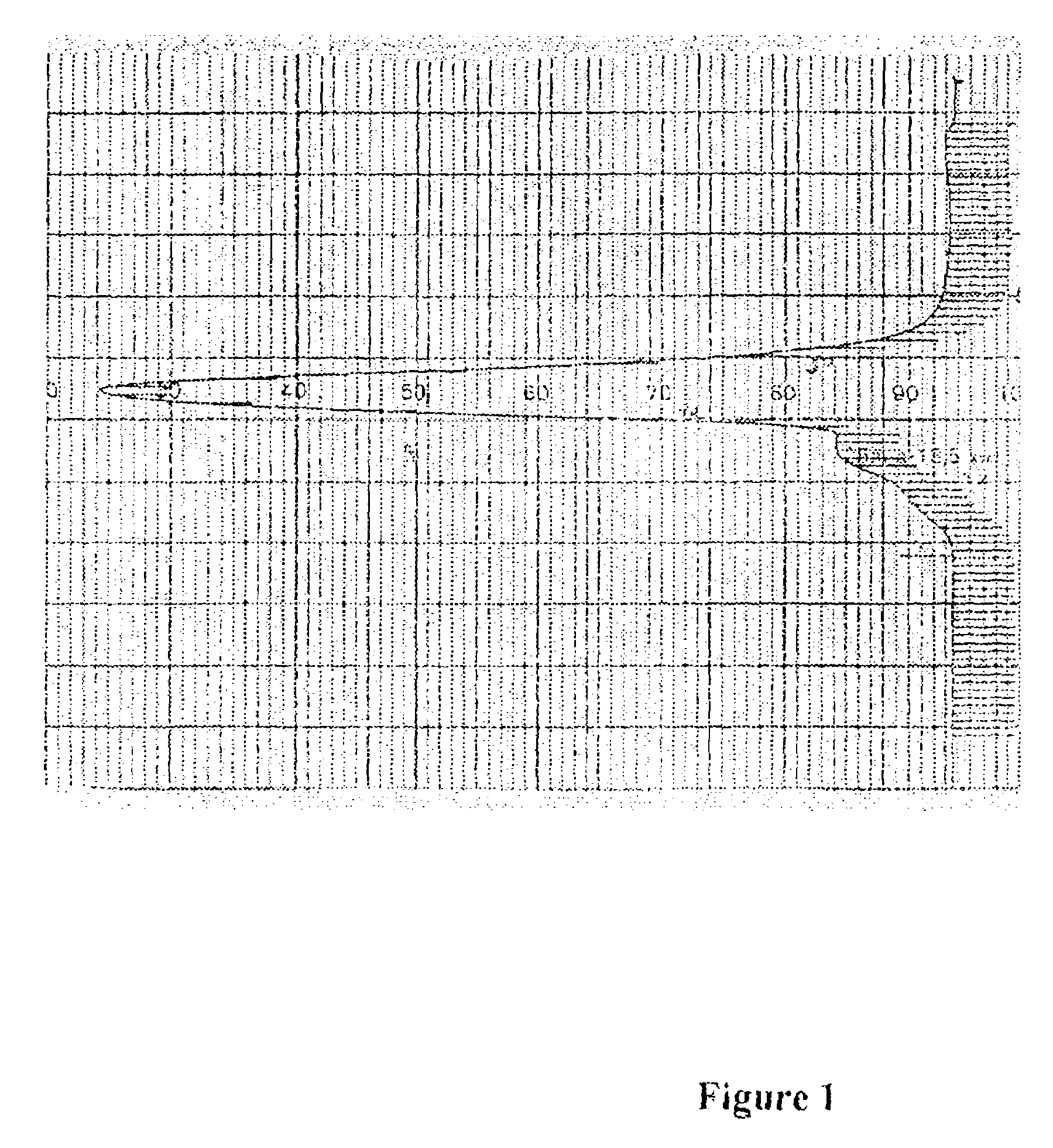
Crystalline (8s,9r)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1h-1,2,4-triazol-5-yl)-8,9-dihydro-2h-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt
Provided herein are (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt forms, including crystalline forms, and methods of their preparation. Pharmaceutical compositions comprising a (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt are also provided, as are methods of using (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt to treat a disease or condition, such as a cancer.
Owner:MEDIVATION TECH INC
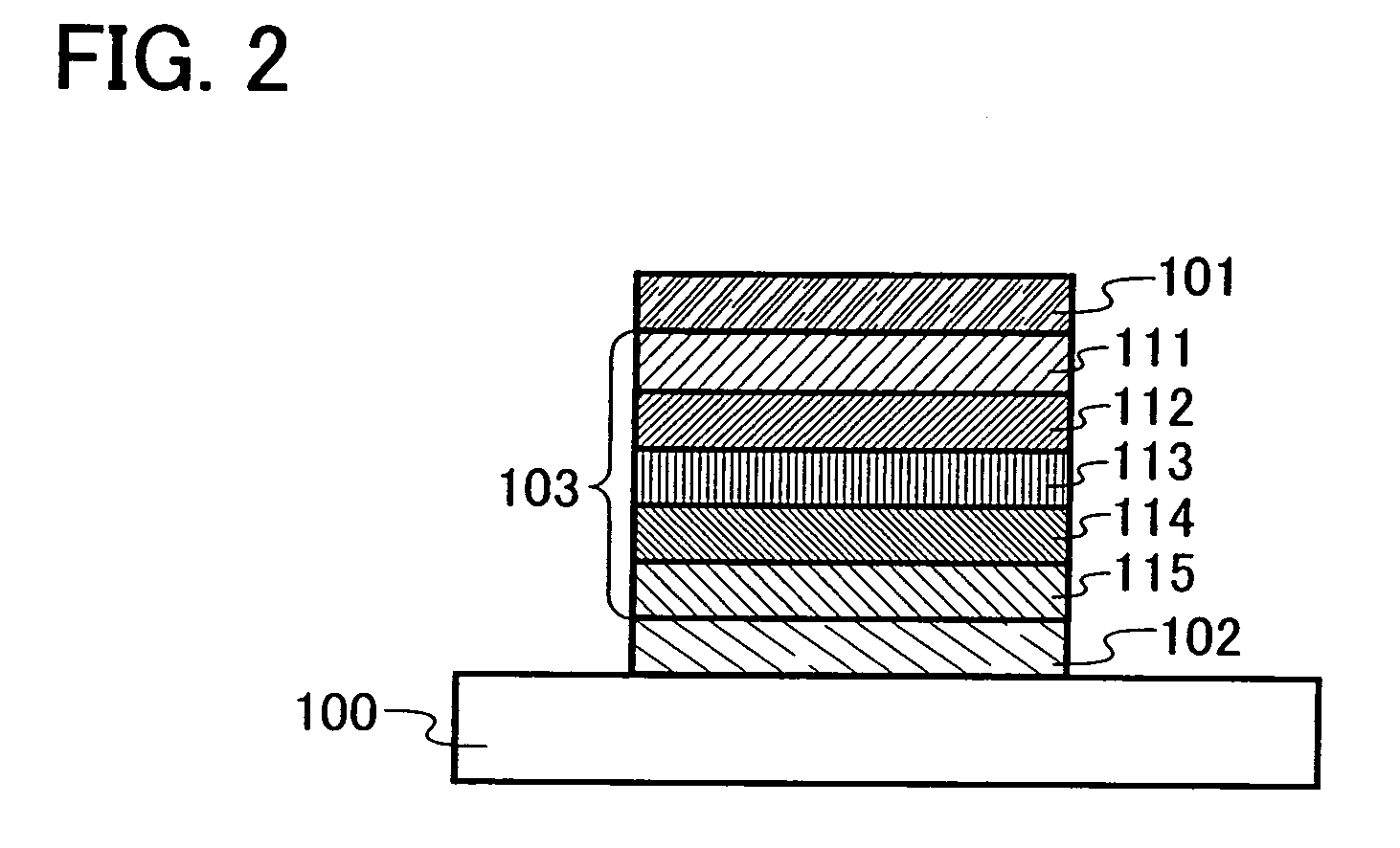
Triazole derivative, and light-emitting device, and electronic device with the use of triazole derivative
ActiveUS20080286607A1High triplet excitation energySolve low luminous efficiencyOrganic chemistryDischarge tube solid anodesArylTriazole derivatives
It is an object of the present invention to provide a novel triazole derivative. Further, it is another object of the present invention to provide a light-emitting element having high luminous efficiency with the use of the novel triazole derivative. Moreover, it is still another object of the present invention to provide a light-emitting device and electronic devices which have low power consumption. A light-emitting element having high luminous efficiency can be manufactured with the use of a triazole derivative which is a 1,2,4-triazole derivative, in which an aryl group or a heteroaryl group is bonded to each of 3-position, 4-position, and 5-position, and in which any one of the aryl group or heteroaryl group has a 9H-carbazol-9-yl group.
Owner:SEMICON ENERGY LAB CO LTD
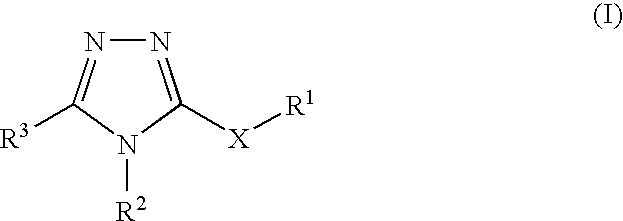
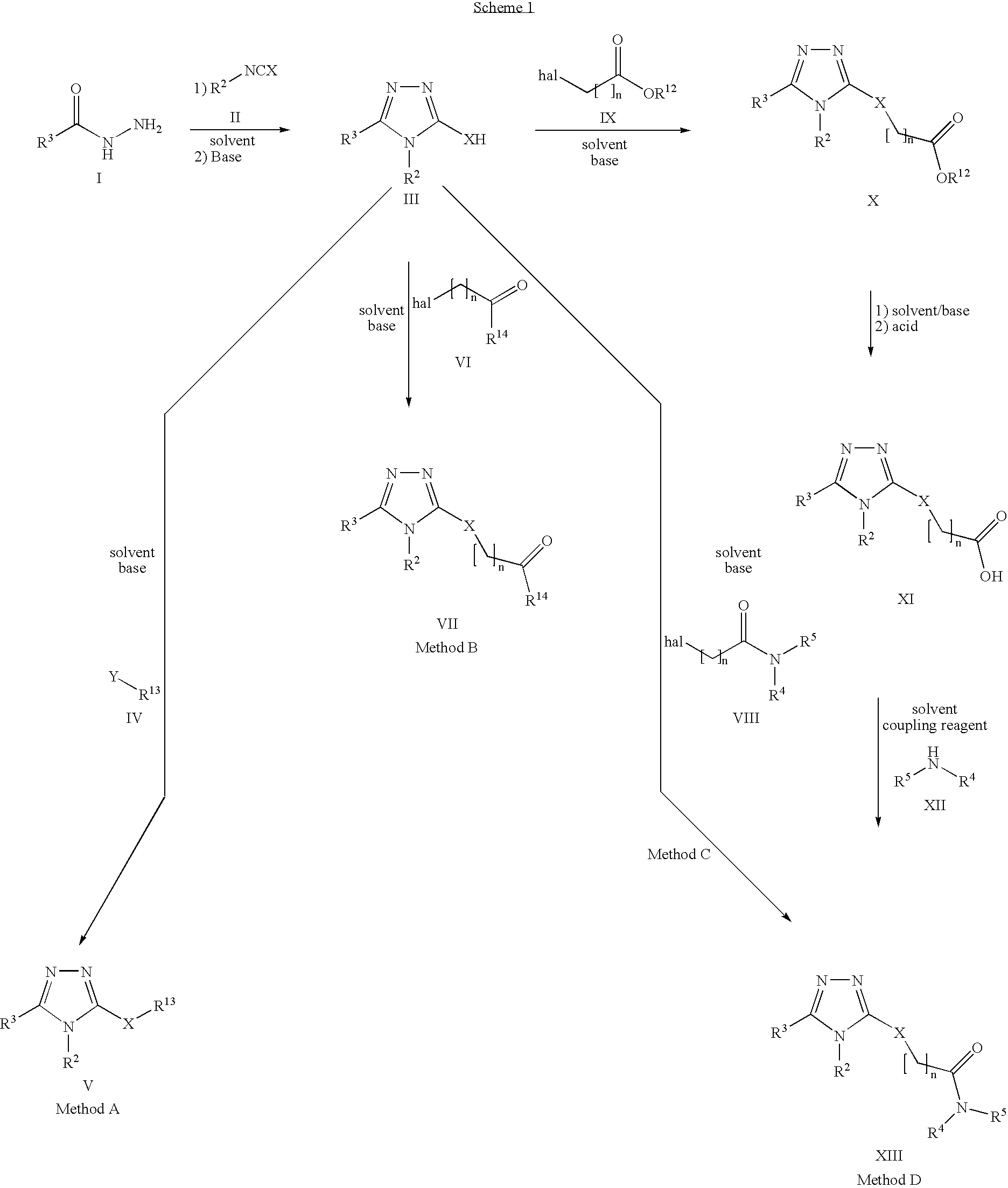
Pharmaceutical use of substituted 1,2,4-triazoles
The use of substituted 1,2,4-triazoles for modulating the activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and the use of these compounds as pharmaceutical compositions has been described. Also a novel class of substituted 1,2,4-triazoles, their use in therapy, pharmaceutical compositions comprising the compounds, as well as their use in the manufacture of medicaments has been described. The present compounds are modulators and more specifically inhibitors of the activity of 11βHSD1 and may be useful in the treatment, prevention and / or prophylaxis of a range of medical disorders where a decreased intracellular concentration of active glucocorticoid is desirable.
