Synthesis method of vilazodone and salt thereof
A synthetic method, the technology of vilazodone, which is applied in the field of drug synthesis, can solve the problems of being unsuitable for large-scale industrial production of vilazodone hydrochloride, reducing the utilization rate of production resources, increasing the cost of equipment use, etc., and achieving simple post-reaction treatment Ease of operation, low price, and the effect of increasing product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
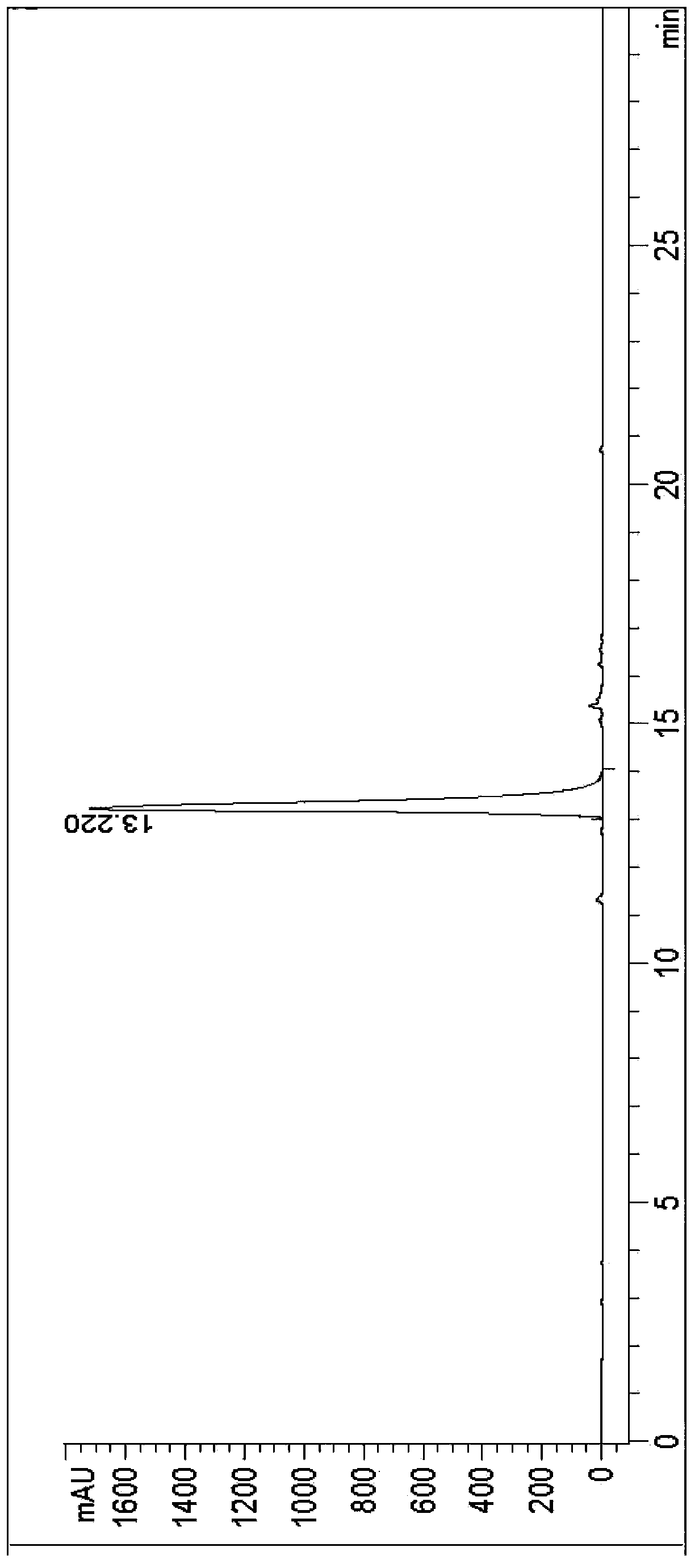
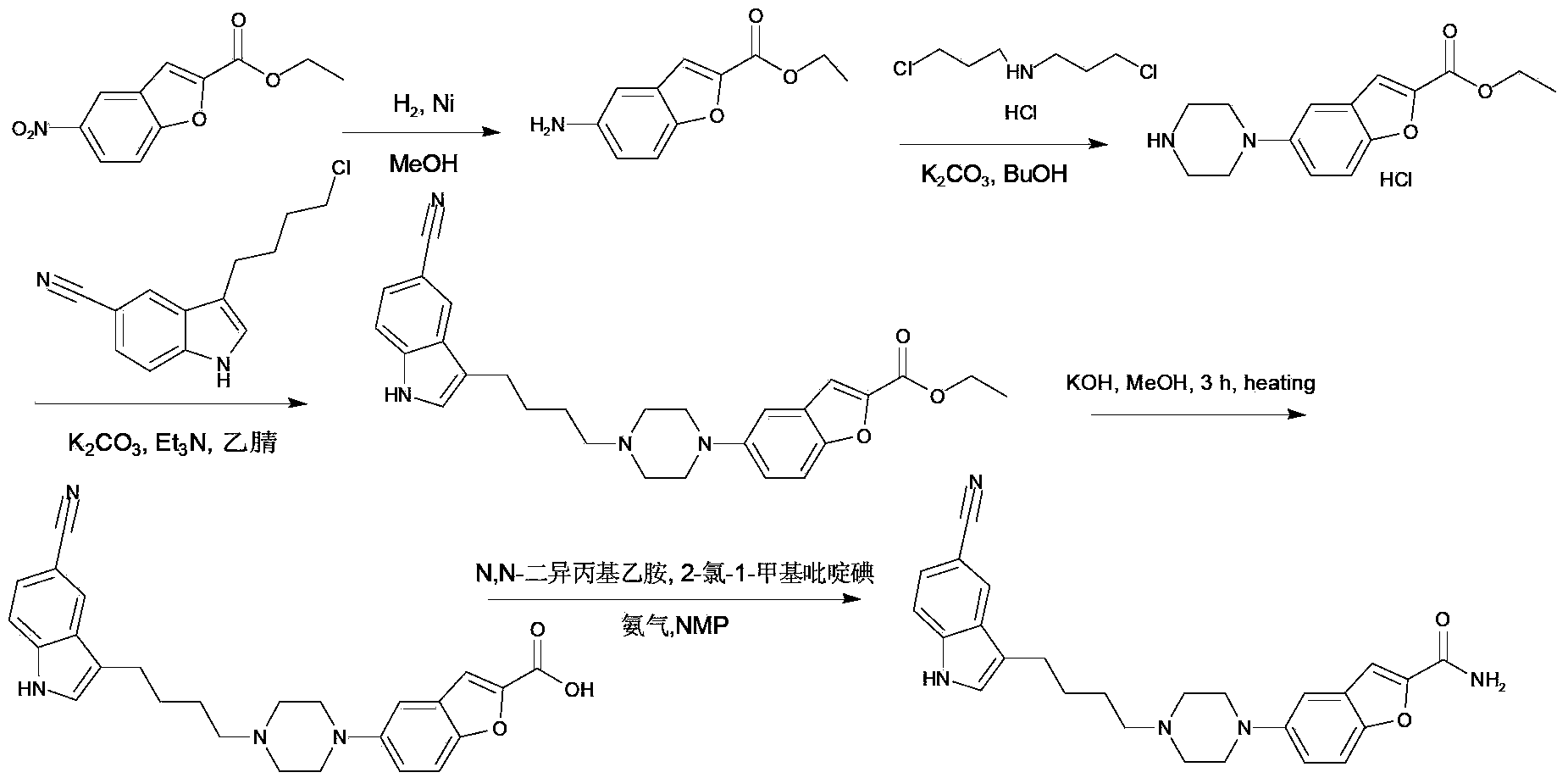
Image
Examples
Embodiment 1
[0049] At room temperature, under nitrogen protection, 2.4 g of 1.1-dimethoxy-6-chlorohexane was dissolved in a mixed solution of 24 mL of ethanol and 12 mL of water, and the temperature was raised to 68° C. to dissolve all. At room temperature, a mixed solvent of 1.9 g of 4-cyanophenylhydrazine hydrochloride, 19 mL of ethanol and 9.5 mL of pure water was slowly added dropwise to 2.3 g of the 1.1-dimethoxy-6-chlorohexane reaction solution. The temperature was kept at 70°C for 0.8 hours, and after the reaction solution was lowered to room temperature, a large amount of solid was precipitated, suction filtered, and the filter cake was recrystallized with 50% ethanol aqueous solution to obtain 1.98g of 3-(4-chlorobutyl)-5-cyanoindole indoles.
[0050] Under nitrogen protection, 4.0 g of 5-fluoro-2-hydroxybenzaldehyde was dissolved in 15 mL of acetonitrile to form a reaction solution. Under stirring, 3.26 g of cesium carbonate was added to the reaction solution. After stirring for...
Embodiment 2
