Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3812 results about "Pyridyne" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pyridyne in chemistry is the pyridine analogue of benzyne. This reactive intermediate is of some importance to scientific research. Pyridynes are the class of compounds sharing the pyridyne building motif. Two isomers exist, the 2,3-pyridine (2,3-didehydropyridine) and the 3,4-pyridyne (3,4-didehydropyridine). The reaction of 3-bromo-4-chloropyridine with furan and lithium amalgam gives 1,4-epoxy-dihydroquinoline through the 2,3-pyridyne intermediate. The reaction of 4-bromopyridine with sodium in liquid ammonia gives both 3-aminopyridine and 4-aminopyridine through the 3,4-pyridyne intermediate and an E1cB-elimination reaction.
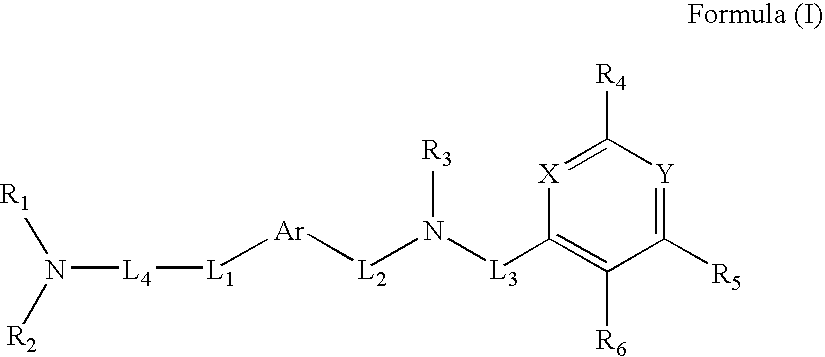
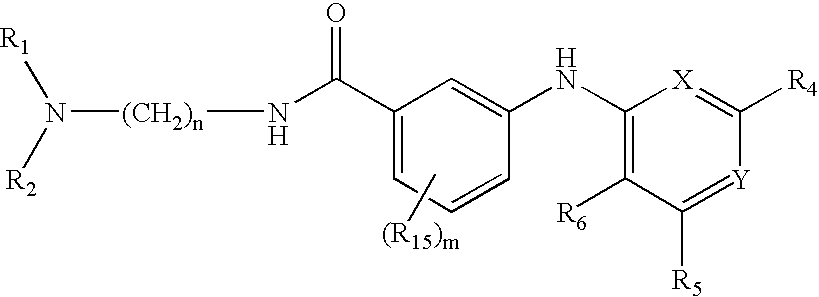
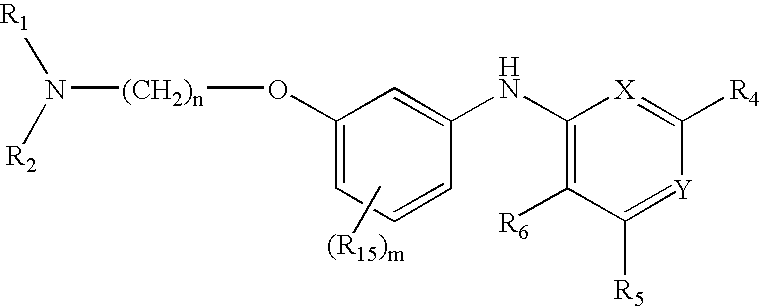
Novel Thieno-Pyridine and Thieno-Pyrimidine Derivatives and Their Use as Positive Allosteric Modulators of Mglur2-Receptors
The present invention relates to novel compounds, in particular novel thieno-pyridine and thieno-pyrimidine derivatives according to Formula (I), wherein all radicals are defined in the application. The compounds according to the invention are positive allosteric modulators of metabotropic receptors-subtype 2 ("mGluR2") which are useful for the treatment or prevention of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which the mGluR2 subtype of metabotropic receptors is involved. In particular, such diseases are central nervous system disorders selected from the group of anxiety, schizophrenia, migraine, depression, and epilepsy. The invention is also directed to pharmaceutical compositions and processes to prepare such compounds and compositions, as well as to the use of such compounds for the prevention and treatment of such diseases in which mGluR2 is involved.
Owner:ORTHO MCNEIL JANSSEN PHARMA
2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones
The present invention provides substituted 2-aminopyridines useful in treating cell proliferative disorders. The novel compounds of the present invention are potent inhibitors of cyclin-dependent kinases 4 (cdk4)
Owner:WARNER LAMBERT CO LLC
Substituted pyrrolopyridines and pyrazolopyridines as kinase modulators
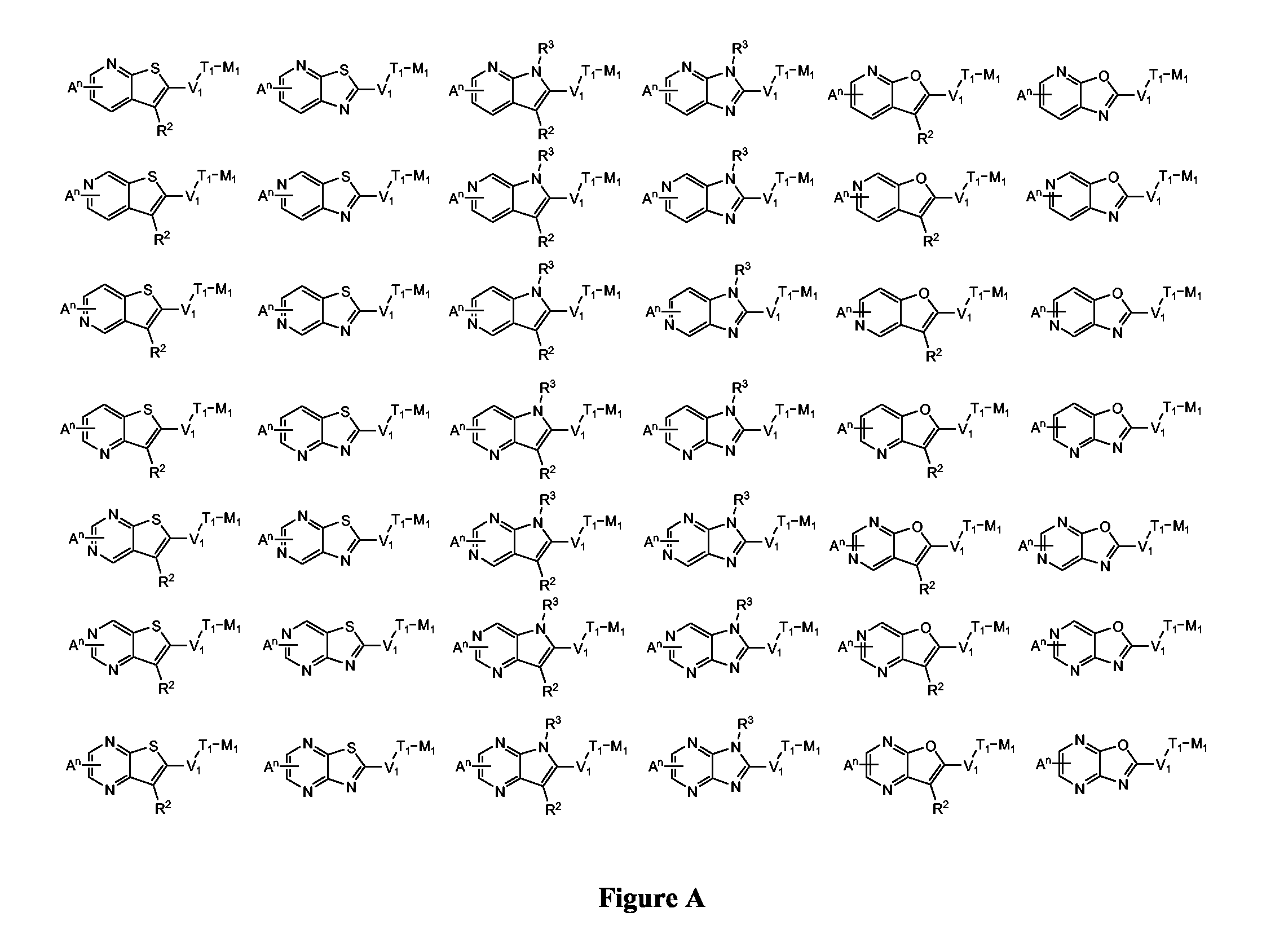
Provided herein are substituted pyrrolopyridine heterocycles and substituted pyrazolopyridine heterocycles, pharmaceutical compositions comprising said heterocycles and methods of using said heterocycles in the treatment of disease. The heterocycles disclosed herein function as kinase modulators and have utility in the treatment of diseases such as cancer, allergy, asthma, inflammation, obstructive airway disease, autoimmune diseases, metabolic disease, infection, CNS disease, brain tumor, obesity, asthma, hematological disorder, degenerative neural disease, cardiovascular disease, or disease associated with angiogenesis, neovascularization, or vasculogenesis.