Owner:NOVO NORDISK AS
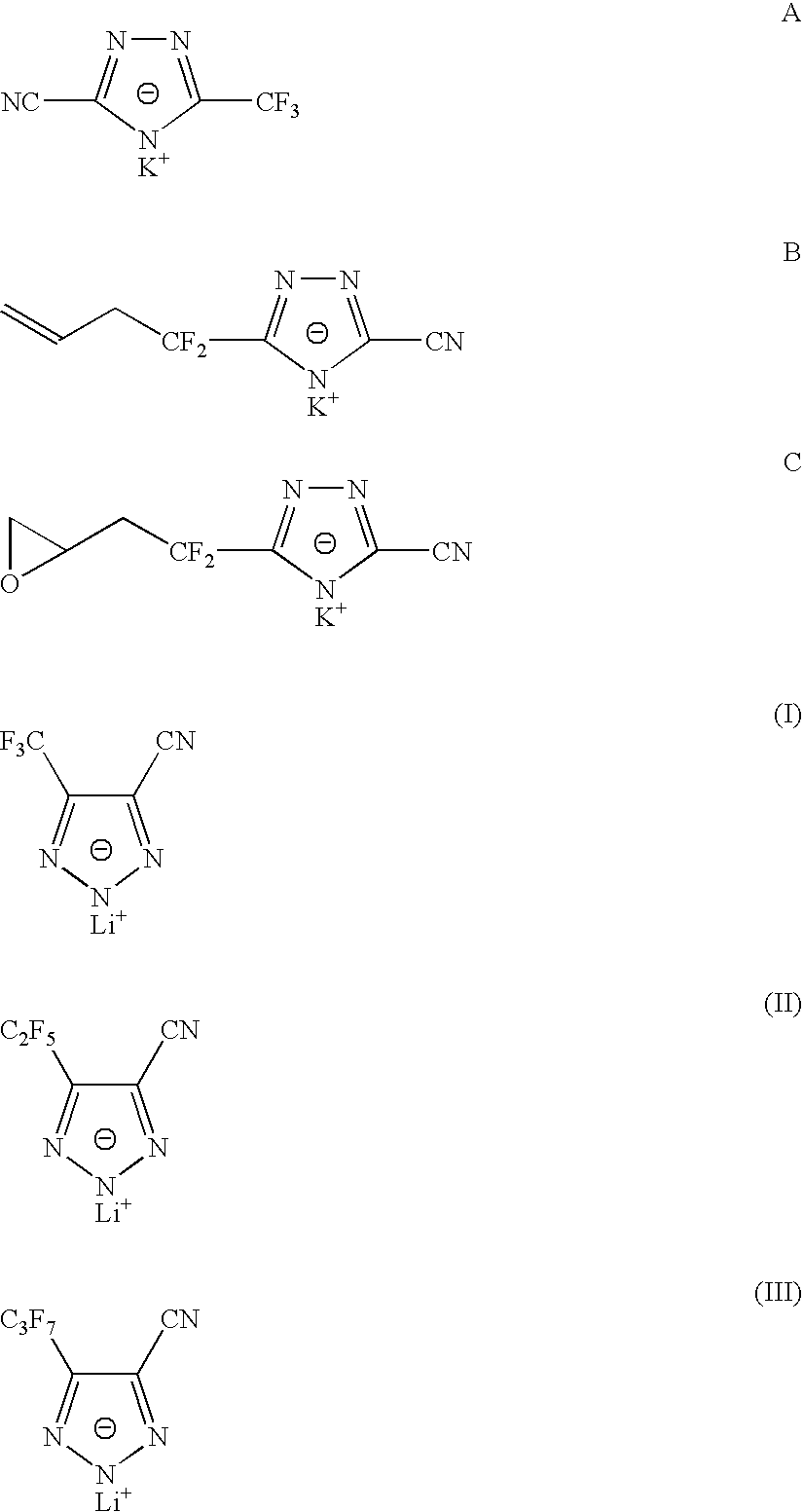
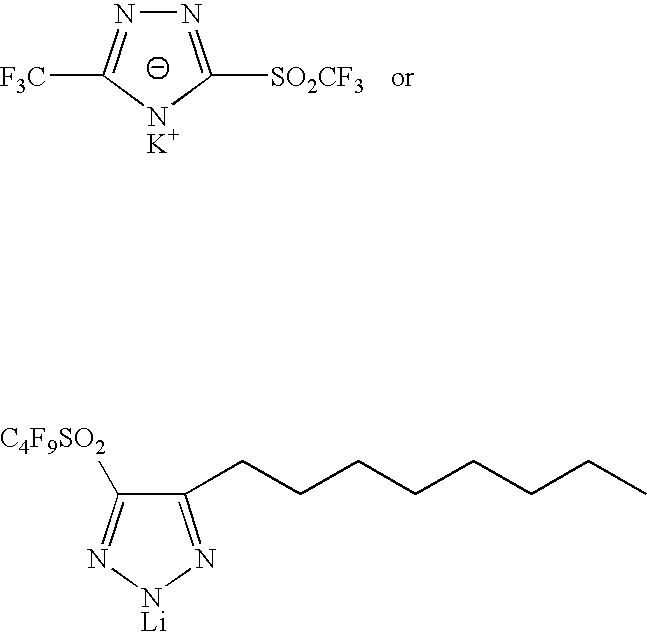
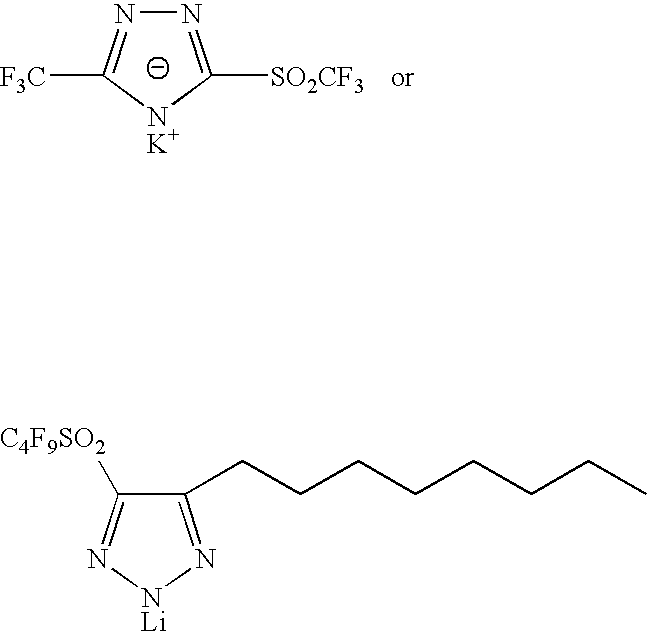
Sulphonyl-1,2,4-triazole salts
InactiveUS7919629B2Easy to prepareImprove conductivityOrganic chemistryChemical reactionSupercapacitor
The invention relates to triazole salts, to their preparation and to applications thereof. The salts have at least one anionic triazolium group which carries at least one chlorosulphonyl, fluorosulphonyl or alkoxyfluorosulphonyl group, each of the anionic groups being combined with a proton or a cation that has a valency of less than or equal to 4. The salts are useful as synthesis reagents, as chemical-reaction or polymerization catalysts, and as ion-conducting materials for electrochemical generators, supercapacitors and electrochromic devices.
Owner:PHOSTECH LITHIUM +1
Sulphonyl-1,2,4-triazole salts
InactiveUS20090292105A1Readily dispersibleHigh viscosityOrganic chemistryChemical reactionSupercapacitor
The invention relates to triazole salts, to their preparation and to applications thereof. The salts have at least one anionic triazolium group which carries at least one chlorosulphonyl, fluorosulphonyl or alkoxyfluorosulphonyl group, each of the anionic groups being combined with a proton or a cation that has a valency of less than or equal to 4. The salts are useful as synthesis reagents, as chemical-reaction or polymerization catalysts, and as ion-conducting materials for electrochemical generators, supercapacitors and electrochromic devices.
Owner:PHOSTECH LITHIUM +1
Pharmaceutical use of fused 1,2,4-triazoles
The use of fused 1,2,4-triazoles for modulating the activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and the use of these compounds as pharmaceutical compositions has been described. Also a novel class of fused 1,2,4-triazoles, their use in therapy, pharmaceutical compositions comprising the compounds, as well as their use in the manufacture of medicaments has been described. The present compounds are modulators and more specifically inhibitors of the activity of 11βHSD1 and may be useful in the treatment, prevention and / or prophylaxis of a range of medical disorders where a decreased intracellular concentration of active glucocorticoid is desirable.
Owner:HIGH POINT PHARMA
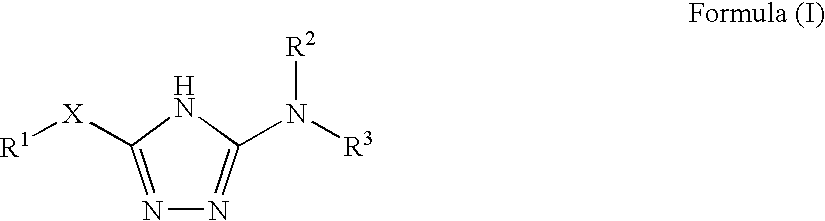
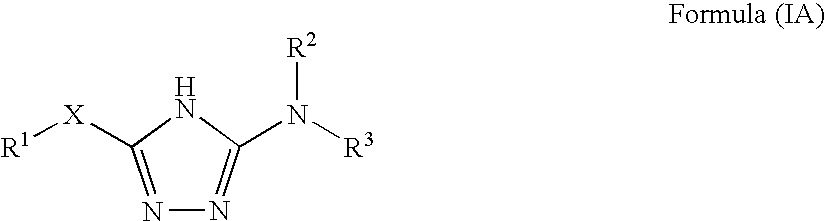
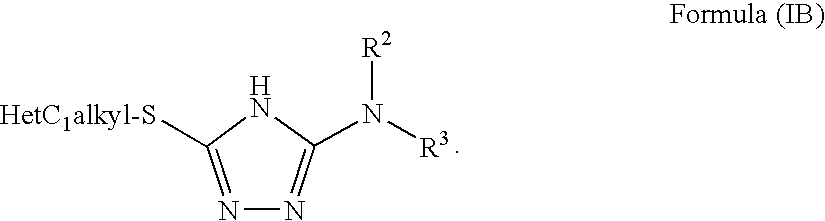
1,2,4-triazole derivatives, compositions, process of making and methods of use
Compounds of this invention are non-peptide, reversible inhibitors of type 2 methionine aminopeptidase, useful in treating conditions mediated by angiogenesis, such as cancer, haemangioma, proliferative retinopathy, rheumatoid arthritis, atherosclerotic neovascularization, psoriasis, ocular neovascularization and obesity.