[0055] At room temperature, under nitrogen protection, 6.5 g of 1.1-dimethoxy-6-chlorohexane was dissolved in a mixed solution of 65 mL of methanol and 35 mL of water, and the temperature was raised to 70° C. to dissolve all. At room temperature, a mixed solvent of 6.0 g of 4-cyanophenylhydrazine hydrochloride, 60 mL of methanol and 30 mL of pure water was slowly added dropwise to 5.9 g of the 1.1-dimethoxy-6-chlorohexane reaction solution. Incubate for 1.0 hours under the condition of ℃, when the reaction solution is lowered to room temperature, a large amount of solid is precipitated, suction filtration, and the filter cake is recrystallized with 53% ethanol aqueous solution to obtain 8.0g of 3-(4-chlorobutyl)-5-cyanoindole .
[0056]Under the protection of nitrogen, dissolve 7.0g of 5-fluoro-2-hydroxybenzaldehyde in 70mL of N,N-dimethylformamide to form a reaction solution, add 3.0g of triethylamine to the reaction solution under stirring, and stir for 9 Minutes later, 4.1...
Embodiment 3
[0061] At room temperature, 4.5g of 1.1-dimethoxy-6-chlorohexane was dissolved in a mixed solution of 45mL of ethanol and 25mL of water under the protection of nitrogen, and the temperature was raised to 72°C to completely dissolve. At room temperature, a mixed solvent of 3.9g of 4-cyanophenylhydrazine hydrochloride, 39mL of ethanol and 10mL of pure water was slowly added dropwise to the reaction solution of 3.9g of 1.1-dimethoxy-6-chlorohexane, at 72 Keep warm at ℃ for 1.1 hours, wait for the reaction liquid to drop to room temperature, a large amount of solids precipitate out, filter with suction, and recrystallize the filter cake with 55% ethanol aqueous solution to obtain 6.0 g of 3-(4-chlorobutyl)-5-cyanindole .
[0062] Under the protection of nitrogen, dissolve 4.5g of 5-fluoro-2-hydroxybenzaldehyde in 45mL of tetrahydrofuran to form a reaction solution, add 3.9g of N-methylmorpholine to the reaction solution under stirring, stir for 11 minutes under the protection of n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com