Owner:SGX PHARMA INC
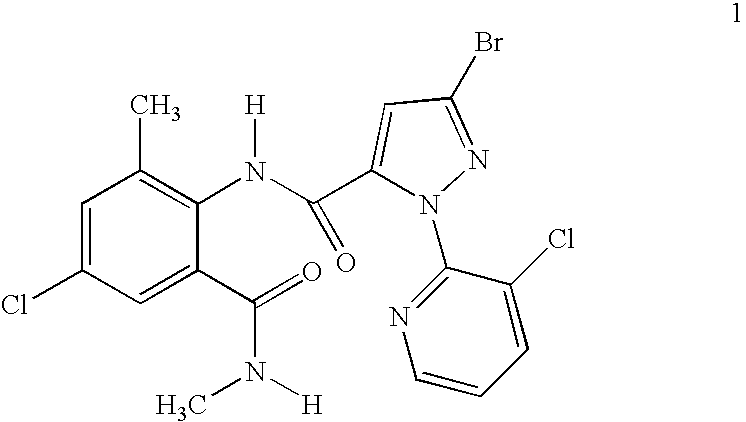
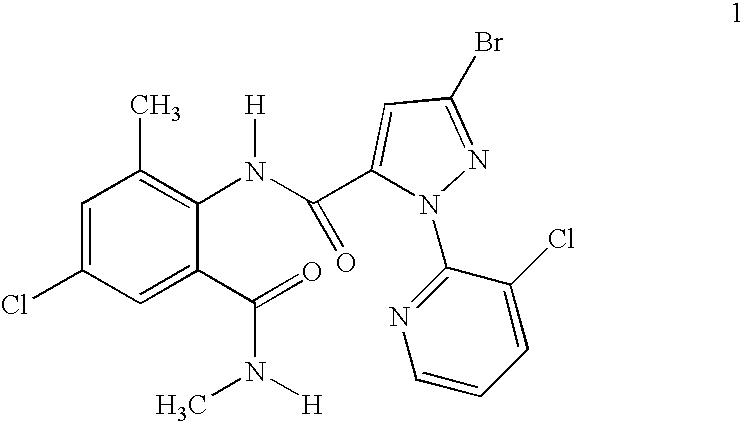
Synergistic Mixtures of Anthranilamide Invertebrate Pest Control Agents
Disclosed are mixtures and compositions for controlling invertebrate pests relating to combinations comprising (a) 3-bromo-N-[4-chloro-2-methyl-6-[(methylamino)carbonyl]phenyl]-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide, and its N-oxides, and suitable salts thereof and a component (b) wherein the component (b) is at least one compound or agent selected from neonicotinoids, cholinesterase inhibitors, sodium channel modulators, chitin synthesis inhibitors, ecdysone agonists, lipid biosynthesis inhibitors, macrocyclic lactones, GABA-regulated chloride channel blockers, juvenile hormone mimics, ryanodine receptor ligands, octopamine receptor ligands, mitochondrial electron transport inhibitors, nereistoxin analogs, pyridalyl, flonicamid, pymetrozine, dieldrin, metaflumizone, biological agents, and suitable salts of the foregoing. Also disclosed are methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a mixture or composition of the invention.
Owner:FMC CORP
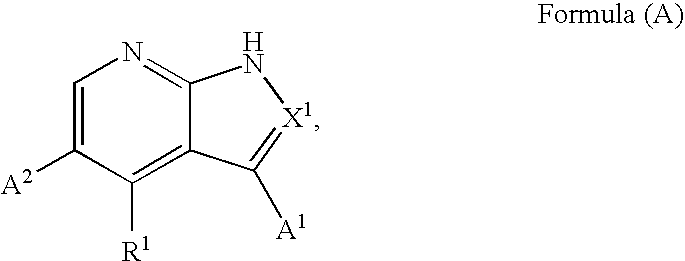
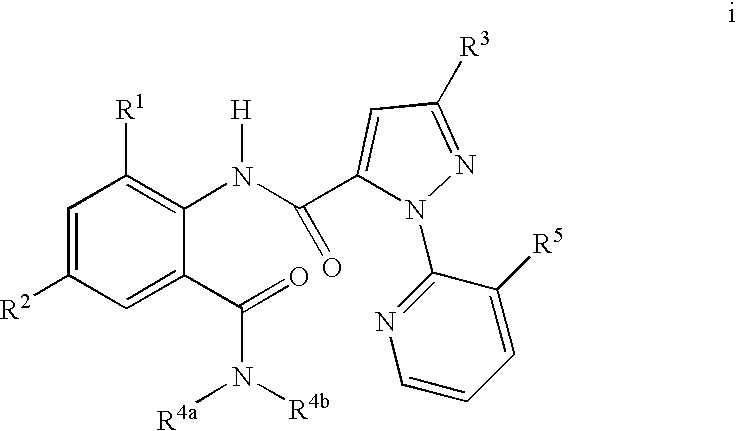
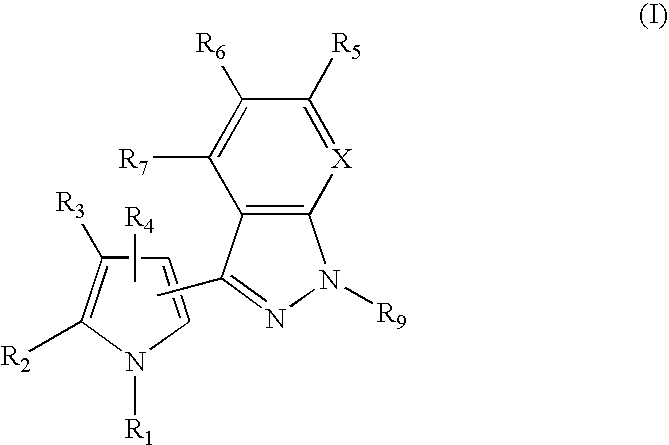
1H-pyrazolo[3,4-b]pyridines and therapeutic uses thereof
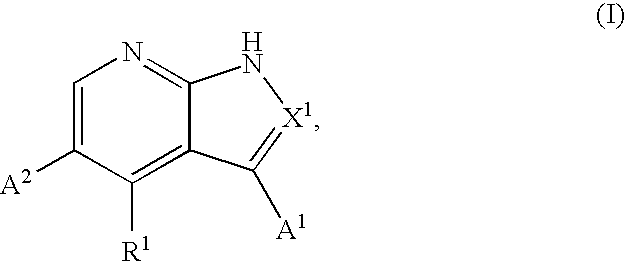
Provided herein are compounds according to Formula I and pharmaceutically acceptable salts thereof, and compositions comprising the same, for use in various methods, including treating cancers such as colon, ovarian, pancreatic, breast, liver, prostate and hematologic cancers:
Owner:BIOSPLICE THERAPEUTICS INC
Pyridine, pyrimidine, quinoline, quinazoline, and naphthalene urotensin-II receptor antagonists
The present invention relates to urotensin II receptor antagonists, pharmaceutical compositions containing them and their use.
Owner:ENCYSIVE PHARMA INC
7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy
Owner:ONCOCEUTICS INC
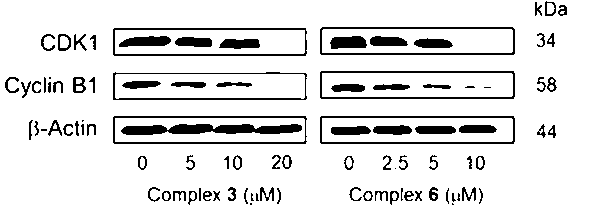
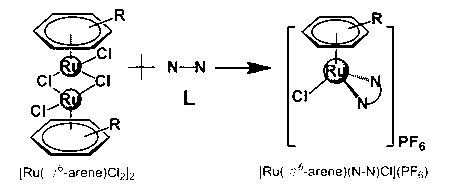
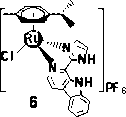
Aryl ruthenium-beta-carboline complex and its preparation method and application
InactiveCN103254239AEasy to modifyGood water solubilityOrganic active ingredientsGroup 8/9/10/18 element organic compoundsCancer cellAlkaloid
Owner:SUN YAT SEN UNIV
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto
Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol compounds are disclosed that are inhibitors of the vesicular monoamine transporter 2 (VMAT2). The compounds of this invention have the structure:wherein R1 is as defined herein, including stereoisomers and pharmaceutically acceptable salts and solvates thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
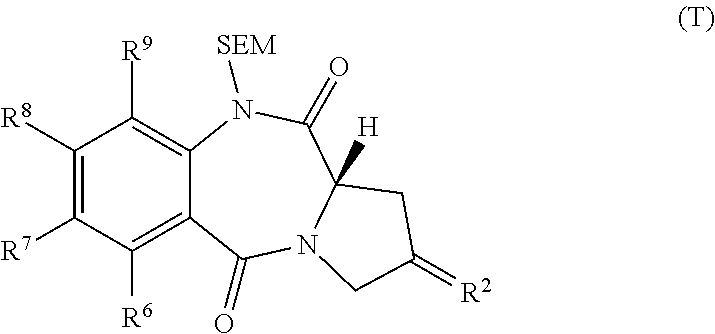
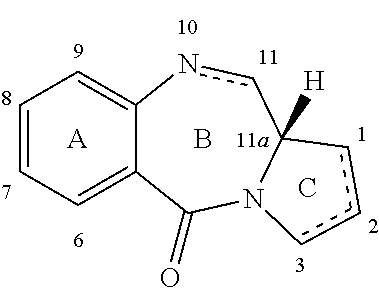
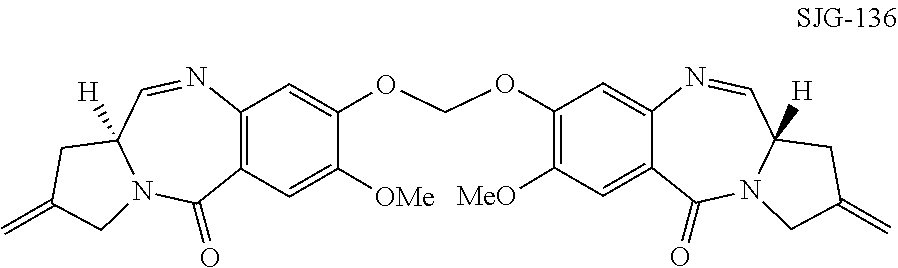
Pyrrolobenzodiazepines
The invention relates to certain pyrrolobenzodiazepines (PBDs), and in particular pyrrolobenzodiazepine dimers bearing C2 substitutions, including compounds of formula (T): wherein: R2 is CHR2A, and R2A is independently selected from H, R, CO2R, COR, CHO, CO2H, and halo; R6 and R9 are independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, NO2, Me3Sn and halo; R7 is independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, NO2, Me3Sn and halo; R8 is independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR′, NO2, Me3Sn and halo; R is independently selected from optionally substituted C1-12 alkyl, C3-20 heterocyclyl and C5-20 aryl groups; or the compound is a dimer with each monomer being of formula (M), where the R7 groups or R8 groups of each monomer form together a dimer bridge having the formula —X—R″—X— linking the monomers; wherein R″ is a C3-12 alkylene group, which chain may be interrupted by one or more heteroatoms, e.g. O, S, N(H), and / or aromatic rings, e.g. benzene or pyridine; and each X is independently selected from O, S, or N(H); or any pair of adjacent groups from R6 to R9 together form a group —O—(CH2)p—O—, where p is 1 or 2, and salts and solvates thereof, and their use as intermediates for the preparation of other PBD compounds.