Owner:GLAXO SMITHKLINE LLC
Pharmaceutical use of fused 1,2,4-triazoles
The use of fused 1,2,4-triazoles for modulating the activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and the use of these compounds as pharmaceutical compositions has been described. Also a novel class of fused 1,2,4-triazoles, their use in therapy, pharmaceutical compositions comprising the compounds, as well as their use in the manufacture of medicaments has been described. The present compounds are modulators and more specifically inhibitors of the activity of 11βHSD1 and may be useful in the treatment, prevention and / or prophylaxis of a range of medical disorders where a decreased intracellular concentration of active glucocorticoid is desirable.
Owner:HIGH POINT PHARMA
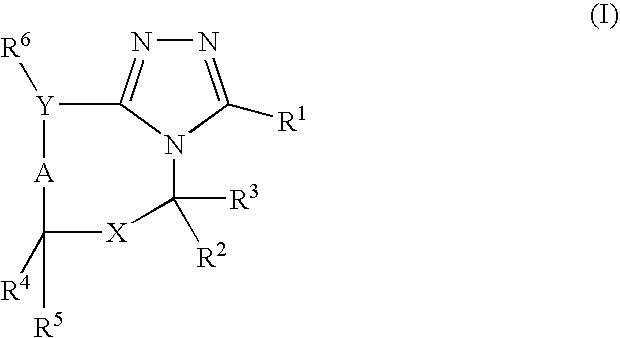
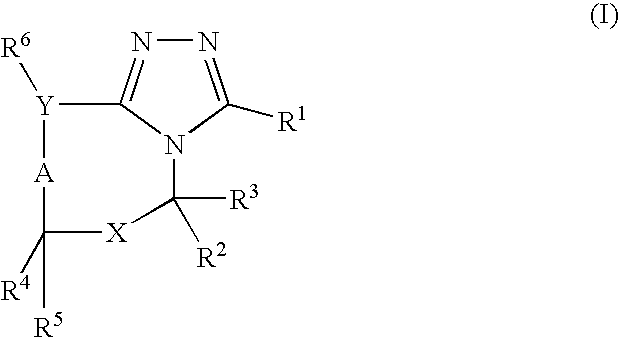
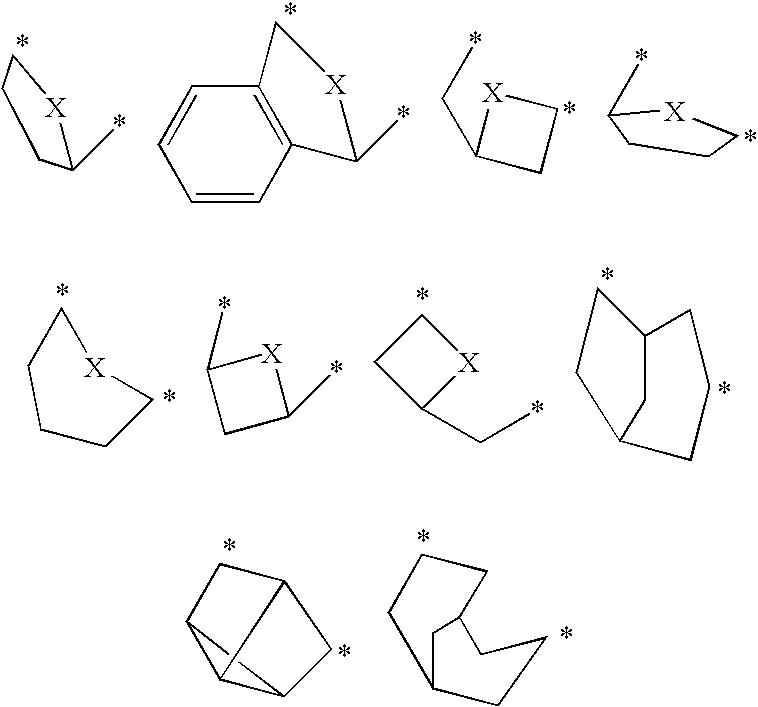
7-ARYL-1,2,4-TRIAZOLO[4,3-a]PYRIDINE DERIVATIVES AND THEIR USE AS POSITIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS
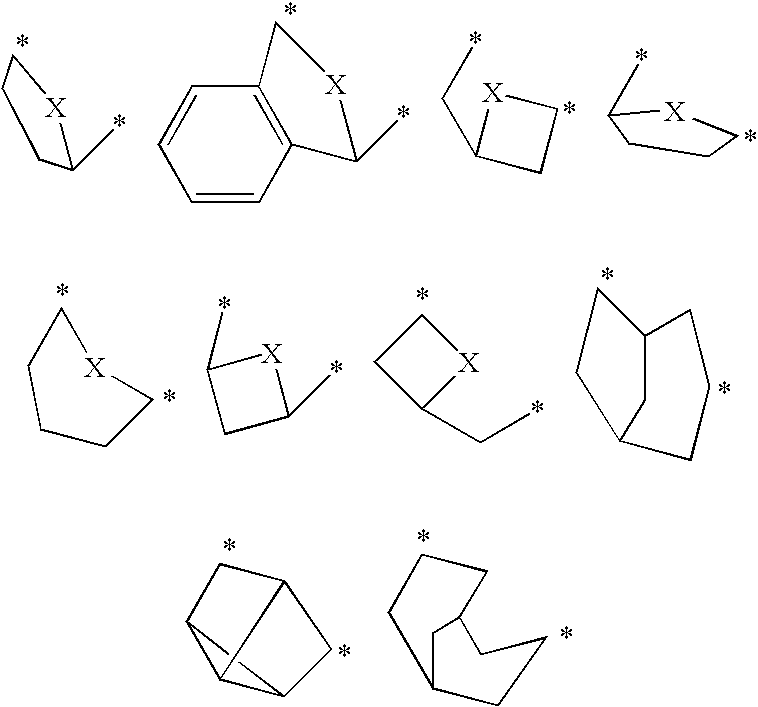
The present invention relates to novel triazolo[4,3-a]pyridine derivatives of Formula (I) wherein all radicals are as defined in the claims. The compounds according to the invention are positive allosteric modulators of the metabotropic glutamate receptor subtype 2 (“mGluR2”), which are useful for the treatment or prevention of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which the mGluR2 subtype of metabotropic receptors is involved. The invention is also directed to pharmaceutical compositions comprising such compounds, to processes to prepare such compounds and compositions, and to the use of such compounds for the prevention or treatment of neurological and psychiatric disorders and diseases in which mGluR2 is involved.
Owner:JANSSEN PHARMA INC +1
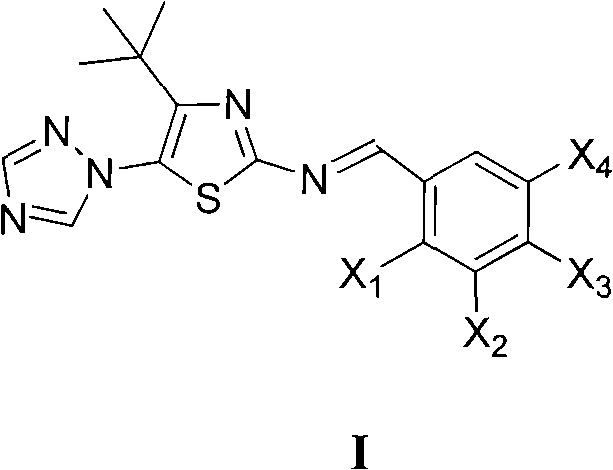
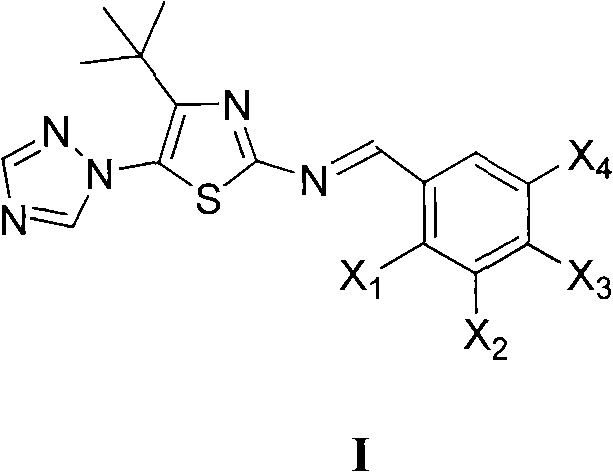
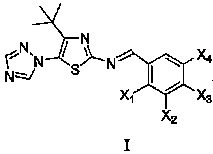
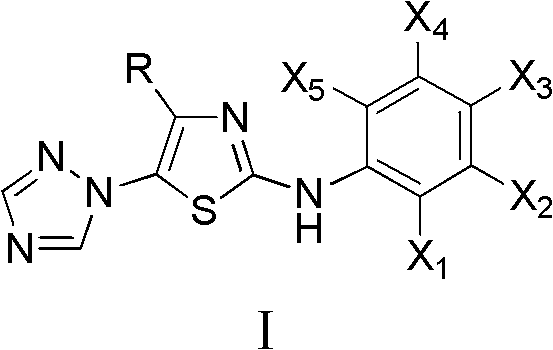
Medication application of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzyliminothiazole
InactiveCN101836979AStrong inhibitory activityOrganic active ingredientsAntineoplastic agentsHydrogenBromine
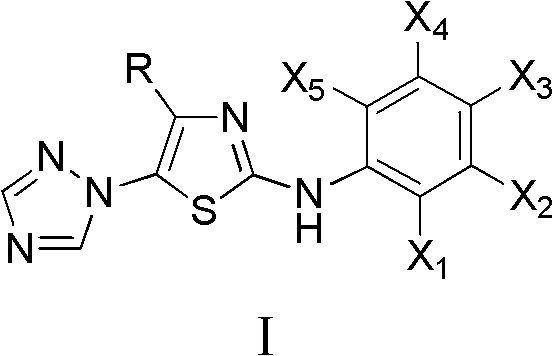
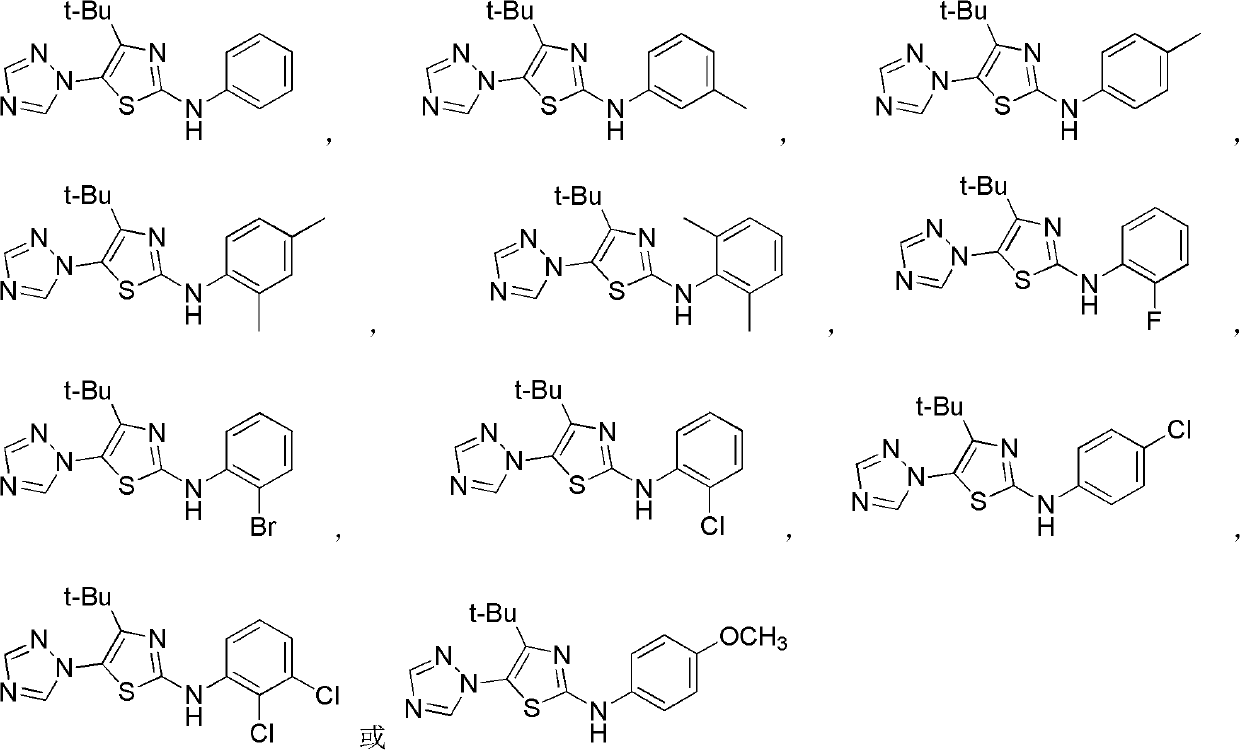
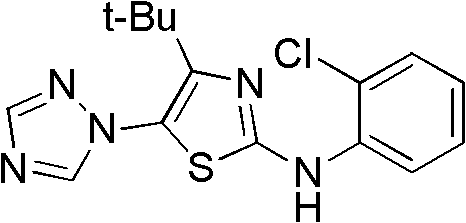
The invention discloses a medical application of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzyliminothiazole as shown in a chemical structural formula (I), wherein the X in the formula (I) is selected from hydrogen, hydroxyl, methoxyl, nitryl, amido and chlorine; the X2 is selected from hydrogen, nitryl, amido, chlorine, bromine and iodine; the X3 is selected from hydrogen, methyl, ethyl, nitryl, methoxyl, chlorine, bromine, amido and dimethylamino; and the X4 is selected from hydrogen, nitryl, amido, chlorine, bromine and iodine. The 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzyliminothiazole has better inhibitory activity to human cervical carcinoma cells, human hepatoma cells, human nasopharyngeal carcinoma cells and the like and can be used for preparing antitumor drugs.
