N-imidazolyl acetate derivative, and preparation method and application thereof
A technology of imidazolyl acetate and derivatives, which is applied in the field of N-imidazolyl acetate derivatives and its preparation, can solve the problem that the solution does not meet the requirements of national standards, the mutual influence is difficult to accurately grasp, and the carbon deposit in the engine increases too fast and other problems to achieve the effect of saving the preparation process, avoiding competition or antagonism, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
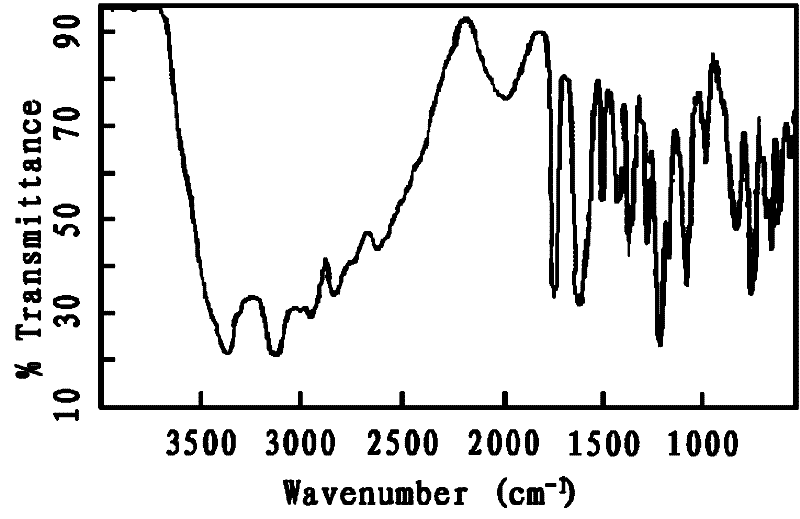
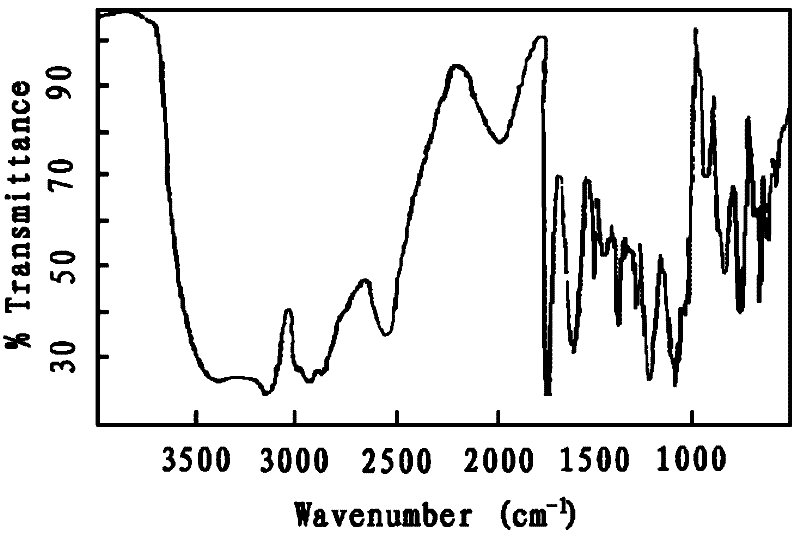
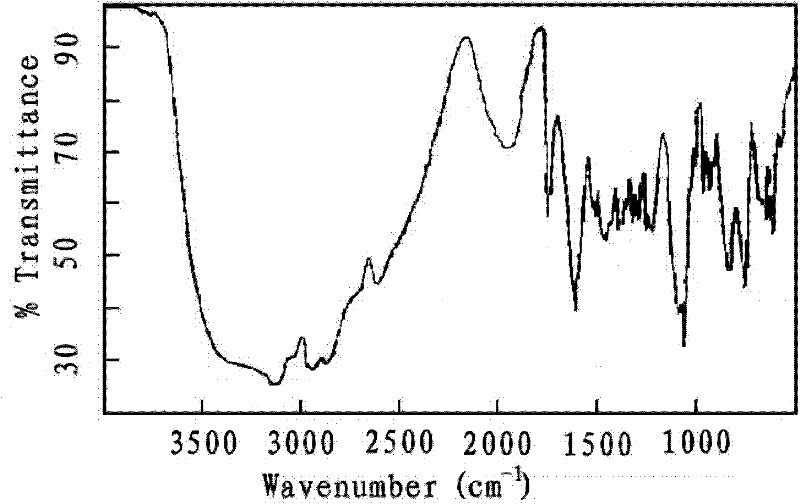
Image
Examples
preparation example Construction
[0051] The preparation method of N-imidazolyl acetate derivative of the present invention comprises the following reaction steps:
[0052] 1) Synthesis of chloroacetate:
[0053] With return line, CaCl 2 In the round bottom flask of the drying tube, add chloroacetic acid and lower carbon alcohol with a molar ratio of 1:1.05~1.1, heat in an oil bath, slowly raise the temperature to reflux, reflux for 6~8h, stop heating, stand for 6~8h and cool to room temperature , the reactant was poured into distilled water, static layered, and the organic layer was separated; with NaHCO 3 Wash the organic layer with a saturated aqueous solution for 2-4 times, then dry the organic layer with anhydrous calcium chloride, leave it for 11-15 hours, and distill and refine under reduced pressure to obtain a colorless chloroacetate liquid;
[0054] 2) Synthesis of N-imidazolyl acetate derivatives: in a reflux tube, CaCl 2 In the three-neck round-bottomed flask of the drying tube and the constant ...
Embodiment 1
[0056] Synthesis of methyl N-imidazolyl acetate
[0057] (1) Synthesis of methyl chloroacetate
[0058] In a fume hood, assemble with reflux condenser, CaCl 2 Add 94.5g (1mol) of chloroacetic acid and 35g (about 1.1mol) of methanol to the 500mL round bottom flask of the drying tube, heat in an oil bath, slowly raise the temperature, reflux for 6h, stop heating, let stand for 6h to cool to room temperature, and react The mixture was poured into 1.2 L of distilled water, and the organic phase was separated. with NaHCO 3 The organic phase was washed with saturated aqueous solution 4 times, 60 mL each time, and the organic phase was separated. Then dry the organic phase with anhydrous calcium chloride, place it for 12 hours, and refine it by distillation under reduced pressure. Obtain 93.6g of methyl chloroacetate colorless liquid, relative density d 20 4 1.2218, refractive index n 20 D 1.4586, boiling point 136.8°C.
[0059] (2) Synthesis of N-imidazolyl acetate
[0060...
Embodiment 2
[0065] Synthesis of ethyl N-imidazolyl acetate
[0066] (1) Synthesis of ethyl chloroacetate
[0067] In a fume hood, assemble with reflux condenser, CaCl 2 In a 500mL round bottom flask with a drying tube, add 94.5g (1mol) of chloroacetic acid and 49.5g (about 1.075mol) of ethanol into the flask, heat in an oil bath, slowly raise the temperature, reflux for 7h, stop heating, let stand for 7h to cool to room temperature, and The reactant was poured into 1.6 L of distilled water, and the organic phase was separated. with NaHCO 3 The organic phase was washed with saturated aqueous solution 3 times, 70 mL each time, and the organic phase was separated. Dry the organic phase with anhydrous calcium chloride, place it for 11 hours, and distill it under reduced pressure for purification. Obtain 98.3g of ethyl chloroacetate colorless liquid, relative density d 20 4 1.2319, refractive index n 20 D 1.4714, boiling point 143.7°C.
[0068] (2) Synthesis of ethyl N-imidazolyl acet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com