Owner:MEDIMMUNE LTD
Fused amino pyridine as hsp90 inhibitors
The present invention relates to HSP90 inhibitors containing fused amino pyridine core that are useful as inhibitors of HSP90 and their use in the treatment of HSP90 related diseases and disorders such as cancer, an autoimmune disease, or a neurodegenerative disease.
Owner:CURIS INC
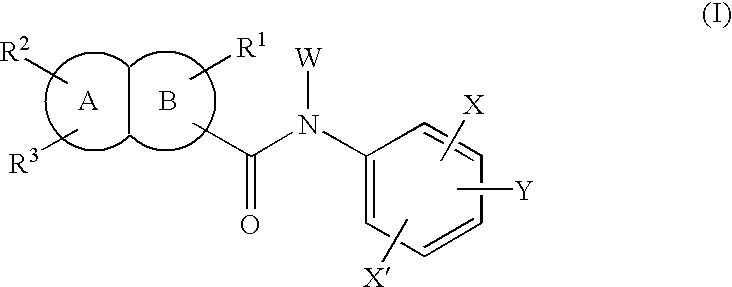
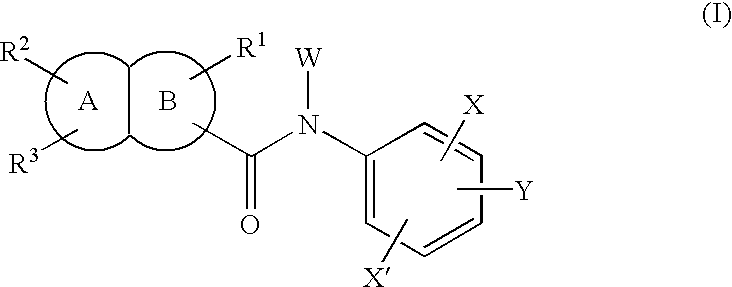
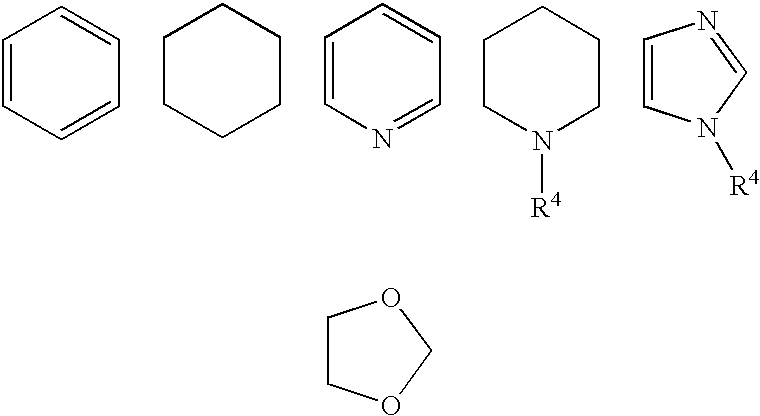
Fused bicyclic amide compounds and medicinal use thereof
Compounds represented by the following general formula (I) wherein ring A is benzene, cyclohexane, pyridine, piperidine, a derivative thereof, imidazole, a derivative thereof, etc.; the ring B represents benzene, cyclohexane, pyrrole or a derivative thereof, furan, thiophene, etc.; R1, R2 and R3 represent each hydrogen, alkyl, halogen, hydroxyl, alkoxy, etc.; W represents hydrogen, alkyl or hydroxycarbonylalkyl; X represents halogen, cyano, nitro, etc.; X′ represents hydrogen, halogen, etc.; and Y represents alkyl, hydroxyalkyl, hydroxycarbonylalkyl, aminoalkyl, etc.; salt thereof, and drugs comprising these compounds. Because of having an exellent effect of inhibiting activated lymphocyte proliferation, these compounds are usefull as preventives or remedies for various autoimmune diseases.
Owner:MITSUBISHI TANABE PHARMA CORP
Novel compound useful for the treatment of degenerative and inflammatory diseases
ActiveUS20120277247A1Modify activityModulate activityOrganic active ingredientsOrganic chemistryAutoimmune conditionAutoimmune disease
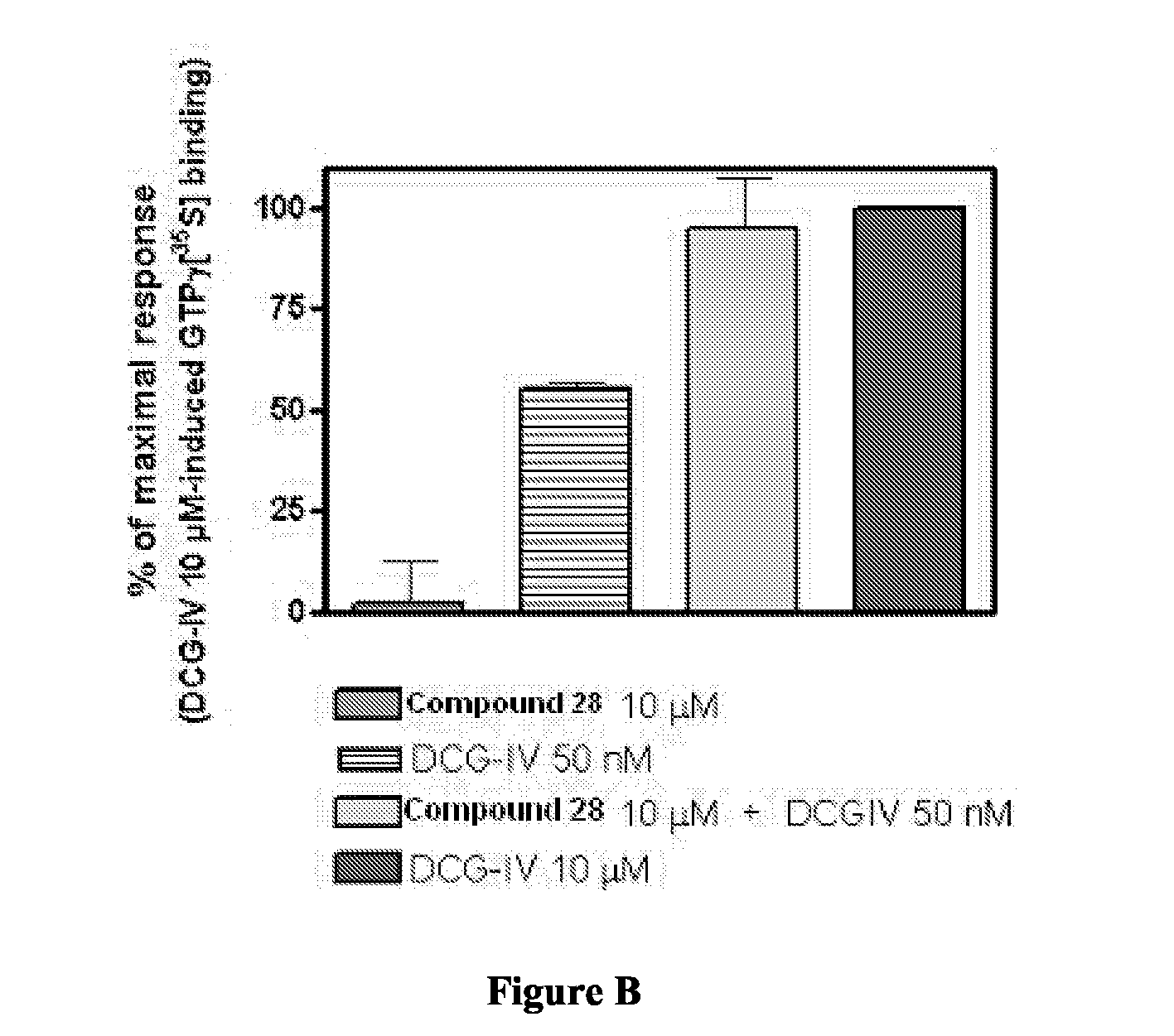
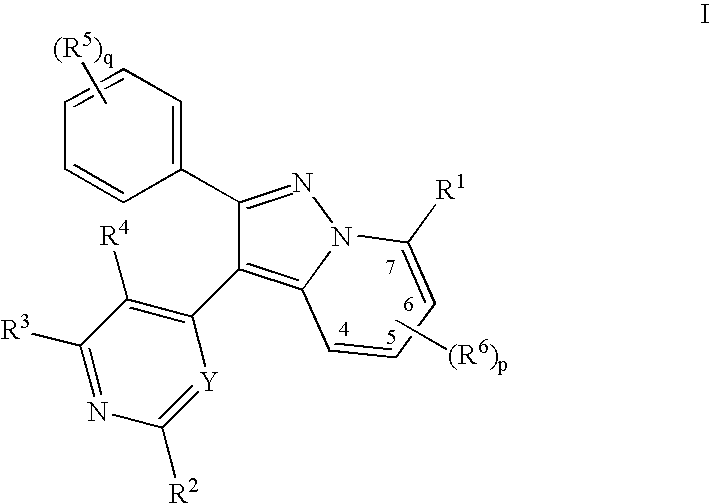
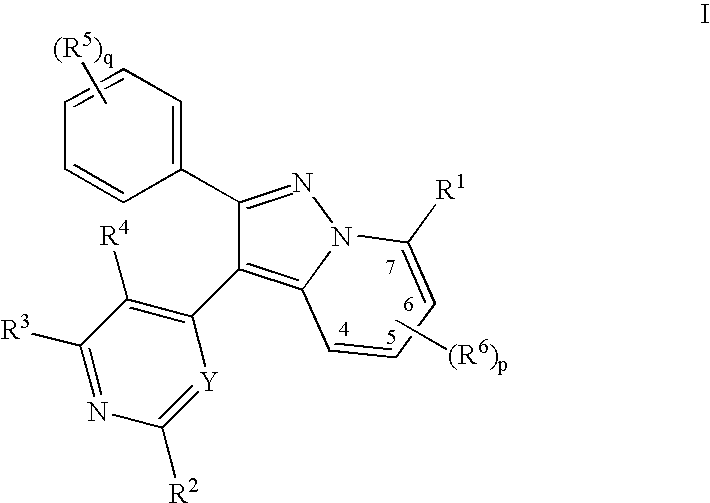
A pyrazolopyridine compound according to Formula I, able to inhibit JAK is disclosed, as well as pharmaceutically acceptable salts, a solvate thereof, solvates of the pharmaceutically acceptable salts and biologically active metabolites thereof. The compound may be prepared as a pharmaceutical composition, and may be used for the treatment or prophylaxis of a variety of conditions in mammals including humans, and particularly, such conditions as may be associated with aberrant JAK activity, including by way of non-limiting example, allergy, inflammatory conditions, autoimmune diseases, proliferative diseases, transplant rejection, diseases involving impairment of cartilage turnover, congenital cartilage malformations, and / or diseases associated with hypersecretion of IL6.