Owner:HUNAN UNIV
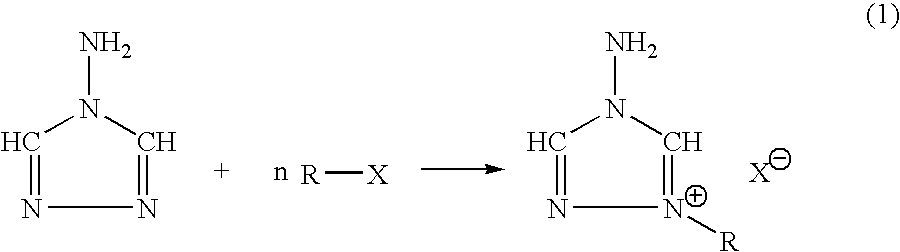
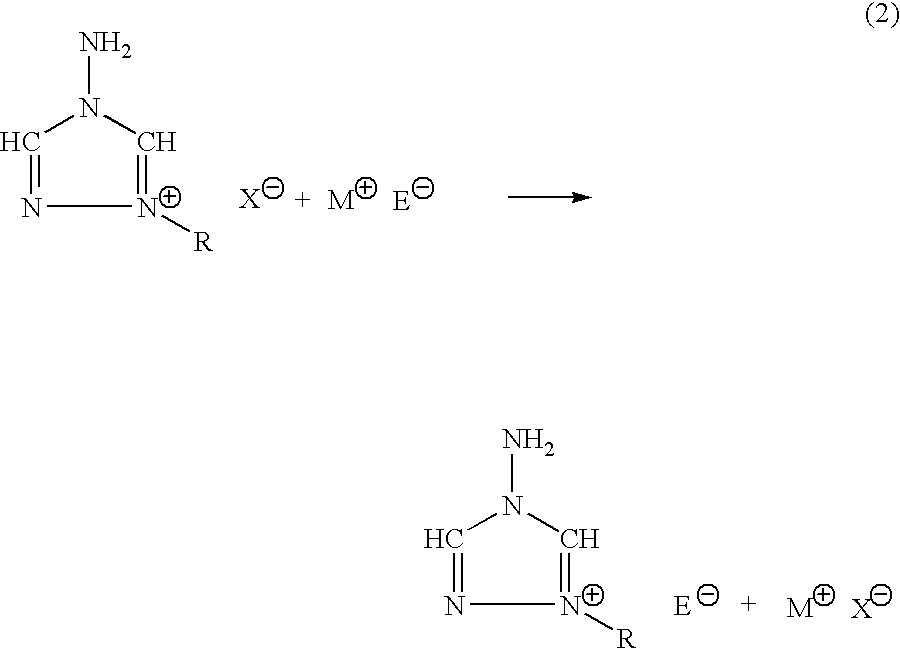
Energetic ionic salts
InactiveUS7745635B1High yieldHigh purityOrganic chemistryNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsNitrateIodide
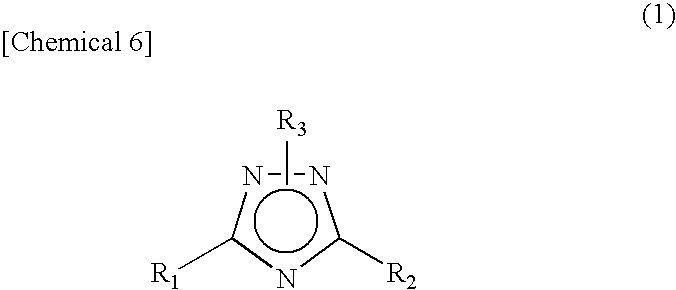
The invention provides a) a method for making improved ionic halide salts, e.g., 1-methyl-4-amino-1,2,4-triazolium iodide and b) a method for making energetic ionic salts, e.g., 1-methyl-4-amino-1,2,4-triazolium nitrate, in high yield and purity from triazolium precursors. Also provided are the resulting novel salts from the above methods.
Owner:THE GOVERNMENT OF THE US SEC THE AIR FORCE +1
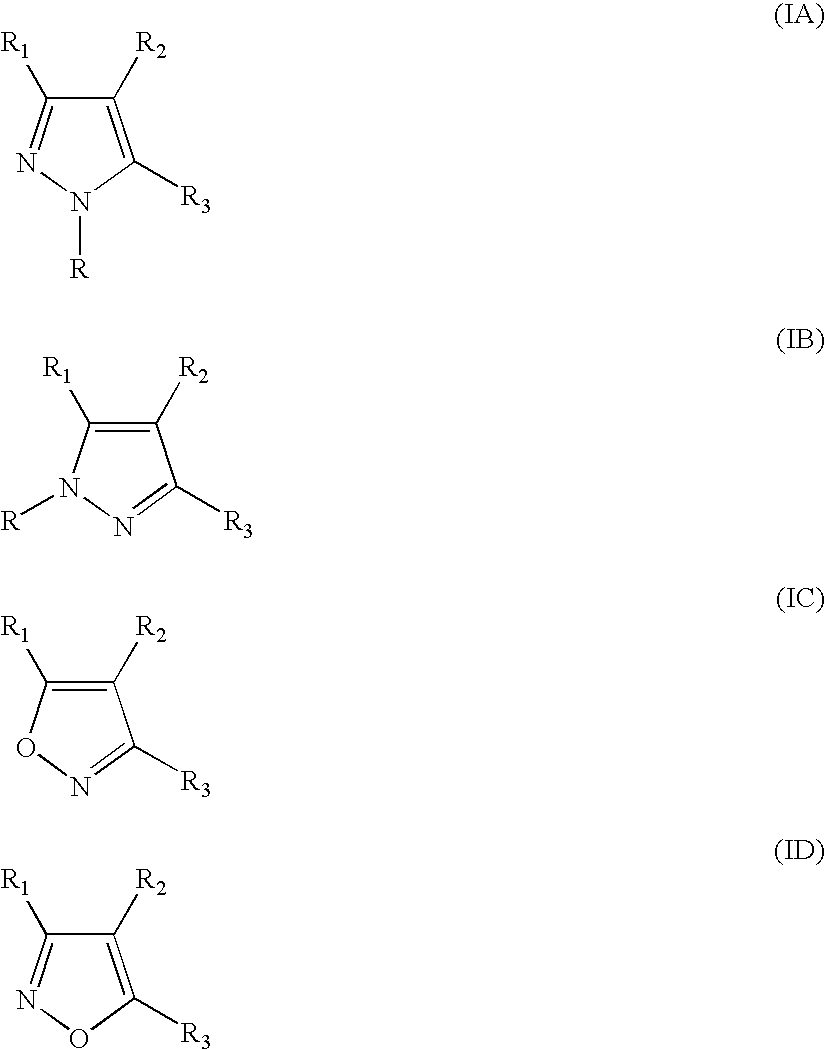
Substituted 5-membered ring compounds and their use
Compound of a compound of formula (1) or a salt, N-oxide, hydrate or solvate thereof, in the preparation of a composition for inhibition of HSP90 activity: wherein ring A is an aromatic or non-aromatic carbocyclic or heterocyclic ring having 5 ring atoms, for example 1,2,3-triazolyl or a 1,2,4-triazolyl or a tetrazolyl ring; and R1 R2 R3 are as defined in the specification are inhibitors of HSP90 and therefore of use in the treatment of, for example, cancers, viral disease, inflammatory diseases such as rheumatoid arthritis, asthma, multiple sclerosis, Type I diabetes, lupus, psoriasis and inflammatory bowel disease; cystic fibrosis angiogenesis-related disease such as diabetic retinopathy, haemangiomas, and endometriosis; or for protection of normal cells against chemotherapy-induced toxicity; or diseases where failure to undergo apoptosis is an underlying factor, or protection from hypoxia-ischemic injury due to elevation of Hsp70 in the heart and brain; scrapie / CJD, Huntingdon's and Alzheimer's disease.
Owner:VERNALIS (R&D) LTD +2
1 2 4-triazole compound
A novel 1,2,4-triazole compound which is useful as a therapeutic agent for hyperuricemia and gout due to hyperuricemia is provided. A compound is represented by the following general formula (1):wherein R2 represents an unsubstituted or substituted pyridyl group, R1 represents a similar pyridyl group, a pyridine-N-oxide group corresponding to these pyridyl groups, or a phenyl group, and R3 represents hydrogen or a lower alkyl group substituted with pivaloyloxy group and R3 bonds to a nitrogen atom in the ring. A process for production of a compound by reacting a nitrile and a hydrazide, and a therapeutic agent, particularly a xanthine oxidase inhibitor are also provided.
Owner:FUJI YAKUHIN CO LTD
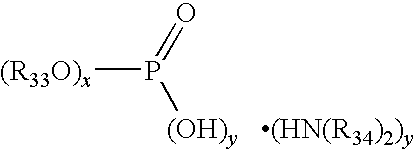
Multiple metal corrosion inhibitor
Disclosed is a lubricant composition comprising a) a lubricant, b) a triazole metal deactivator, c) a borate ester and optionally d) an amine phosphate. The lubricants, e.g. engine oils or functional fluids exhibit low corrosion of lead, copper, iron and zinc. The metal deactivators are 1,2,4-triazoles, for example 1-(di-isooctylaminomethyl)-1,2,4-triazole or 1-(di-(2-ethylhexyl)aminomethyl)-1,2,4-triazole, or are benzotriazoles, for example 1-(2-methoxyprop-2-yl)tolyltriazole, 1-(1-cyclohexyloxypropyl)tolyltriazole, 1-(1-cyclohexyloxyheptyl)tolyltriazole or 1-(1-cyclohexyloxybutyl)tolyltriazole or 1-[bis(2-ethylhexyl)aminomethyl-4-methylbenzotriazole. The borate esters are for example triethyl borate, tripropyl borate, triisopropyle borate, tributyl borate, tripentyl borate, trihexyl borate, tricyclohexyl borate, trioctyl borate, triisooctyl borate, tridecyl borate, tri(C8C10) borate, tri (C12-C15) borate or oleyl borate. The amine phosphate are for examplewherein R33 is n-hexyl and R34 is C11-C14 branched alkyl, and when x=1 then y=2; when x=2 then y=1.
