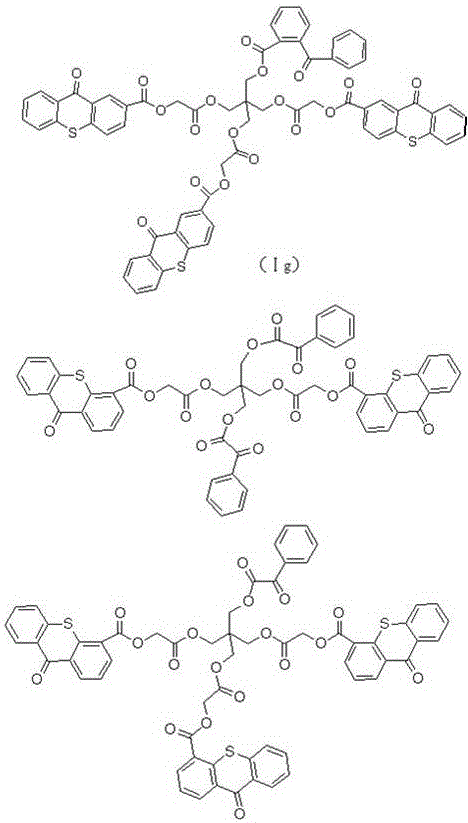
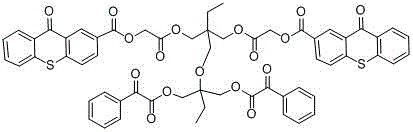
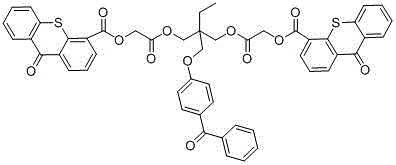
Hydrogen self-supply type photoinitiator and preparation method thereof
A photoinitiator, self-supplying hydrogen technology, applied in applications, coatings, inks, etc., can solve problems such as environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1: methyl thioxanthone-4-formyloxyacetate
[0104] In a 250ml four-neck flask equipped with mechanical stirring, add 25.6g thioxanthone-4-carboxylic acid, 250 ml tetrahydrofuran, 12.1g triethylamine, 13.1 g methyl chloroacetate, heat to 50~60°C for 8 hours , suction filtration, the filtrate was cooled to 0~5°C, stirred and crystallized for 2 hours, and then the crude product was obtained by suction filtration. The crude product was recrystallized with toluene, and after drying, 23.9g of yellow flaky crystals were obtained. The yield was 73%, and the content was ≥98.0%. 1 H NMR (DMSO) δ: 8.25 (m, 1H), 8.16 (m, 1H), 8.01 (m, 1H), 7.83 (d,1H), 7.66 (m, 2H), 7.42 (m, 1H), 5.17 (m, 2H), 3.55 (s, 3H).
Embodiment 2
[0105] Embodiment 2: methyl thioxanthone-4-formyloxyacetate
[0106]In a 250ml four-necked flask equipped with mechanical stirring, add 25.6g thioxanthone-4-carboxylic acid, 250 ml tetrahydrofuran, 18.4g diisopropylethylamine, 14.8g methyl chloroacetate, and heat to reflux for 8 hours. Cool down to 40~50°C and filter with suction, cool the filtrate to 0~5°C, stir and crystallize for 2 hours, and then filter with suction to obtain the crude product. The crude product is recrystallized with toluene, and after drying, 23.0g of yellow flaky crystals are obtained. The yield is 70%, and the content is ≥ 98.0%.
Embodiment 3
[0107] Embodiment 3: methyl thioxanthone-2-formyloxyacetate
[0108] In a 250ml four-necked flask equipped with mechanical stirring, add 25.6g thioxanthone-2-carboxylic acid, 200 ml tetrahydrofuran, 12.1g triethylamine, 13.1 g methyl chloroacetate, heat to 50~60°C for 8 hours , suction filtration, the filtrate was cooled to 0~5°C, stirred and crystallized for 2 hours, and then the crude product was obtained by suction filtration. The crude product was recrystallized with toluene, and after drying, 27.2g of light yellow flaky crystals were obtained. The yield was 83%, and the content was ≥97.5%. 1 H NMR (DMSO) δ: 8.16 (d, 1H), 8.04 (m, 1H), 7.91 (m, 1H), 7.63 (d,1H), 7.51 (m, 2H), 7.22 (m, 1H), 5.14 (m, 2H), 3.52 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com