Owner:GALAPAGOS NV
Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity
ActiveUS9708322B2High activityPromote disseminationOrganic active ingredientsOrganic chemistryQuinoxalineDisease
The invention relates to substituted heterocycle fused gamma-carbolines of the Formula Q as described herein, in free base or pharmaceutically acceptable salt form, and / or pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving the 5-HT2A receptor pathway, the serotonin transporter (SERT) pathway and / or the dopamine D2 receptor pathway signaling systems.
Owner:INTRA CELLULAR THERAPIES INC
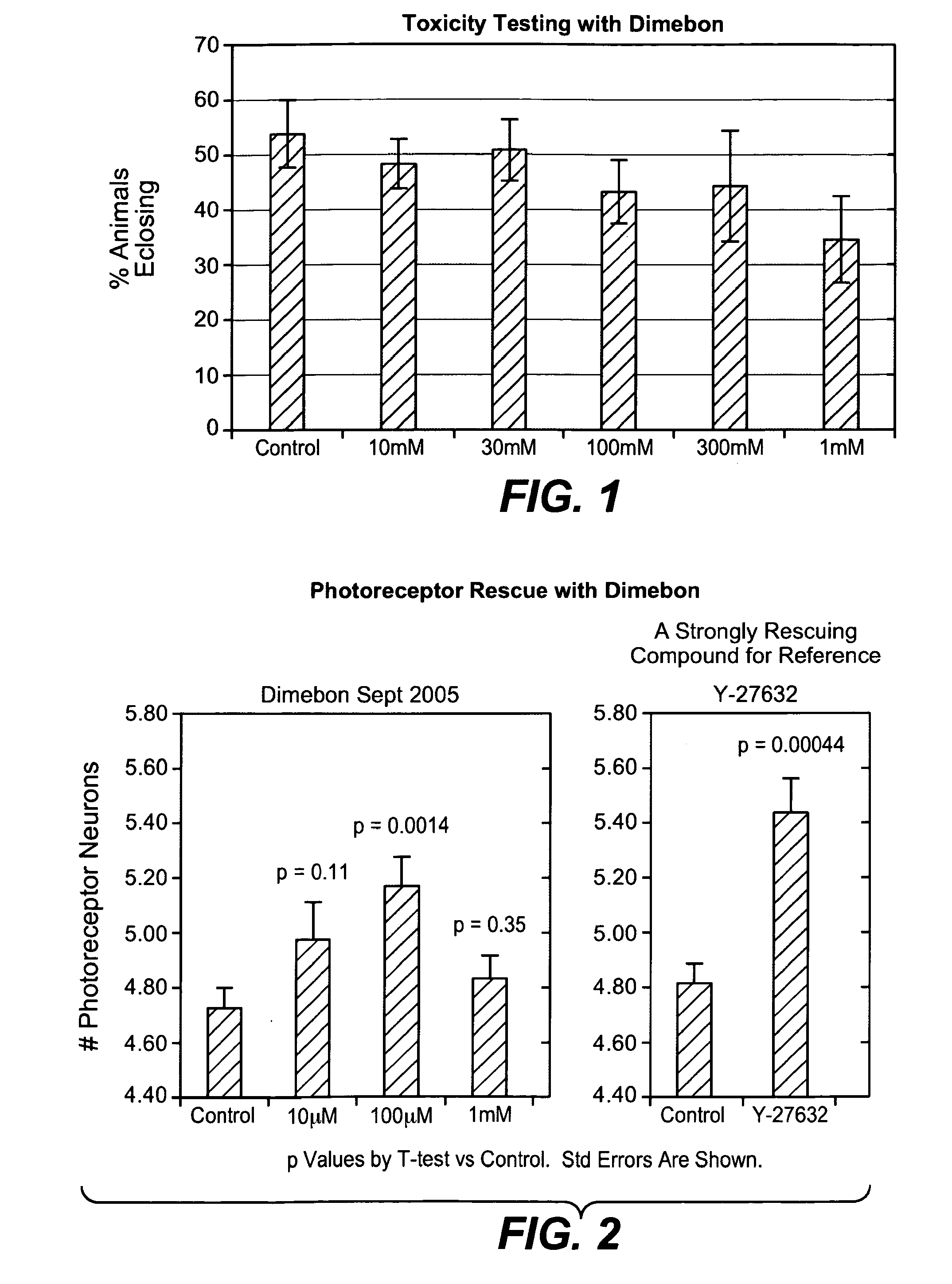
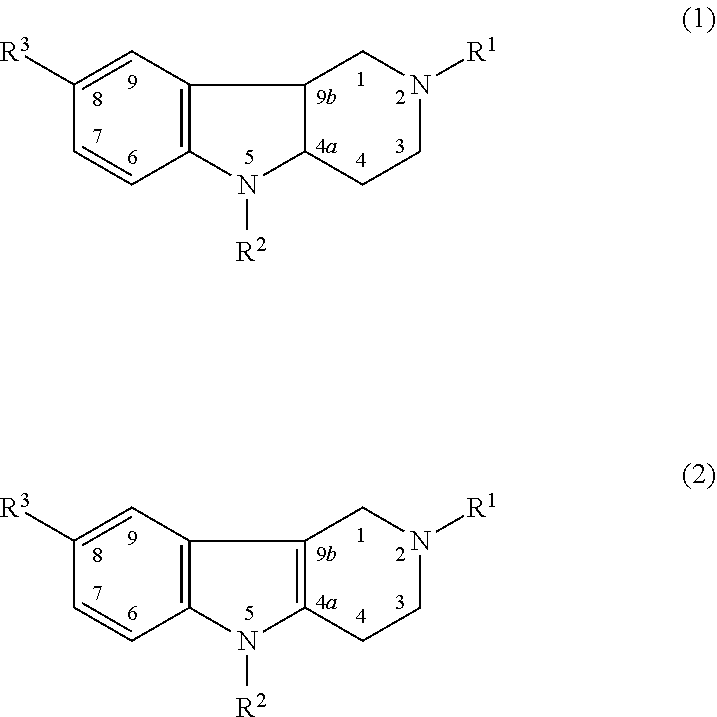
Hydrogenated pyrido (4,3-b) indoles for treating amyotrophic lateral sclerosis (ALS)
InactiveUS20100099700A1Quality improvementProlong survival timeBiocideNervous disorderAmyotrophic lateral sclerosisMedicine
Owner:MEDIVATION TECH INC
Substituted pyrrolo[2.3-B]pyridines
Compounds represented by Formula (I):or stereoisomers or pharmaceutically acceptable salts thereof, are inhibitors of least two of the Abl, Aurora-A, Blk, c-Raf, cSRC, Src, PRK2, FGFR3, Flt3, Lck, Mek1, PDK-1, GSK3β, EGFR, p70S6K, BMX, SGK, CaMKII, Tie-2, IGF-1R, Ron, Ret, and KDR kinases in animals, including humans, for the treatment and / or prevention of various diseases and conditions such as cancer.