Owner:CHASAN DAVID E +1
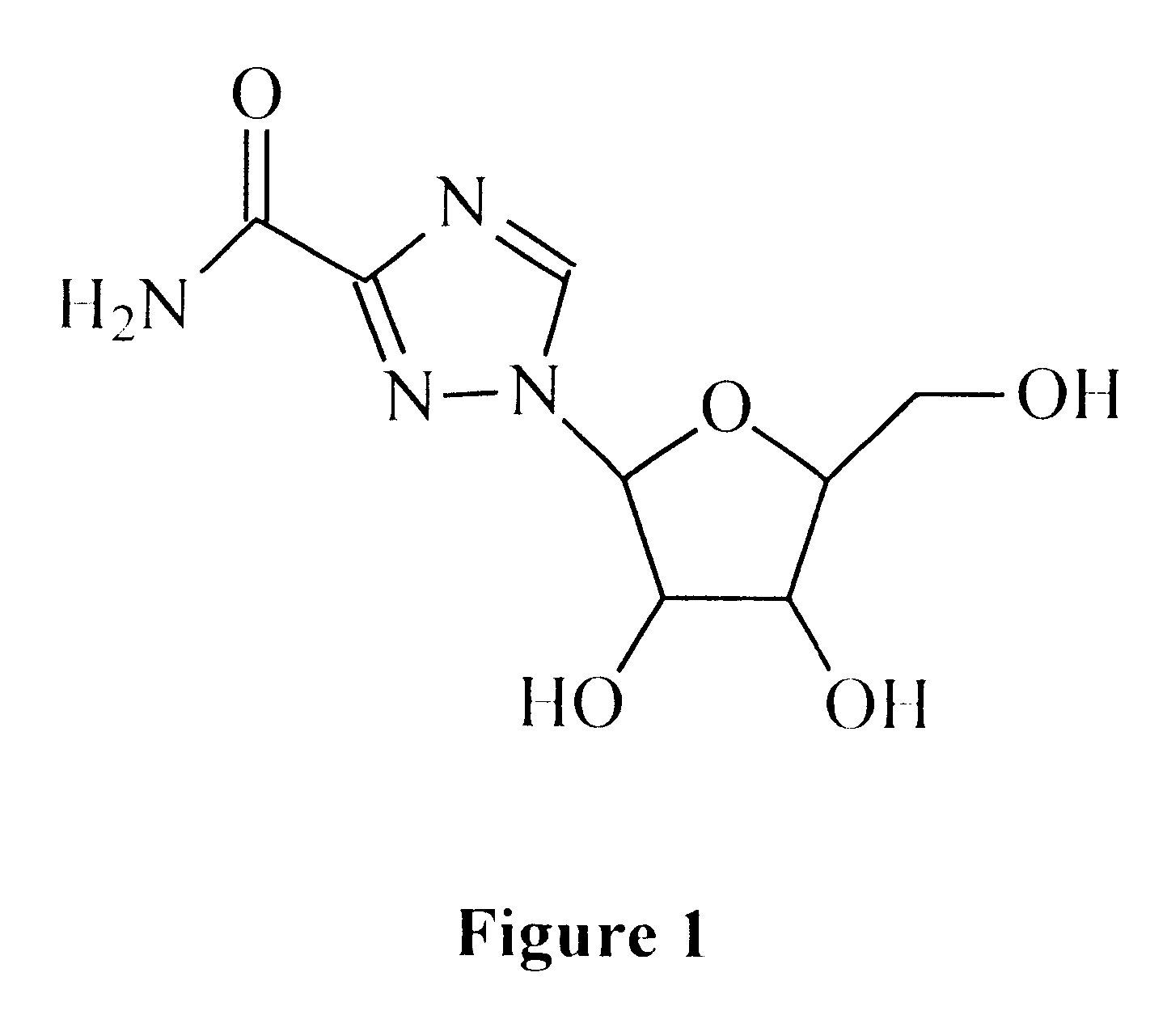
Treatment of viral infections using the L-isomer of ribavirin
A 1-(beta-L-ribofuranosyl)-1,2,4-triazole-3-carboxamide is administered in a method of treatment of a viral infection in a patient, including HIV infection, HCV infection, or BHV infection.
Owner:VALEANT RES & DEV
2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-ylthio)acetic acid and methyl ester
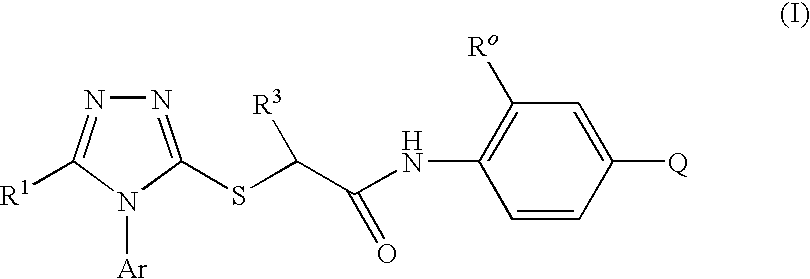
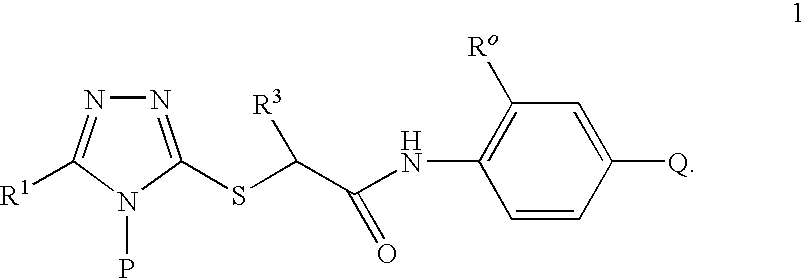
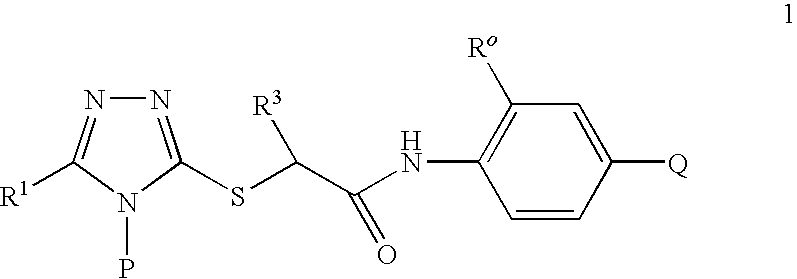
A series of S-triazolyl α-mercaptoacetanilides having general structure (I) are provided, where Q is CO2H, CONR2, SO3H, or SO2NR2. The compounds inhibit several variants of the reverse transcriptase of HIV, and are useful in the treatment of HIV infections.
Owner:ARDEA BIOSCI
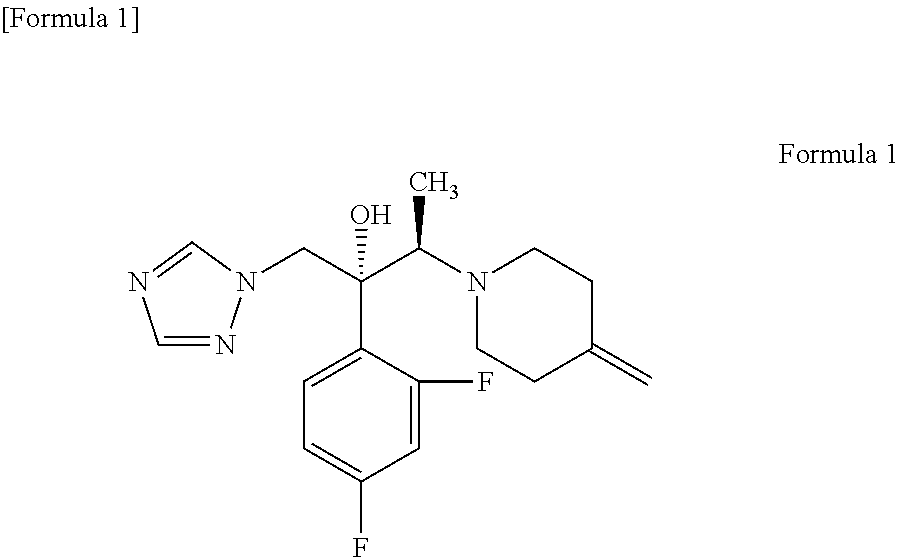
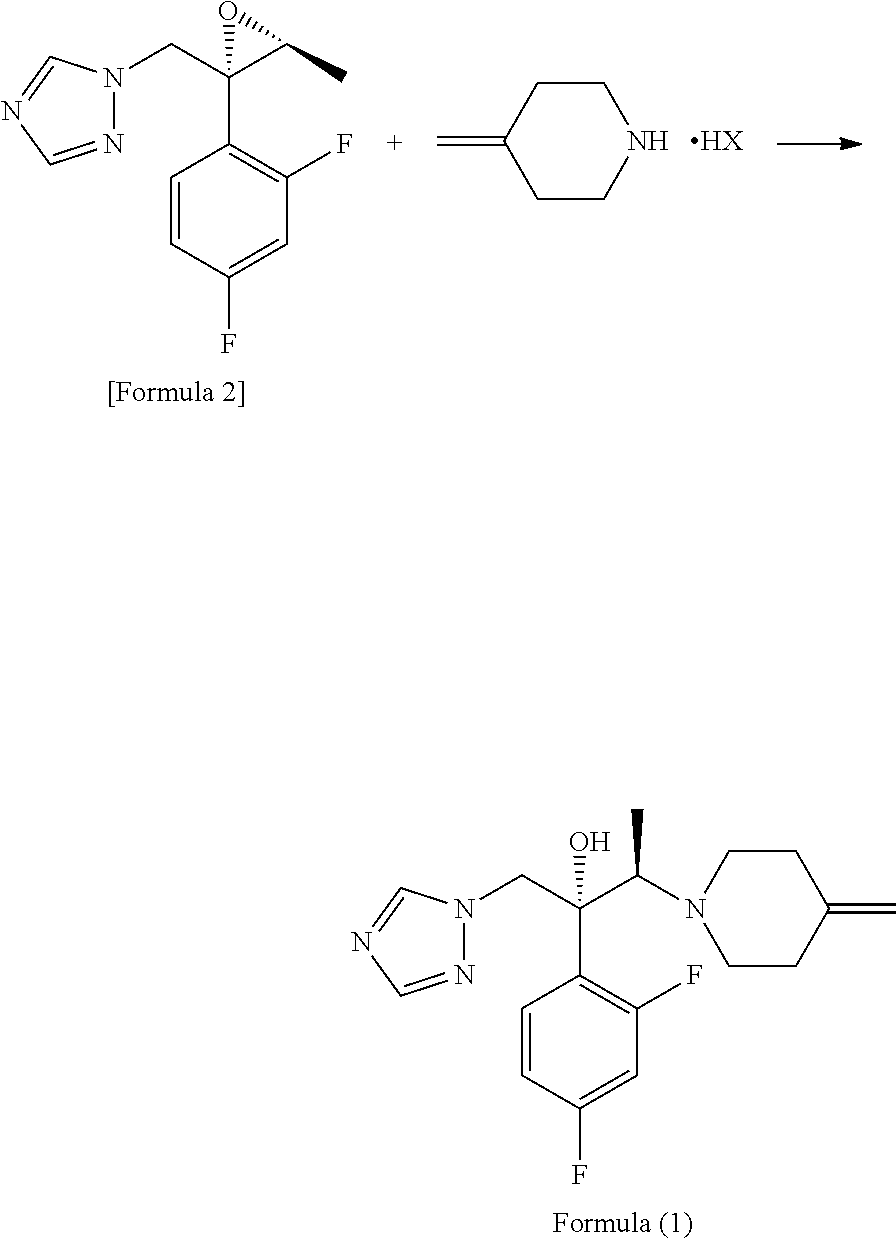
Process for producing 1-triazole-2-butanol derivatives
ActiveUS20130150586A1High yieldReduce generationAntimycoticsOrganic chemistryAlkaline earth metalCycloaddition
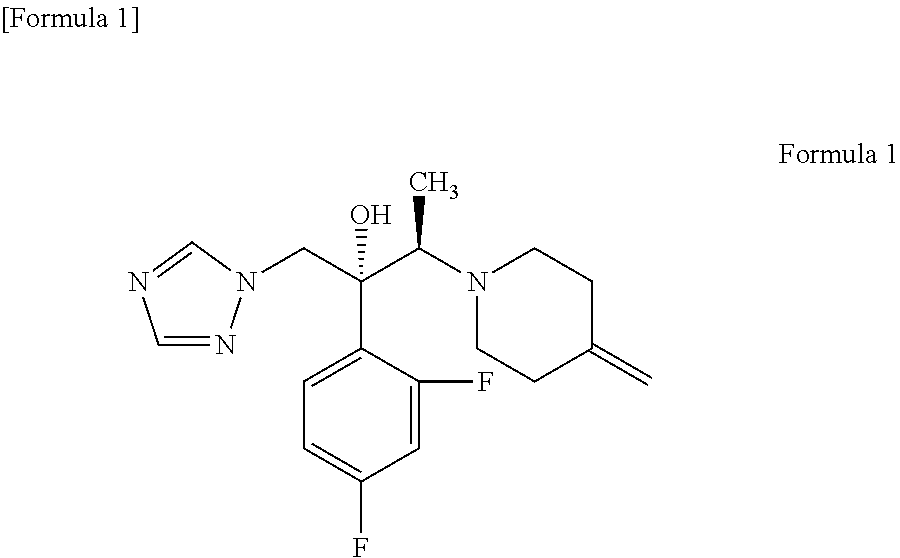
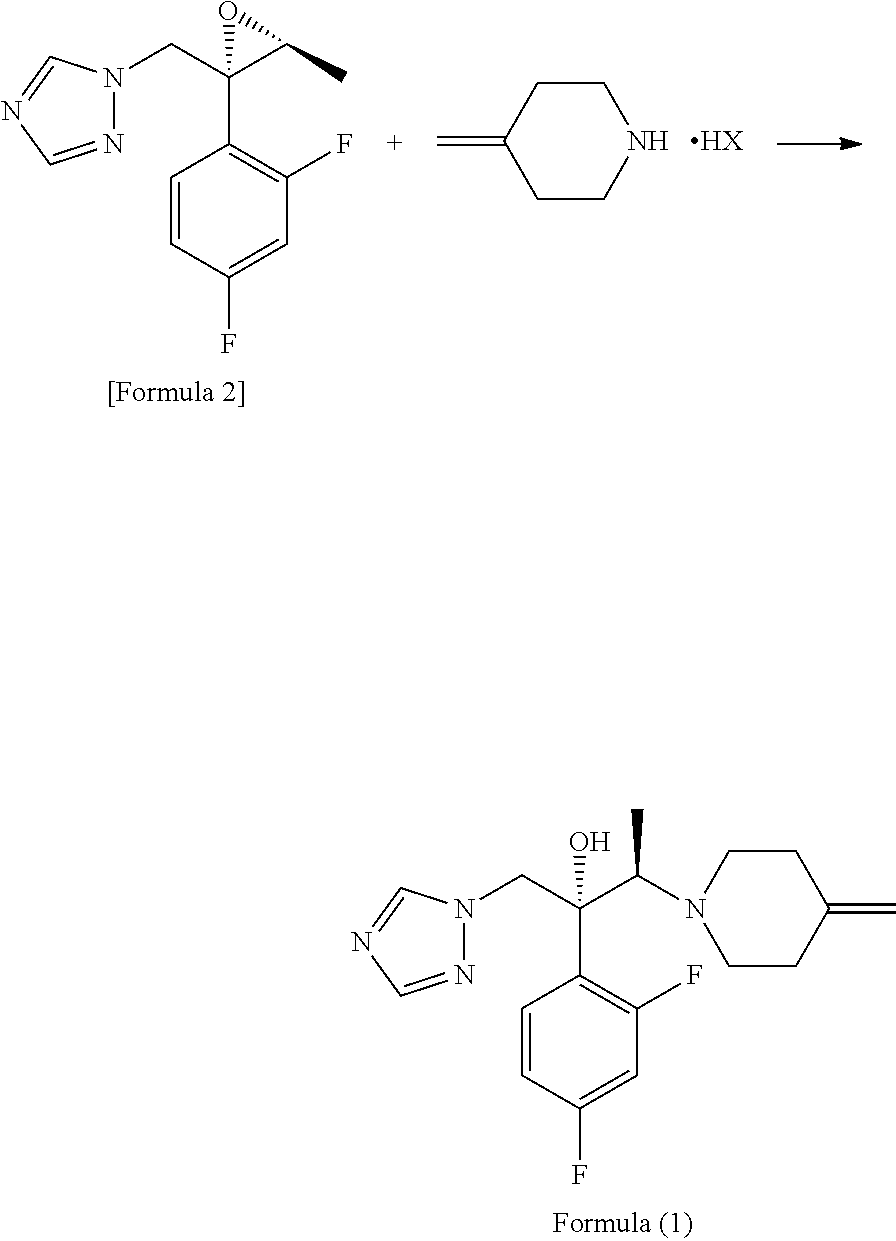
An object is to provide a process for producing the compound of formula 1 in higher yield by the ring-opening addition reaction of epoxytriazole with amine under mild conditions without using a large excess of 4-methylenepiperidine. The process for producing (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol or an acid addition salt thereof comprises reacting (2R,3S)-2-(2,4-difluorophenyl)-3-methyl-2-[(1H-1,2,4-triazol-1-yl)methyl]oxirane with an acid addition salt of 4-methylenepiperidine in a reaction solvent in the presence of a hydroxide of an alkali metal or an alkaline earth metal selected from the group consisting of lithium, sodium, calcium, and strontium, or a hydrate thereof.
Owner:KAKEN PHARMA CO LTD
4-alkyl-2-aryl amino-5-(1,2,4-triazol-1-yl)thiazole and preparation method and application thereof
InactiveCN102603728AHas antitumor activityOrganic active ingredientsOrganic chemistryHydrobromideThiourea
Owner:HUNAN UNIV
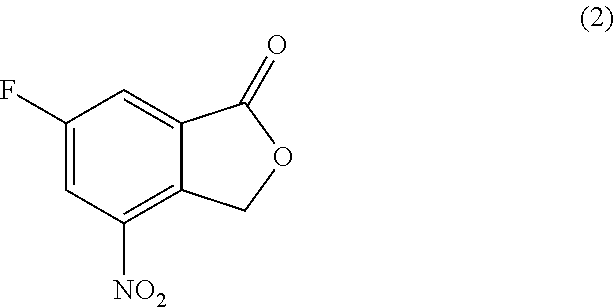
Processes of synthesizing dihydropyridophthalazinone derivatives
Provided herein are processes for synthesizing dihydropyridophthalazinone derivatives, such as for example, 5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one and its stereoisomers, which are potent poly(ADP-ribose)polymerase (PARP) inhibitors as well as novel synthetic intermediate compounds.
Owner:MEDIVATION TECH INC
CMP Fluid and Method for Polishing Palladium
ActiveUS20120100718A1Improve polishing rateStable polishing rateOther chemical processesSemiconductor/solid-state device manufacturingOrganic solventPhosphorus acid
