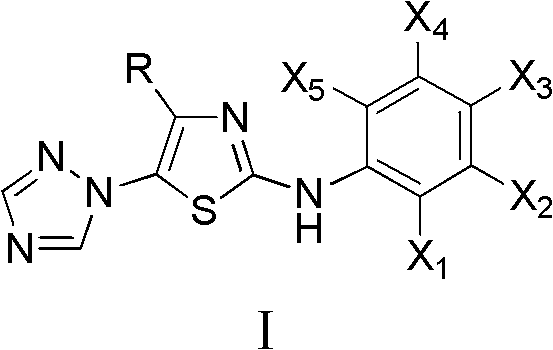
4-alkyl-2-aryl amino-5-(1,2,4-triazol-1-yl)thiazole and preparation method and application thereof
A technology of arylamino and alkyl, applied in the field of new compounds and their preparation, can solve the problems of no research and development reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
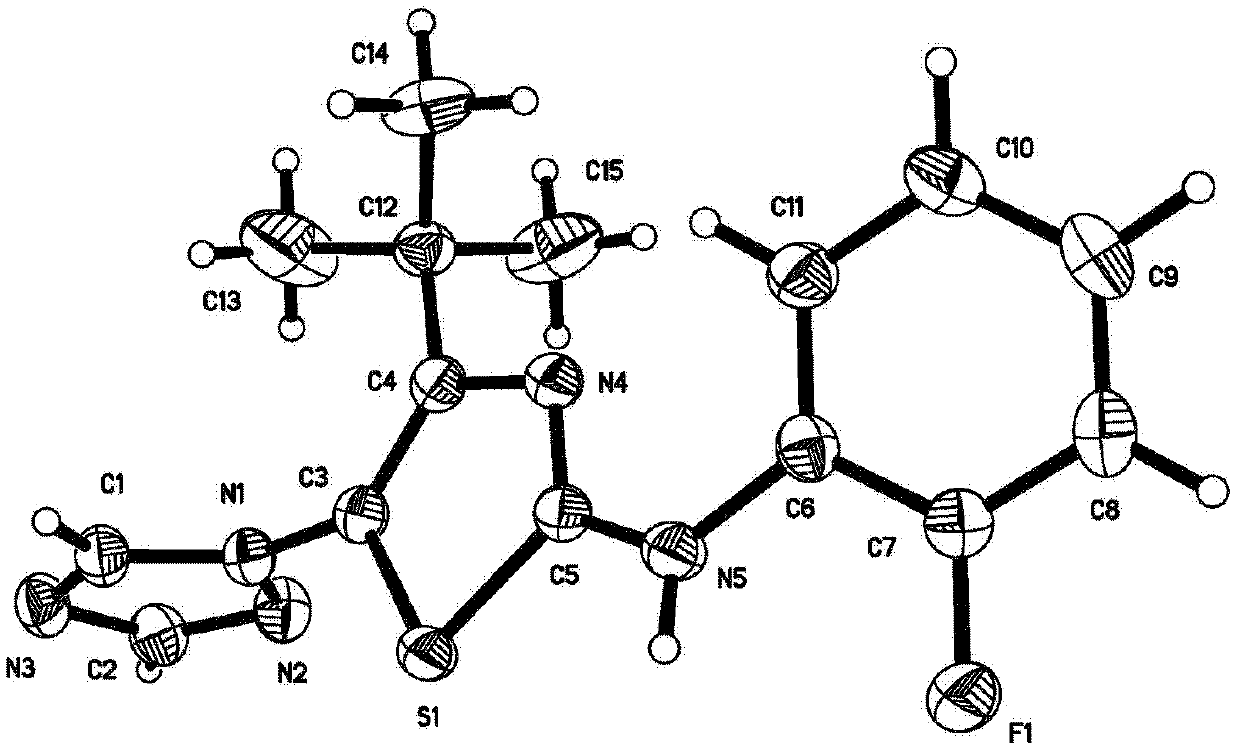
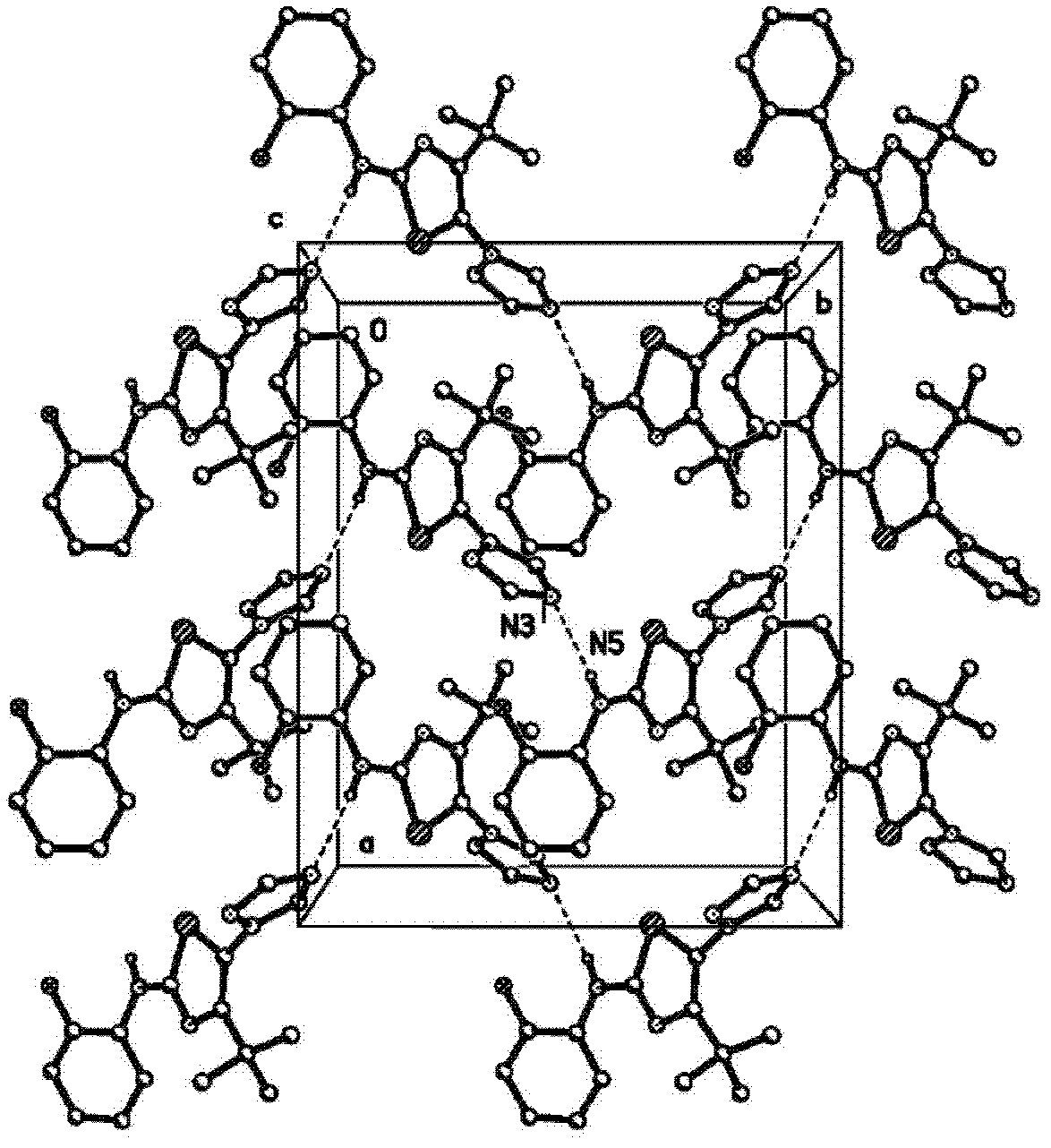
Image
Examples
Embodiment 1
[0036] Preparation of 4-tert-butyl-2-phenylamino-5-(1,2,4-triazol-1-yl)thiazole and its hydrobromide
[0037]
[0038] 3,3-Dimethyl-1-(1,2,4-triazol-1-yl)-1-bromo-2-butanone and phenylthiourea compounds are fed in a molar ratio of 1:1, in ethanol Medium reflux reaction. After the reaction was completed, the solvent was distilled off under reduced pressure, washed with ethyl acetate, filtered, and dried to obtain 4-tert-butyl-2-phenylamino-5-(1,2,4-triazol-1-yl)thiazole hydrobromide Salt; hydrobromide neutralized with ammonia water, extracted with ethyl acetate, and distilled under reduced pressure to obtain 4-tert-butyl-2-phenylamino-5-(1,2,4-triazol-1-yl)thiazole, melting point 186~188℃. 1 HNMR (CDCl 3 , 400MHz), δ: 1.17(s, 9H, 3×CH 3 ), 1.83 (brs, 1H, NH), 7.10~7.40 (m, 5H, C 6 h 5 ), 8.10(s, 1H, C 2 h 2 N 3 3-H), 8.26(s, 1H, C 2 h 2 N 3 5-H).