Owner:OSI PHARMA INC
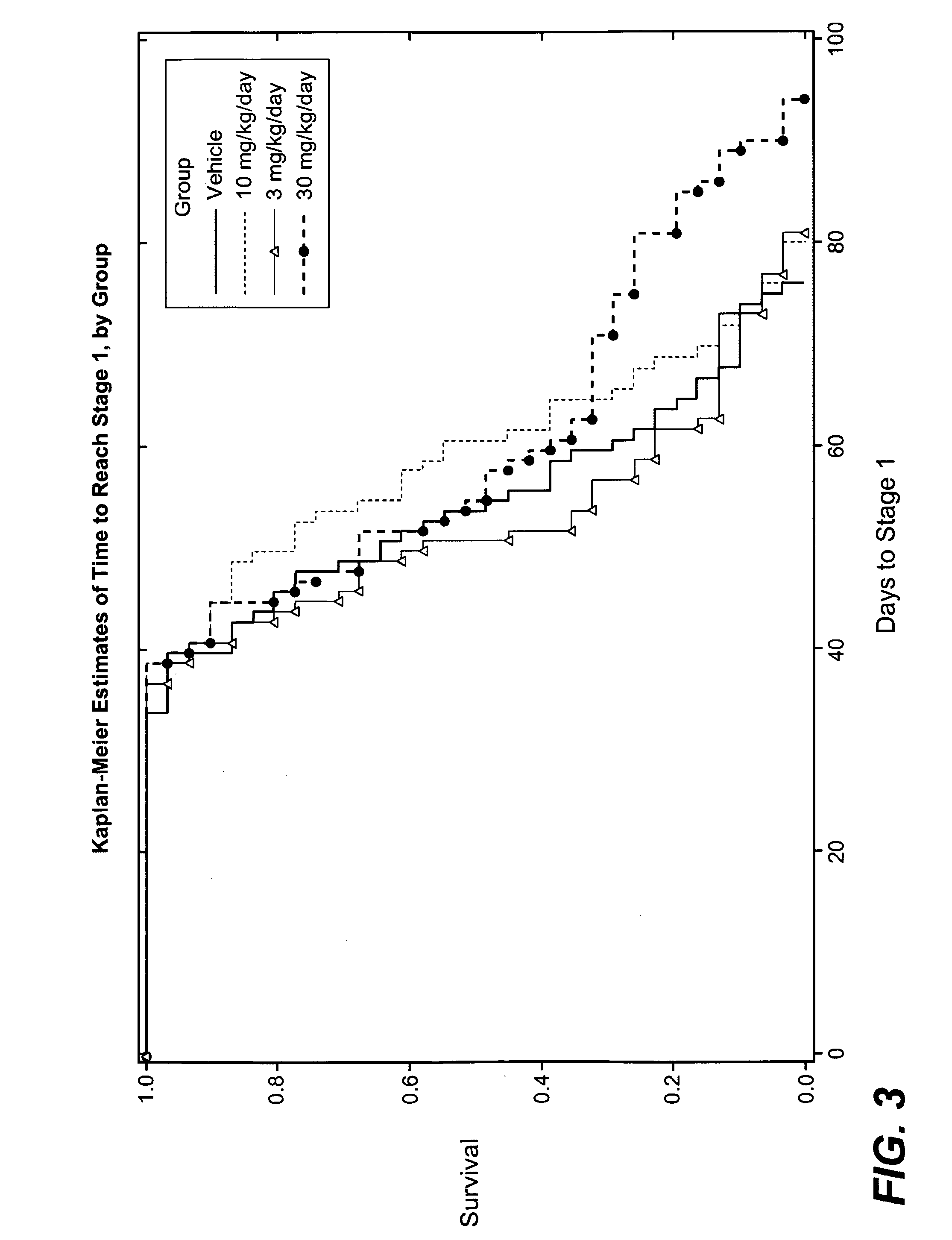
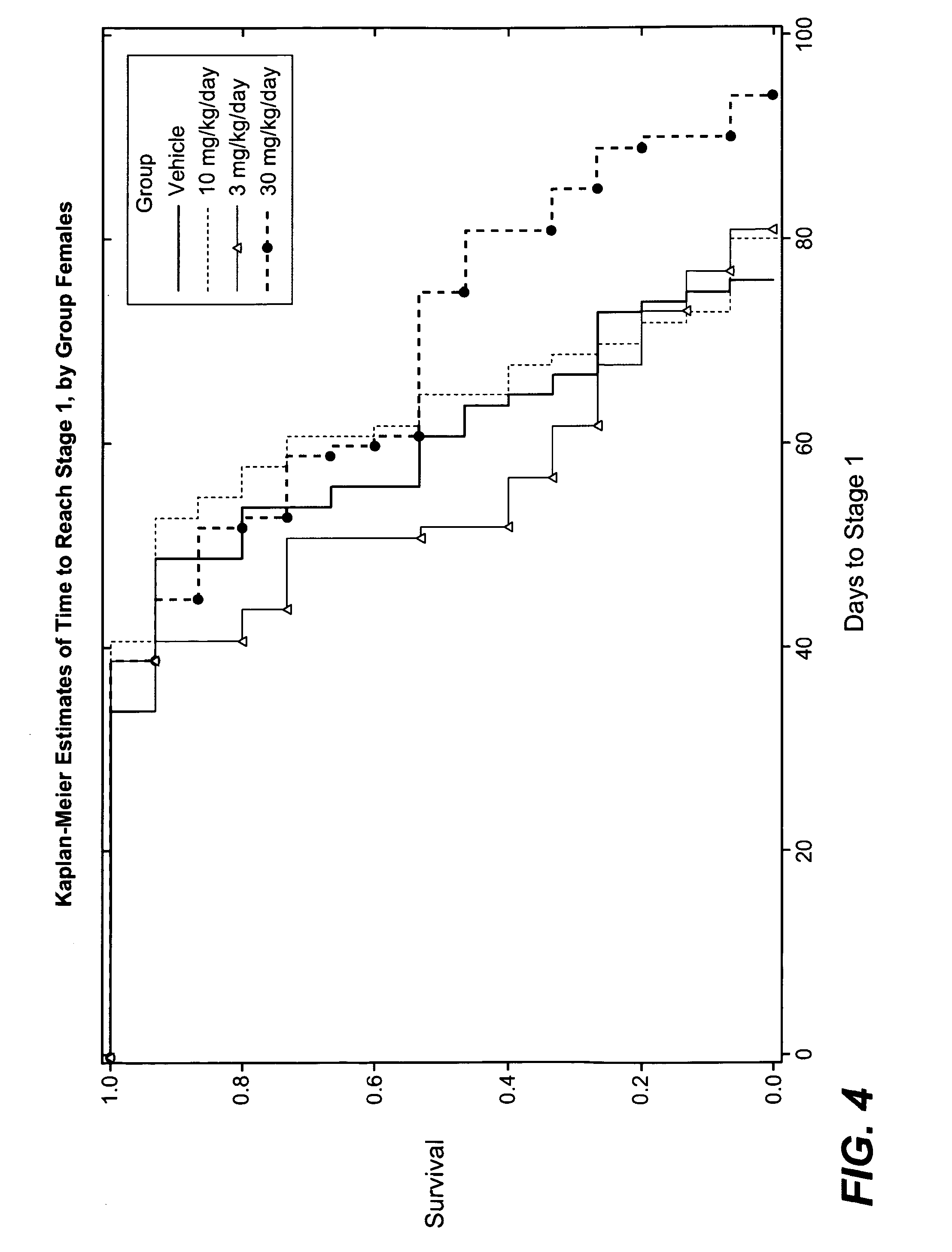
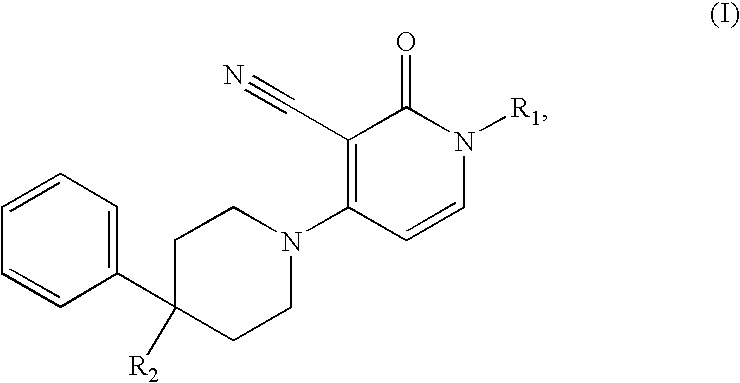
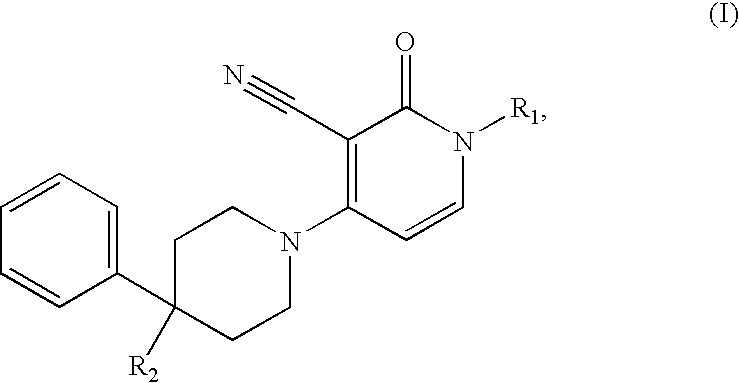
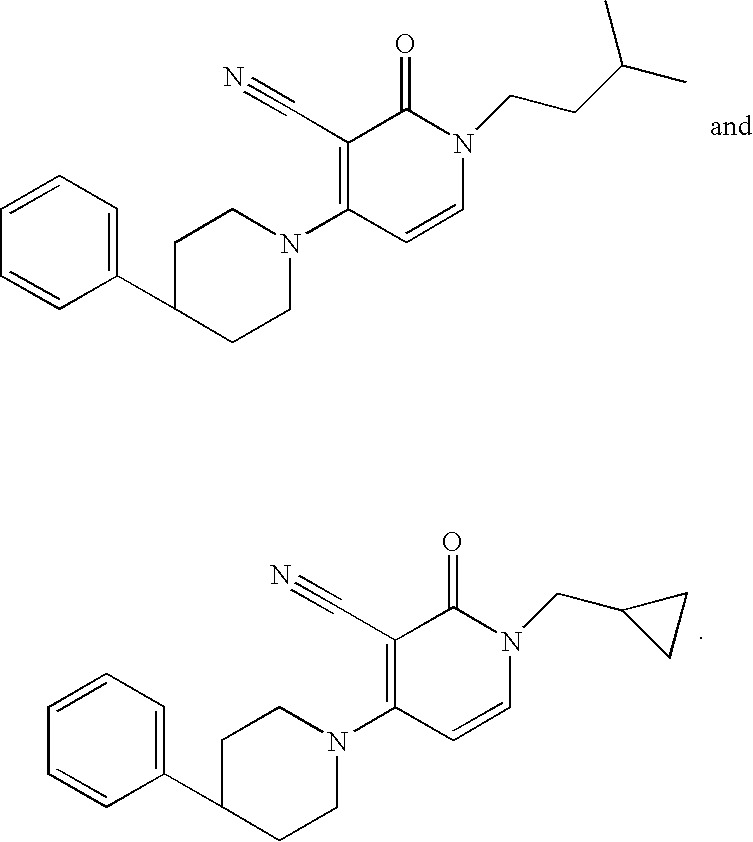
3-cyano-4-(4-phenyl-piperidin-1-yl)-pyridin-2-one derivatives
The present invention relates to novel compounds, in particular novel pyridinone derivatives according to Formula (I) including any stereochemically isomeric form thereof, or a pharmaceutically acceptable salt thereof or a solvate thereof, wherein all radicals are defined in the application and claims. The compounds according to the invention are positive allosteric modulators of metabotropic glutamate receptors subtype 2 (“mGluR2”) which are useful for the treatment or prevention of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which the mGluR2 subtype of metabotropic receptors is involved. In particular, such diseases are central nervous system disorders selected from the group of anxiety, schizophrenia, migraine, depression, and epilepsy. The invention is also directed to pharmaceutical compositions and processes to prepare such compounds and such compositions, as well as to the use of such compounds for the prevention and treatment of such diseases in which mGluR2 is involved.