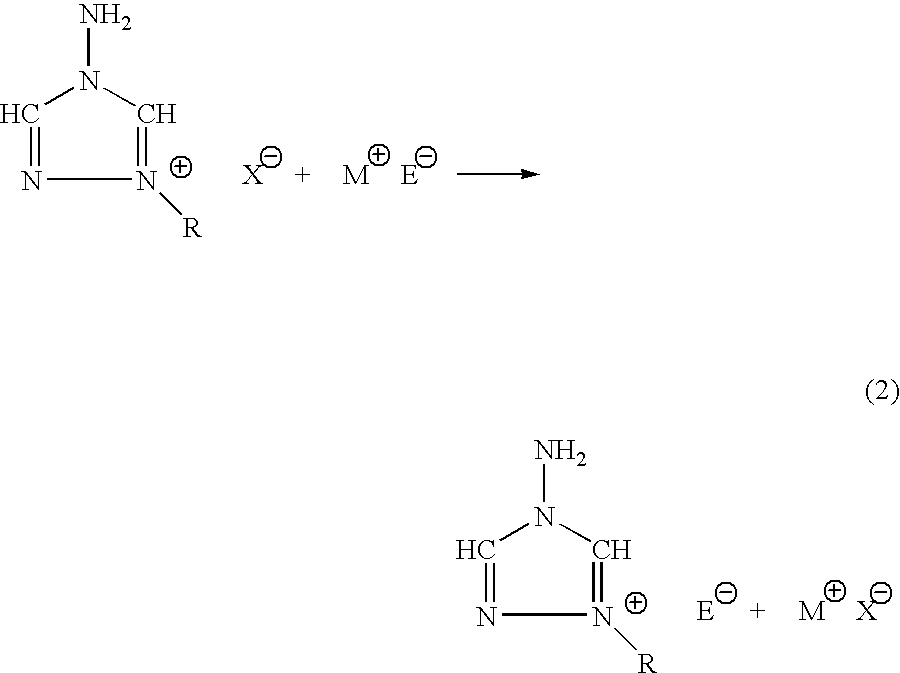
The CMP polishing liquid for polishing palladium of this invention comprises an organic solvent, 1,2,4-triazole, a phosphorus acid compound, an oxidizing agent and an abrasive. The substrate polishing method is a method for polishing a substrate with a polishing cloth while supplying a CMP polishing liquid between the substrate and the polishing cloth, wherein the substrate is a substrate with a palladium layer on the side facing the polishing cloth, and the CMP polishing liquid is a CMP polishing liquid comprising an organic solvent, 1,2,4-triazole, a phosphorus acid compound, an oxidizing agent and an abrasive.
Owner:RESONAC CORP
4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole and application thereof to preparation of medicaments for resisting cancer
InactiveCN102675303AAnti-cervical cancerCtiveOrganic chemistryAntineoplastic agentsHydrobromidePhosphate
The invention discloses 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole and salts thereof shown in a formula I, wherein R is selected from H, alkyl groups of C1-C2 and straight-chain alkyl groups or branched-chain alkyl groups of C3-C4; X1, X2, X3, X4 and X5 are selected from hydrogen, alkyl groups of C1-C2, hydroxide group, methoxy group, oxyethyl group, trifluoromethyl, fluorine, chlorine, bromine, nitryl, amino group, acetyl amino group, methanesulfamide, ethoxycarbonyl or carboxyl; and the salts of the 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole are selected from hydrochloride, hydrobromide, nitrate, sulfate, phosphate, mesylate, p-toluene sulfonate, tartrate, lactate or malate. The 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole is applied to preparation of medicaments for resisting cervical cancer or lung neoplasms.
Owner:HUNAN UNIV
Method for increasing and regulating light emission from a chemiluminescent reaction
ActiveUS9040252B2Microbiological testing/measurementChemiluminescene/bioluminescencePeroxidase1-Methylimidazole
Method for increasing and regulating the emission of light from a chemiluminescent reaction including luminol, a peroxidase enzyme, an oxidant and an electron mediator (primary enhancer) through the use of an acylation catalyst (secondary enhancer) belonging to the class of N-azoles, i.e., a class of five-membered nitrogen heteroaromatic ring compounds containing at least one other atom of nitrogen. N-azoles, which are especially useful as secondary enhancers are imidazole, 1-methylimidazole, 1,2,3-triazole and 1,2,4-triazole. The invention also describes the use in diagnostic assays of chemiluminescent substrates containing said N-azoles, as secondary enhancers.
