Preparation method of alpha arylglycine
A radical and aromatic technology, applied in the field of new synthetic process routes of α-aryl amino acids, can solve the problems of long reaction time of phase transfer catalyst, low conversion rate, long synthesis steps, etc., and achieve good optical purity and chemical yield. High efficiency, high ligand recovery, and simple post-reaction treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
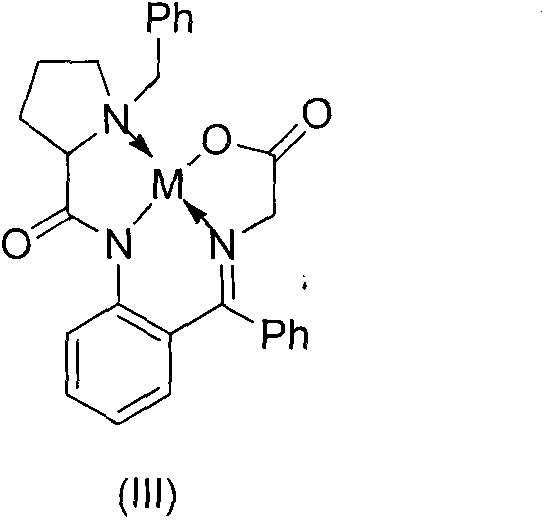
[0027] Synthesis of Phenyl-Substituted Ni Ligands
[0028] Dissolve 50mg Ni(II) chelate, 11μL iodobenzene, 63.68mg potassium phosphate, 11.55mg tetrakistriphenylphosphine palladium and 3.9mg tetrakistriphenylphosphine in 2ml of dioxane, reflux under nitrogen protection for 20 After 1 hour, the reaction solution was filtered, concentrated, and purified by silica gel column to obtain 42.4 mg of the target product with a yield of 74%. 1 H NMR (300MHz, CDCl 3 ): δ8.21-8.10(m, 3H), 7.81-7.72(m, 2H), 7.61-7.35(m, 5H), 7.28-7.23(m, 5H), 7.21-7.19(m, 1H), 7.03 -6.98(m, 1H), 6.69-6.68(m, 2H), 6.13-6.10(m, 1H), 4.84(s, 1H), 4.55-4.51(d, 1H), 3.67-3.59(d, 2H) , 3.57-3.47(m, 3H), 2.87-2.82(m, 2H), 2.59-2.55(m, 1H), 2.09-2.02(m, 2H); ESI-MS m / z=596.1[M+Na] +
Embodiment 2
[0030] Synthesis of 3-Chlorophenyl Substituted Ni Ligands
[0031] Dissolve 50 mg of Ni(II) chelate, 11 μL of 3-chlorobromobenzene, 63.68 mg of potassium phosphate, 11.55 mg of palladium tetrakistriphenylphosphine and 3.9 mg of tetrakistriphenylphosphine in 2 ml of dioxane, under nitrogen protection Under reflux for 20 hours, the reaction solution was filtered, concentrated, and purified by silica gel column to obtain 39.8 mg of the target product with a yield of 65%. 1 H NMR (300MHz, CDCl 3 ): δ8.18-8.08(m, 3H), 8.10(s, 1H), 7.70-7.63(m, 2H), 7.57-7.38(m, 5H), 7.43-7.38(m, 3H), 7.24-7.16 (m, 3H), 7.07-7.02 (m, 1H), 6.68-6.67 (m, 2H), 6.16-6.13 (m, 1H), 4.84 (s, 1H), 4.55-4.51 (d, 1H), 3.67 -3.59(d, 2H), 3.57-3.47(m, 3H), 2.87-2.82(m, 2H), 2.59-2.55(m, 1H), 2.09-2.02(m, 2H); ESI-MS m / z =608.1[M+1] +
Embodiment 3
[0033] Synthesis of 4-methylphenyl substituted Ni ligands
[0034] Dissolve 50 mg of Ni(II) chelate, 11 μL of 4-methylbromobenzene, 63.68 mg of potassium phosphate, 11.55 mg of tetrakistriphenylphosphine palladium and 3.9 mg of tetrakistriphenylphosphine in 2 ml of dioxane, under nitrogen protection Under reflux for 20 hours, the reaction solution was filtered, concentrated and purified by silica gel column to obtain 32.4 mg of the target product with a yield of 56%. 1 H NMR (300MHz, CDCl 3 ): δ8.19-8.08(m, 3H), 7.79-7.77(s, 1H), 7.68-7.66(s, 1H), 7.50-7.45(m, 2H), 7.42-7.35(m, 3H), 7.30 -7.27(m, 2H), 7.23-7.18(m, 2H), 7.11-6.99(m, 2H), 6.67(s, 2H), 6.15-6.09(m, 1H), 4.84(s, 1H), 4.55 -4.51(d, 1H), 3.67-3.59(d, 2H), 3.57-3.47(m, 3H), 2.87-2.82(m, 2H), 2.59-2.55(m, 1H), 2.32(s.3H) , 2.09-2.02 (m, 2H); ESI-MS m / z=610.2[M+Na] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com