Owner:ADDEX PHARM SA
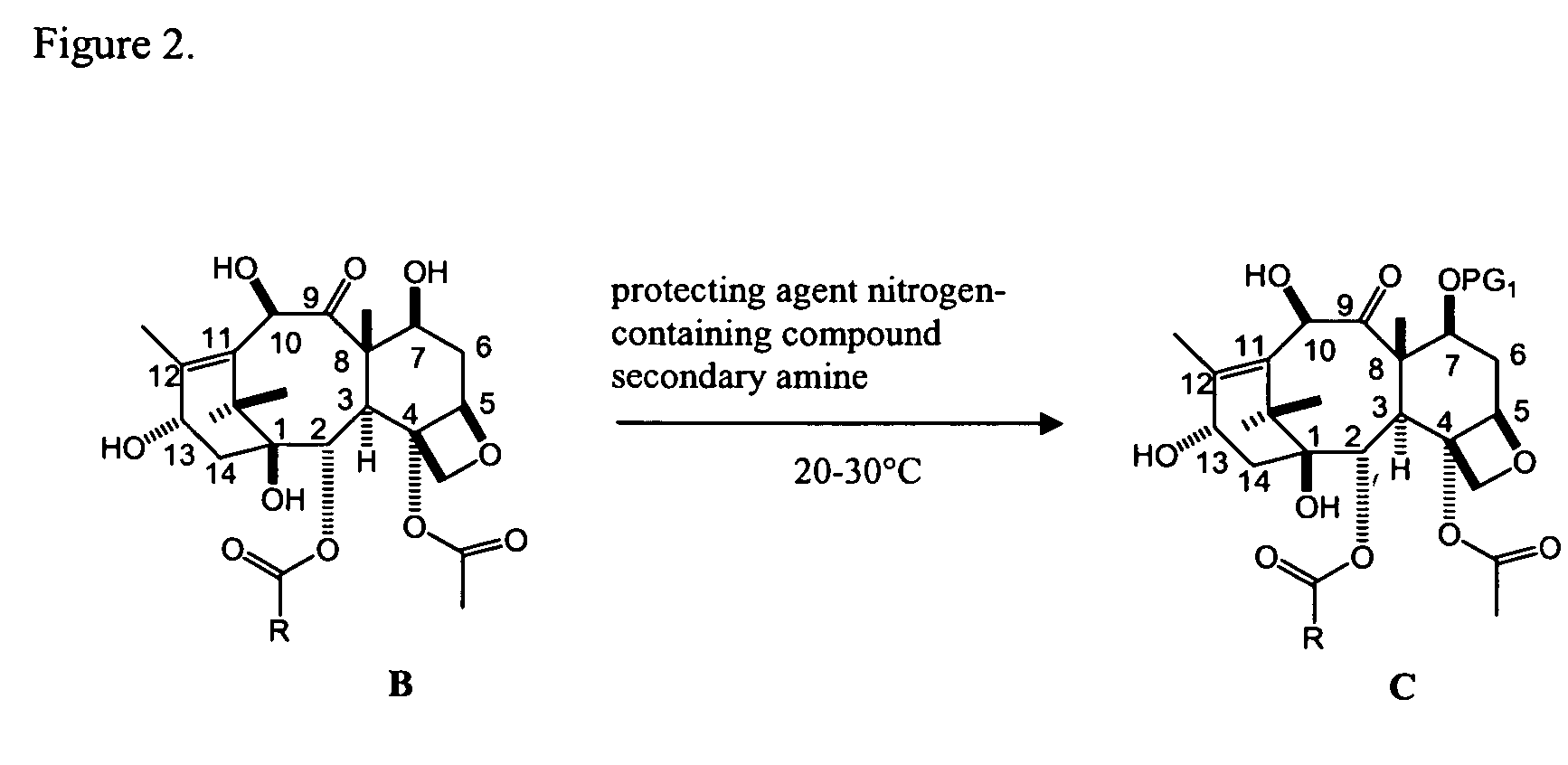
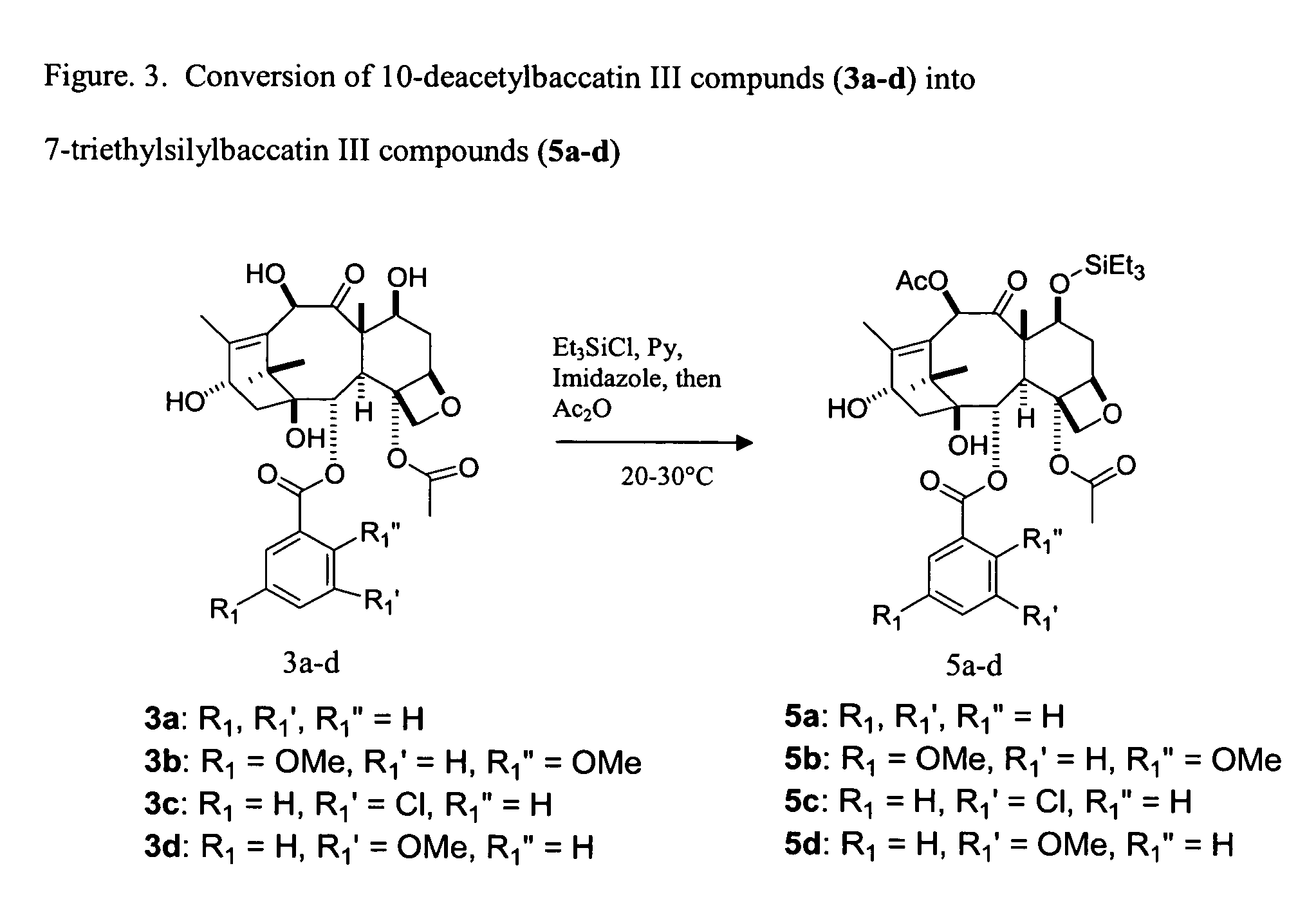
Facile method for synthesizing baccatin III compounds
A process for synthesizing a C-7 protected baccatin III compound represented by formula (A), which comprises reacting a 10-deacetylbaccatin III compound represented by formula (B) with a protecting agent and an acylating agent in the presence of a secondary amine and a nitrogen-containing compound. Also, a process for synthesizing a C-7 protected 10-deacetylbaccatin III compound represented by formula (C), which comprises reacting a 10-deacetylbaccatin III compound represented by formula (B) with a protecting agent in the presence of a secondary amine and a nitrogen-containing compound. In both processes the nitrogen-containing compound is selected from a nitrogen-containing heterocycle or a trialkylamine. When the nitrogen-containing heterocycle is selected, it may be an unsubstituted or a substituted pyridine or an unsubstituted or a substituted pyrazine. When a trialkylamine is selected, it may be, for example, triethylamine or diisopropylethylamine.wherein PG1 represents the organic residue of the protecting agent, PG2 represents the organic residue of the acylating agent, and R represents a simple or substituted aryl group or a heterocyclic group.