Embodiment 2
[0040] Preparation of 4-tert-butyl-2-(3-methylanilino)-5-(1,2,4-triazol-1-yl)thiazole and its hydrobromide
[0041]
[0042] According to the method of Example 1, react for 9.0h; get 4-tert-butyl-2-(3-methylanilino)-5-(1,2,4-triazol-1-yl)thiazole hydrobromide ; The hydrobromide was neutralized with ammonia water, extracted with ethyl acetate, and distilled under reduced pressure to obtain 4-tert-butyl-2-(3-methylanilino)-5-(1,2,4-triazole-1- Base) thiazole; melting point 98 ~ 100 ° C; 1 H NMR (CDCl 3 , 400MHz), δ: 1.16(s, 9H, 3×CH 3 ), 2.36(s, 3H, CH 3 ), 6.94 (d, J=8.0Hz, 1H, C 6 h 4 4-H), 7.12(d, J=6.4Hz, 1H, C 6 h 4 6-H), 7.13(s, 1H, C 6 h 4 2-H), 7.25(t, 1H, J = 7.2Hz, 1H, C 6 h 4 5-H), 8.08(s, 1H, C 2 h 2 N 3 3-H), 8.24(s, 1H, C 2 h 2 N 3 5-H).
Embodiment 3
[0044] Preparation of 4-tert-butyl-2-(4-methylanilino)-5-(1,2,4-triazol-1-yl)thiazole
[0045]
[0046] According to the method of Example 1, react 4.0h; Obtain 4-tert-butyl-2-(4-methylanilino)-5-(1,2,4-triazol-1-yl)thiazole; Melting point 147~ 150°C; 1 HNMR (CDCl 3 , 400MHz), δ: 1.17(s, 9H, 3×CH 3 ), 2.34 (s, 3H, CH 3 ), 7.18 (d, J=8.8Hz, 2H, C 6 h 5 3,5-H), 7.22 (d, J=8.8Hz, 2H, C 6 h 5 2,6-H), 8.08(s,1H,C 2 h 2 N 3 3-H), 8.24(s, 1H, C 2 h 2 N 3 5-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com