Owner:CYANAGEN
Process for producing 1-triazole-2-butanol derivatives
ActiveUS8871942B2Reduce generationHigh yieldAntimycoticsOrganic chemistryAlkaline earth metalCycloaddition
An object is to provide a process for producing the compound of formula 1 in higher yield by the ring-opening addition reaction of epoxytriazole with amine under mild conditions without using a large excess of 4-methylenepiperidine. The process for producing (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol or an acid addition salt thereof comprises reacting (2R,3S)-2-(2,4-difluorophenyl)-3-methyl-2-[(1H-1,2,4-triazol-1-yl)methyl]oxirane with an acid addition salt of 4-methylenepiperidine in a reaction solvent in the presence of a hydroxide of an alkali metal or an alkaline earth metal selected from the group consisting of lithium, sodium, calcium, and strontium, or a hydrate thereof.
Owner:KAKEN PHARMA CO LTD
Gel composition for treating mycosis
ActiveUS20100317695A1Shorten the counting processUniform thicknessBiocideAntimycoticsFordyce's diseaseAlcohol
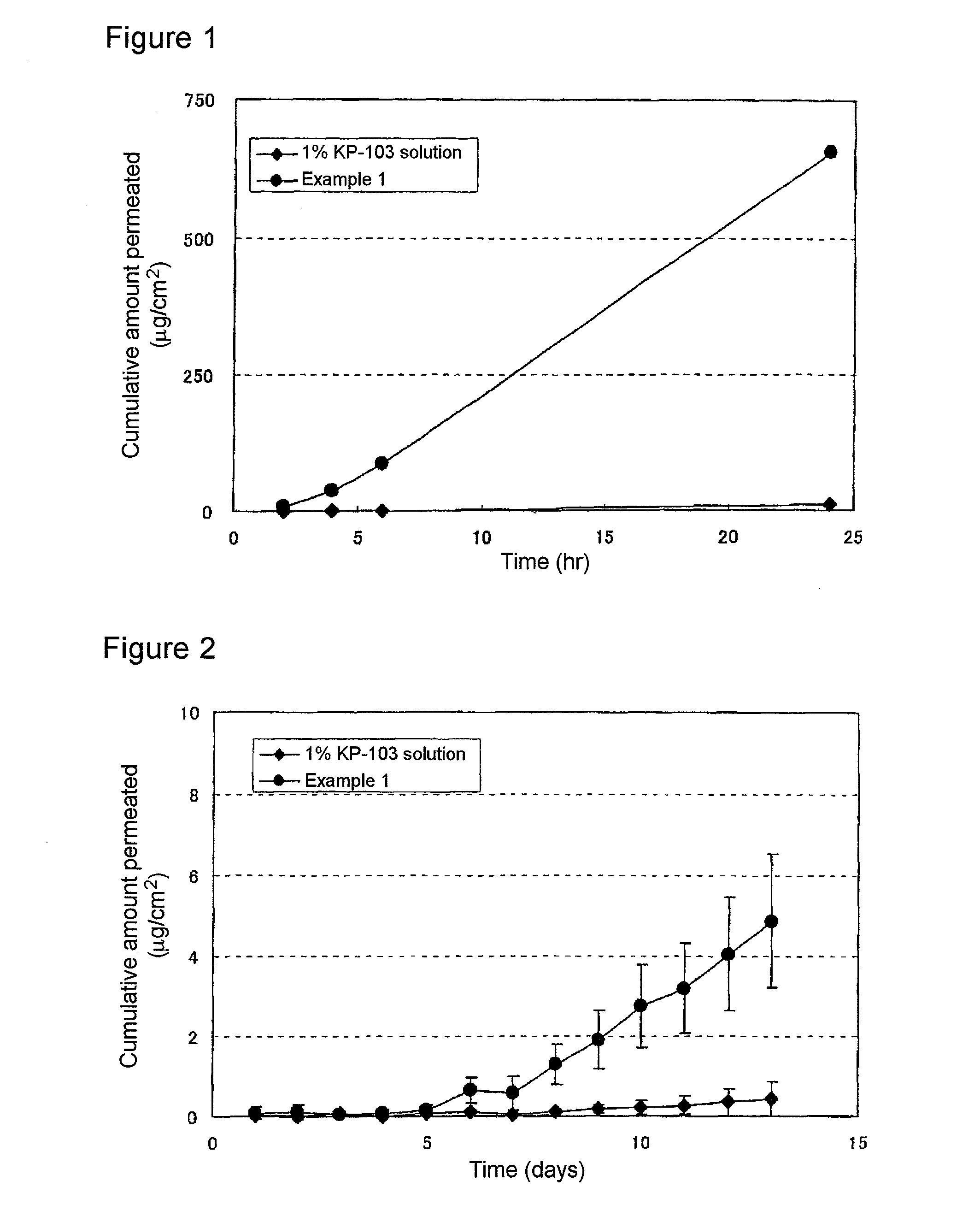
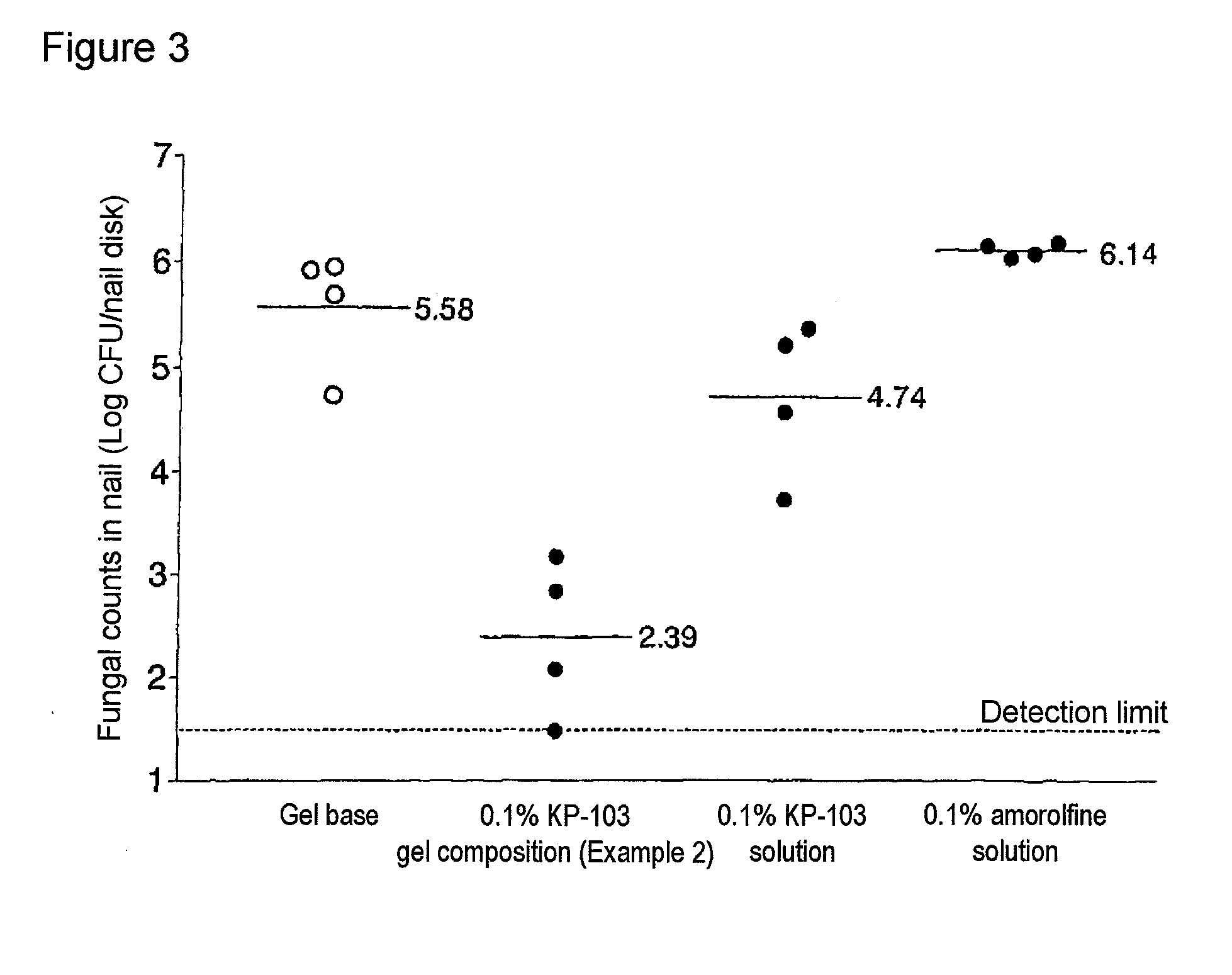
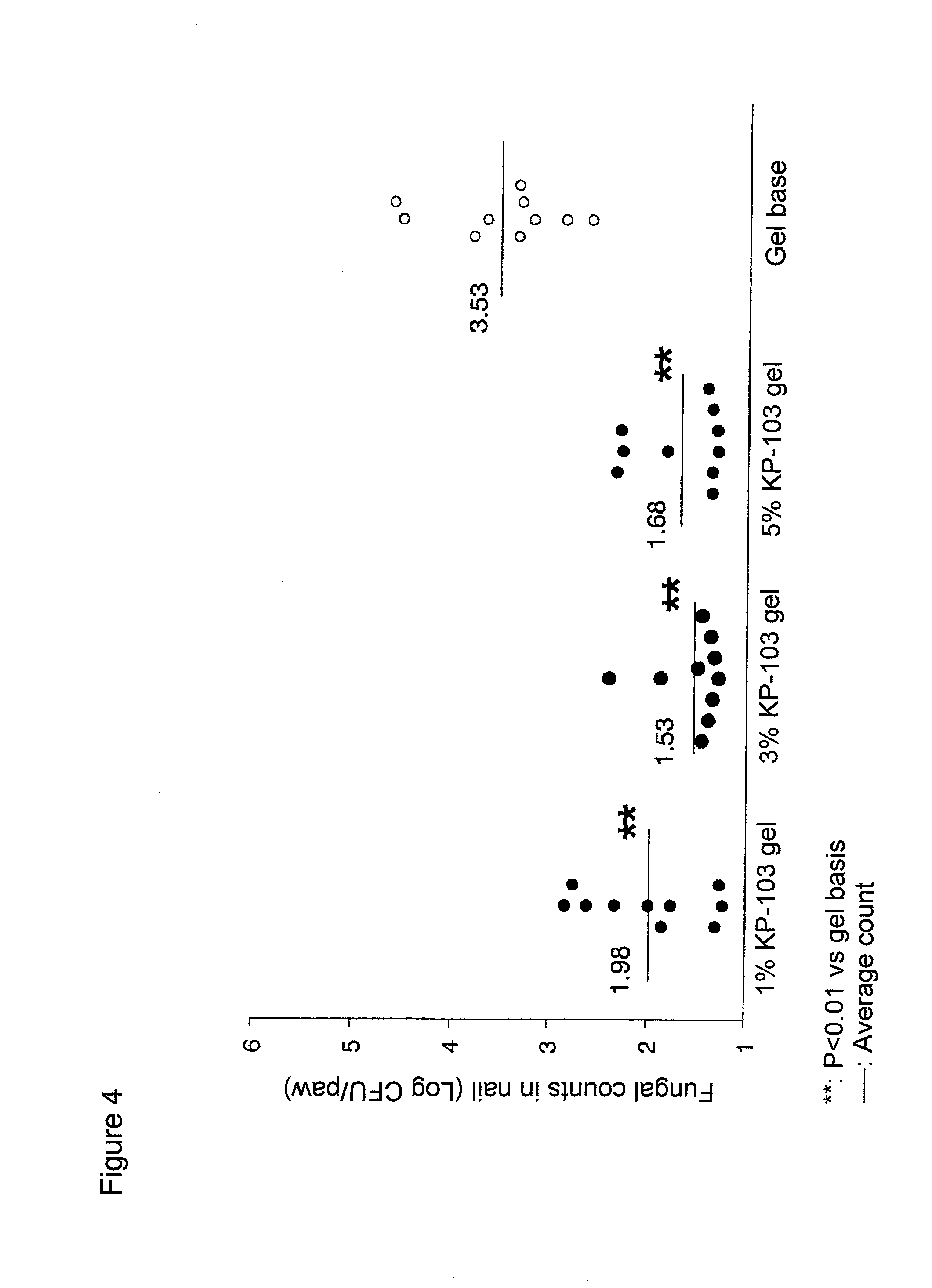
The present invention aims to provide a gel composition for mycosis treatment, which ensures good absorption and permeation of (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)-butan-2-ol into a target site (skin and nail). The present invention also aims to provide a gel composition for mycosis treatment, which is excellent in stability of this drug.It is a gel composition for mycosis treatment, which comprises (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)-butan-2-ol or an acid addition salt thereof, a lower alcohol, a polyhydric alcohol and a gel-forming polymer.The gel composition of the present invention is excellent in permeation of KP-103 into a target site and also excellent in permeation into the nail. Thus, the gel composition of the present invention allows the drug to be directly and rapidly absorbed and permeated into a target site in a constant manner, and thereby produces an excellent effect in mycosis treatment, particularly onychomycosis treatment.