Owner:IMMUNOGEN INC
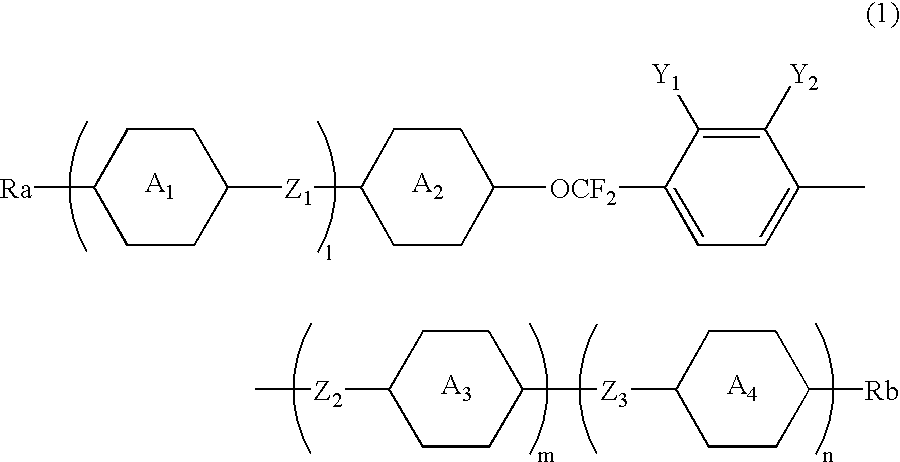
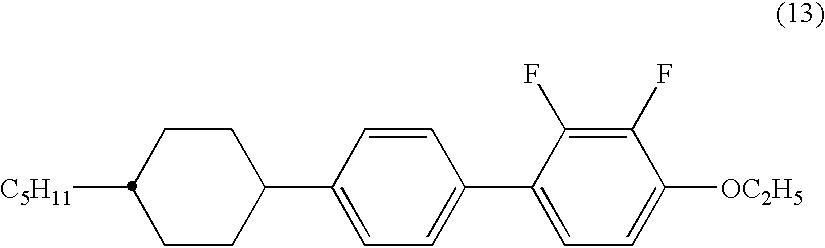
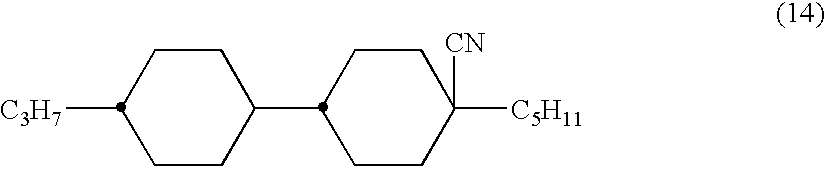
2,3-difluorophenyl derivative having negative dielectric anisotropy, liquid crystal composition, and liquid crystal display device
InactiveUS6548126B1Good compatibilityIncrease resistanceLiquid crystal compositionsOrganic chemistryLiquid crystallineCrystallography
The invention is to provide liquid crystalline compounds having a negative and extremely large dielectric anisotropy value and a small optical anisotropy value at the same time; liquid crystal compositions comprising the compound; and liquid crystal display devices fabricated by using the liquid crystal composition; the liquid crystalline compounds have 2,3-dihalogenophenylene moiety and are expressed by formula (1)wherein Ra and Rb each independently represents a linear or branched alkyl having 1 to 10 carbon, any methylene in the alkyl may be replaced by -O-, -S-, -CH=CH-, or -C=C-, but -O- is not successive, and any hydrogen in the alkyl may be replaced by halogen; rings A1 to A4 each independently represents trans-1,4-cyclohexylene, cyclohexene-1,4-diyl, pyridine-1,4-diyl, pyrimidine-2,5-diyl, or 1,4-phenylene, wherein at least one hydrogen in these rings may be replaced by halogen, and any nonadjacent methylene in cyclohexane ring may be replaced by -O-; Y1 and Y2 each independently represents F or Cl; Z1, Z2 and Z3 each independently represents a single bond, -CH2CH2-, -(CH2)4-, -COO-, -OCO-, -CH2O-, -OCH2-, -CF2O-, or -OCF2-; and l, m and n each independently is 0, 1 or 2, and the sum of l+m+n is 3 and less.
Owner:CHISSO CORP
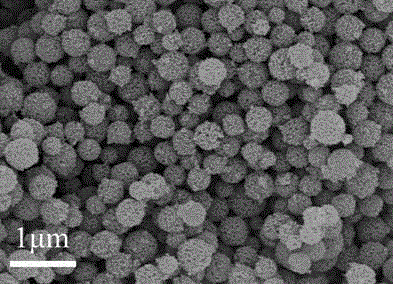
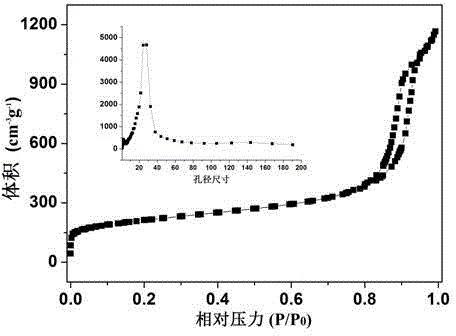
Preparation method and application of iron-nitrogen co-doped porous carbon sphere material
InactiveCN104624154ASimple processSave raw materialsPhysical/chemical process catalystsOther chemical processesPtru catalystPorous carbon
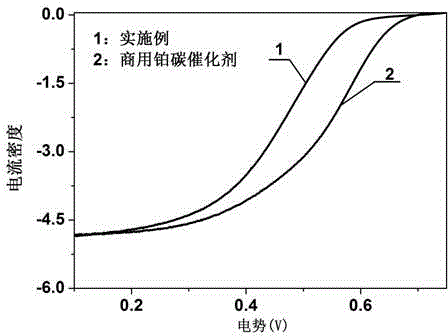
The invention discloses a preparation method for an iron-nitrogen co-doped porous carbon sphere material. The preparation method comprises the following steps: by taking 2-aminopyridine as a monomer and taking ammonium persulfate and ferric chloride as oxidants, performing in-situ polymerization reaction in a duct of a porous silicon dioxide template to obtain a precursor; performing high-temperature carbonization treatment on the precursor in a tubular furnace and an inert gas nitrogen-gas environment; and removing the silicon dioxide template by hydrofluoric acid to obtain the iron-nitrogen co-doped porous carbon sphere material which is taken as an electric catalyst to achieve good catalytic effect in oxygen gas reduction reaction. The preparation method has the advantages that the process is simple and easy to perform and the raw materials are cheap. The prepared carbon material contains a three-dimensional communicated pore structure, has a high specific surface area and a large pore volume, can effectively improve the electric catalytic activity through the heteroatom nitrogen-iron doping, has relatively high electric catalytic efficiency while being applied as a low-price electric catalyst, and has an important value and significance in the fields of doped type porous carbon material preparation and proton membrane fuel battery electric catalysis.
Owner:NANKAI UNIV
Drug demonstrating anxiolytic effect based on hydrogenated pyrido (4,3-b) indoles, its pharmacological compound and application method
Owner:MEDIVATION TECH INC
Pyralopyridines, process for their preparation and use as therapeutic compounds
Owner:SMITHKLINE BECKMAN CORP
2-aminopyrimidin-4-one and 2-aminopyridine derivatives both having bace1-inhibiting activity
InactiveUS20110237576A1Inhibit productionUseful in therapyBiocideNervous disorderDiseaseChemical compound
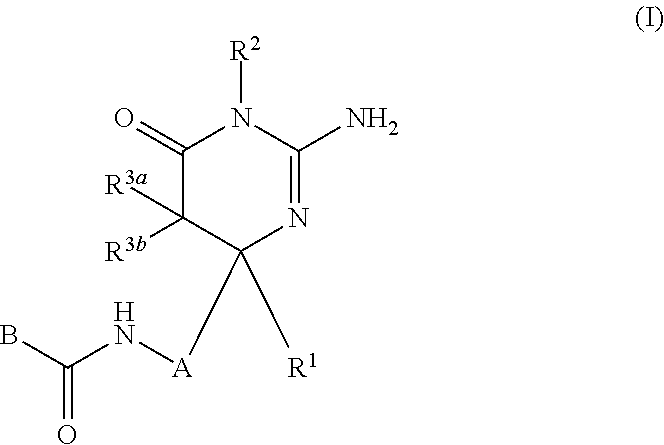
The present invention provides a compound which has an effect of inhibiting amyloid-β production and is useful as a therapeutic agent for diseases induced by production, secretion and / or deposition of amyloid-β proteins. The present invention relates to a compound represented by formula (I) or a pharmaceutically acceptable salt or solvate thereof:wherein A is optionally substituted carbocyclic diyl or optionally substituted heterocyclic diyl; B is an optionally substituted carbocyclic group or an optionally substituted heterocyclic group; R1 is a group such as optionally substituted lower alkyl; R2 is a group such as hydrogen; and R3a and R3b are each independently a group such as hydrogen, provided that the following compound is excluded.