Owner:KAKEN PHARMA CO LTD
Organometallic compound and organic electroluminescence device employing the same
InactiveUS20130033171A1Indium organic compoundsDischarge tube luminescnet screensChemical structureCompound (substance)
Organometallic compounds and organic electroluminescence devices employing the same are provided. The organic compound has a chemical structure as represented below:wherein, A1 is diisopropyl carbodiimide ligand, 5-(2-pyridyl)-1,2,4-triazole ligand, acetylacetone with phenyl group ligand, 2-phenyl-1,3,4-oxadiazole ligand, or derivatives thereof. The organometallic compound of the disclosure can be applied in an organic electroluminescent device for enhancing the electroluminescent efficiency thereof.
Owner:IND TECH RES INST
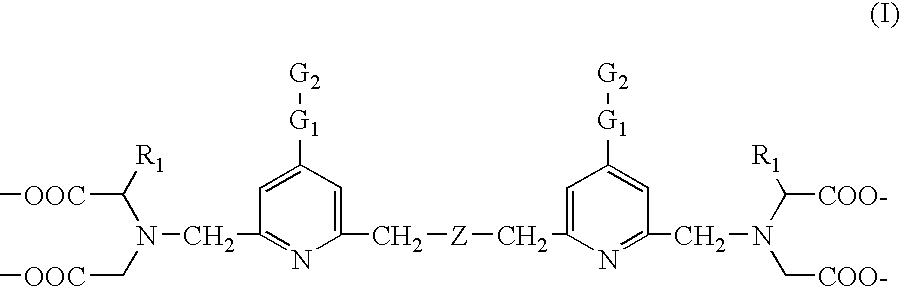
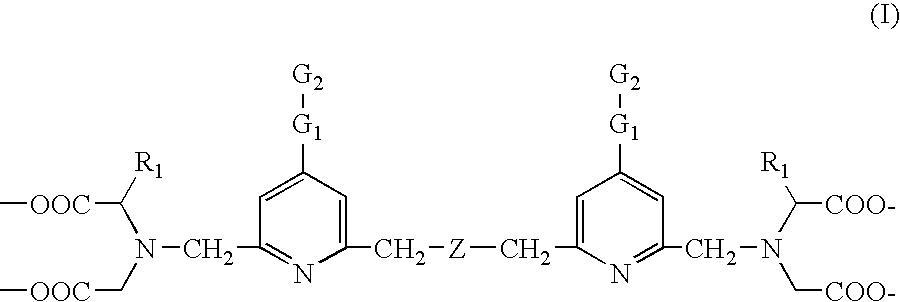
Biospecific binding reactants labeled with new luminescent lanthanide chelates and their use
ActiveUS7018851B2Simple methodImproved labeling methodMicrobiological testing/measurementChemiluminescene/bioluminescenceLanthanideChelating ligands
This invention relates to a luminescent lanthanide chelate comprising a lanthanide ion and a chelating ligand of formula (I) whereinR1 is selected from the group consisting H, —COOH, —COO−, —CH2COOH and —CH2COO−; G1 is a group consisting of one or two moieties each moiety being selected from the group consisting of ethynediyl, ethenylene, phenylene, biphenylene, naphthylene, pyridylene, pyrazinylene, pyrimidinylene, pyridazinylene, furylene, thienylene, pyrrolylene, imidazolylene, pyrazolylene, thiazolylene, isothiazolylene, oxazolylene, isoxazolylene, fyrazanylene, 1,2,4-triazol-3,5-ylene and oxadiazolylene; G2 for coupling to a biospecific binding reactant is selected from the group consisting of amino, aminooxy, carbonyl, aldehyde or mercapto groups and activated forms made of them; Z is selected from the group consisting of carboxyalkyl amine, ether, thioether, carbonyl and unsubstituted or substitute methyl (—CR2—) wherein group R2 is selected from the group consisting of H, methyl, ethyl and carboxylalkyl; and the lanthanide ion is europium(III), terbium(III), dysprosiym(III) or samarium(III). This invention further relates to a detectable molecule comprising the lanthanide chelate and the use of the molecule in a method of carrying out a biospecific binding assay.
Owner:INNOTRAC DIAGNOSTISC
Liquid composition and etching method therewith
The invention relates to a liquid composition and an etching method therewith, wherein the liquid composition is used for etching copper contianing indium, gallium or zinc oxides or metal compounds with copper as main components. The etching method is cahracterized by contacting the liquid composition with the metal compound having copper or with copper as main components. The liquid composition includes A) H2O2; B) acid without fluorine atom; C) more than one from phosphonic acid, phsophates, 1H-tetrazole-1-acetic acid, 1H-tetrazole-5-acetic acid and 4-amino-1,2,4-triazole; and D) water, pH of hte composition is lwoer than 5. THe liquid composition can inhibit damage on teh indium, gallium or zinc oxides and etch copper on the oxides or the metal compounds with copper as main components.
Owner:MITSUBISHI GAS CHEM CO INC
Crystalline (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt
Provided herein are (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt forms, including crystalline forms, and methods of their preparation. Pharmaceutical compositions comprising a (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt are also provided, as are methods of using (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt to treat a disease or condition, such as a cancer.
Owner:MEDIVATION TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


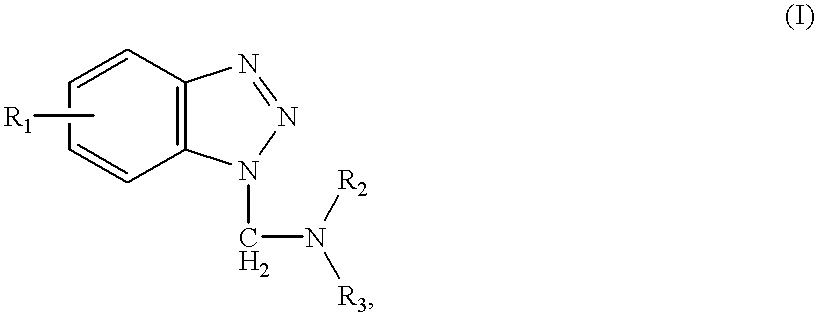


![Crystalline (8s,9r)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1h-1,2,4-triazol-5-yl)-8,9-dihydro-2h-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt Crystalline (8s,9r)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1h-1,2,4-triazol-5-yl)-8,9-dihydro-2h-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/896bfd62-6254-4a38-bbd5-01663f40e774/US20120129865A1-20120524-D00001.png)
![Crystalline (8s,9r)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1h-1,2,4-triazol-5-yl)-8,9-dihydro-2h-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt Crystalline (8s,9r)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1h-1,2,4-triazol-5-yl)-8,9-dihydro-2h-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/896bfd62-6254-4a38-bbd5-01663f40e774/US20120129865A1-20120524-D00002.png)
![Crystalline (8s,9r)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1h-1,2,4-triazol-5-yl)-8,9-dihydro-2h-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt Crystalline (8s,9r)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1h-1,2,4-triazol-5-yl)-8,9-dihydro-2h-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/896bfd62-6254-4a38-bbd5-01663f40e774/US20120129865A1-20120524-D00003.png)
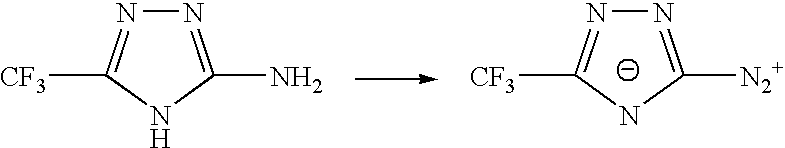
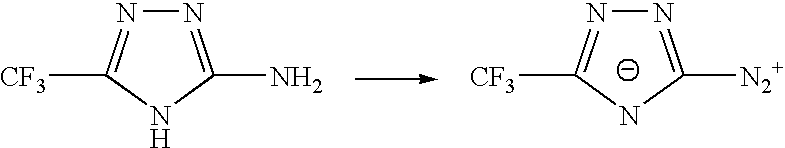
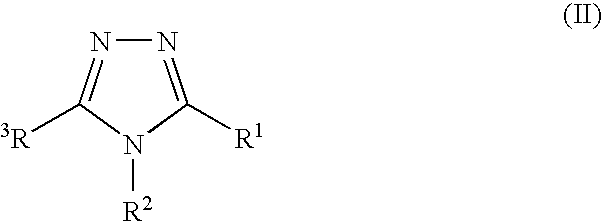
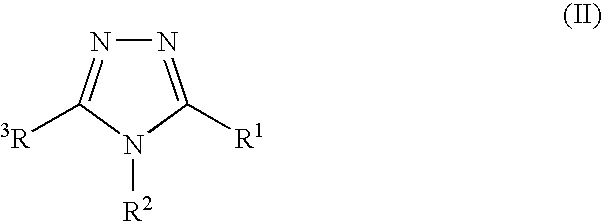
![7-ARYL-1,2,4-TRIAZOLO[4,3-a]PYRIDINE DERIVATIVES AND THEIR USE AS POSITIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS 7-ARYL-1,2,4-TRIAZOLO[4,3-a]PYRIDINE DERIVATIVES AND THEIR USE AS POSITIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31c8fb25-3239-4ae4-bb7d-b77e84d3fc27/US20120184528A1-20120719-C00001.png)
![7-ARYL-1,2,4-TRIAZOLO[4,3-a]PYRIDINE DERIVATIVES AND THEIR USE AS POSITIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS 7-ARYL-1,2,4-TRIAZOLO[4,3-a]PYRIDINE DERIVATIVES AND THEIR USE AS POSITIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31c8fb25-3239-4ae4-bb7d-b77e84d3fc27/US20120184528A1-20120719-C00002.png)
![7-ARYL-1,2,4-TRIAZOLO[4,3-a]PYRIDINE DERIVATIVES AND THEIR USE AS POSITIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS 7-ARYL-1,2,4-TRIAZOLO[4,3-a]PYRIDINE DERIVATIVES AND THEIR USE AS POSITIVE ALLOSTERIC MODULATORS OF MGLUR2 RECEPTORS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31c8fb25-3239-4ae4-bb7d-b77e84d3fc27/US20120184528A1-20120719-C00003.png)








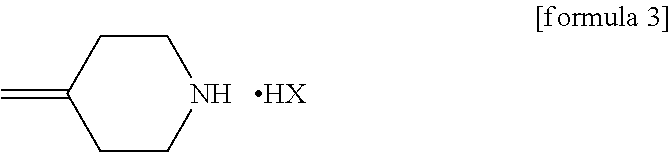





![Crystalline (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt Crystalline (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9b835882-286f-431a-ab40-2c87ce9f4c17/US08735392-20140527-D00001.png)
![Crystalline (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt Crystalline (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9b835882-286f-431a-ab40-2c87ce9f4c17/US08735392-20140527-D00002.png)
![Crystalline (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt Crystalline (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one tosylate salt](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9b835882-286f-431a-ab40-2c87ce9f4c17/US08735392-20140527-D00003.png)