Owner:SHIONOGI & CO LTD
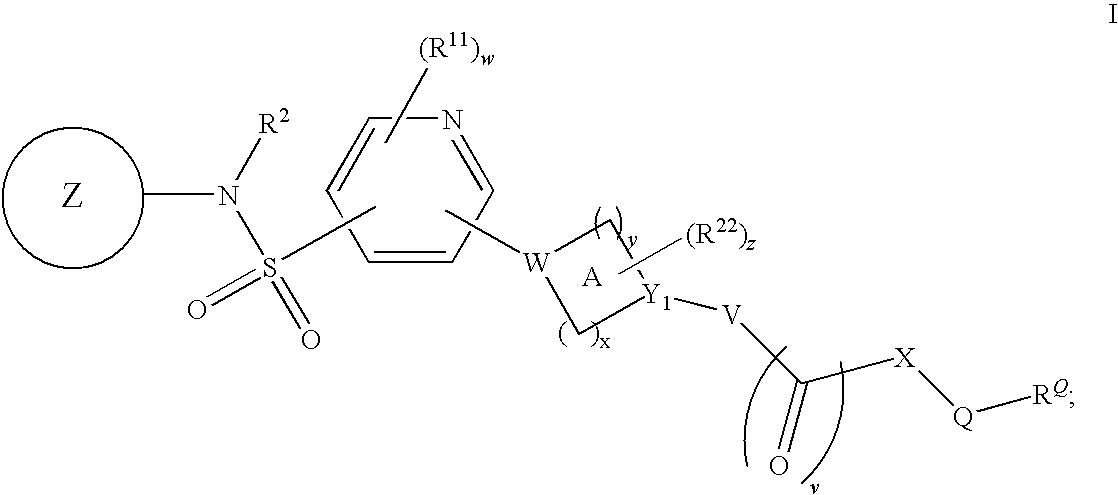
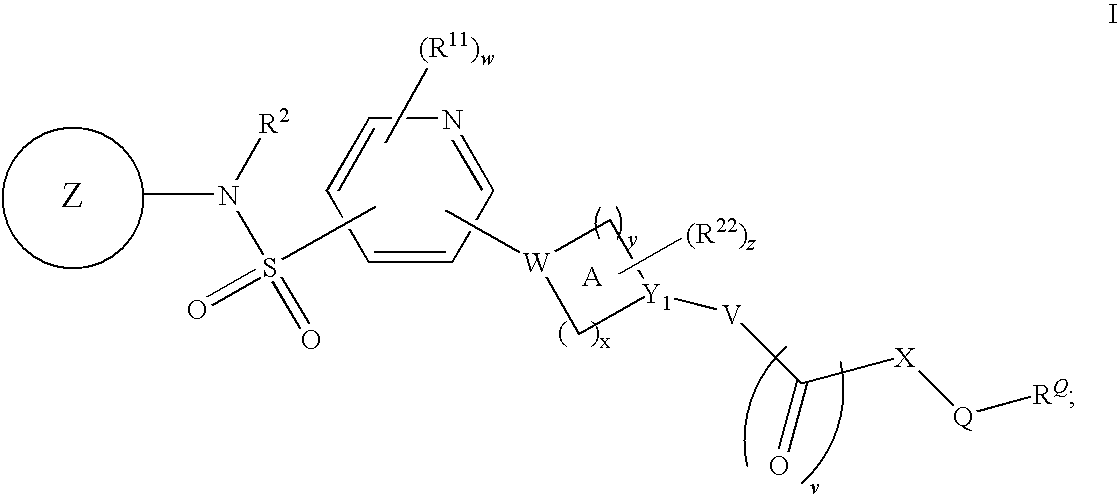
Pyridyl sulfonamides as modulators of ion channels
The present invention relates to pyridyl sulfonamide derivatives useful as inhibitors of ion channels. The invention also provides pharmaceutically acceptable compositions comprising the compounds of the invention and methods of using the compositions in the treatment of various disorders.
Owner:VERTEX PHARMA INC
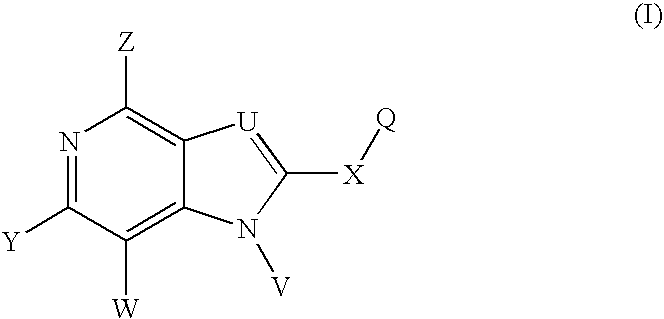
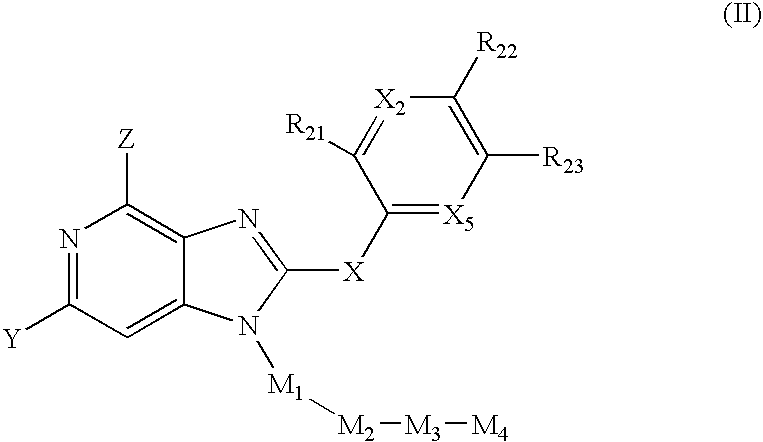
Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds
InactiveUS20090325945A1Promote apoptosisInhibiting cell cycle progressionBiocideOrganic chemistryDiseaseMelanoma
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5 b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formulae: wherein: J is independently —O— or —NRN1−; RN1, if present, is independently —H or a substituent; RN2 is independently —H or a substituent; Y is independently —CH═ or —N═; Q is independently —(CH2)j-M-(CH2)k— wherein: j is independently 0, 1 or 2; k is independently 0, 1, or 2; j+k is 0, 1, or 2; M is independently O—, —S—, —NH—, —NMe-, or —CH2—; each of RP1, RP2, RP5, and RP4 is independently —H or a substituent; and additionally RP1 and RP2 taken together may be CH═CH—CH═CH—; and additionally RP1 and RP5 taken together may be CH═CH—CH═CH—; L is independently: a linker group formed by a chain of 2, 3, or 4 linker moieties; each linker moiety is independently CH2—, —NRN—, —C(═X)—, or —S(═O)2—; either: exactly one linker moiety is —NRN—, or: exactly two linker moieties are —NRN—; either: exactly one linker moiety is —C(═X)—, and no linker moiety is —S(═O)2—, or: exactly one linker moiety is —S(═O)2—, and no linker moiety is —C(═X)—; no two adjacent linker moieties are —NRN—; X is independently ═O or ═S; each RN is independently —H or a substituent; A is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:CANCER RES TECH LTD +1
Substituted 1H-pyrrolo[3,2-b, 3,2-c, and 2,3-c]pyridine-2-carboxamides and related analogs as inhibitors of casein kinase lepsilon
The present invention discloses and claims compounds of formula (I) as inhibitors of human casein kinase IF, and methods of using said compounds of formula (I) for treating central nervous system diseases and disorders including mood disorders and sleep disorders. Pharmaceutical compositions comprising compounds of formula (I) and methods for the preparation of compounds of formula (I) are also disclosed and claimed.
Owner:AVENTISUB LLC
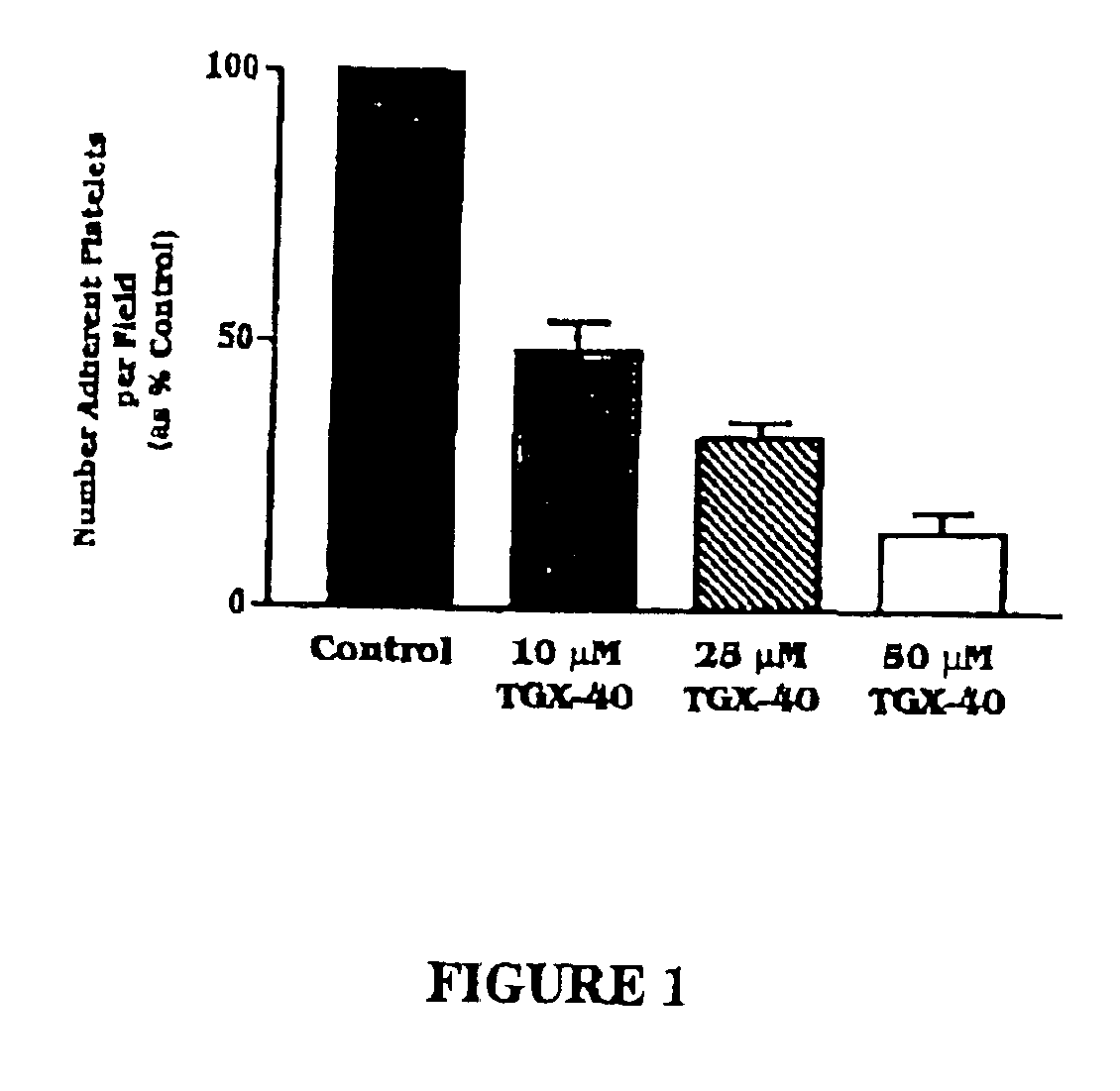
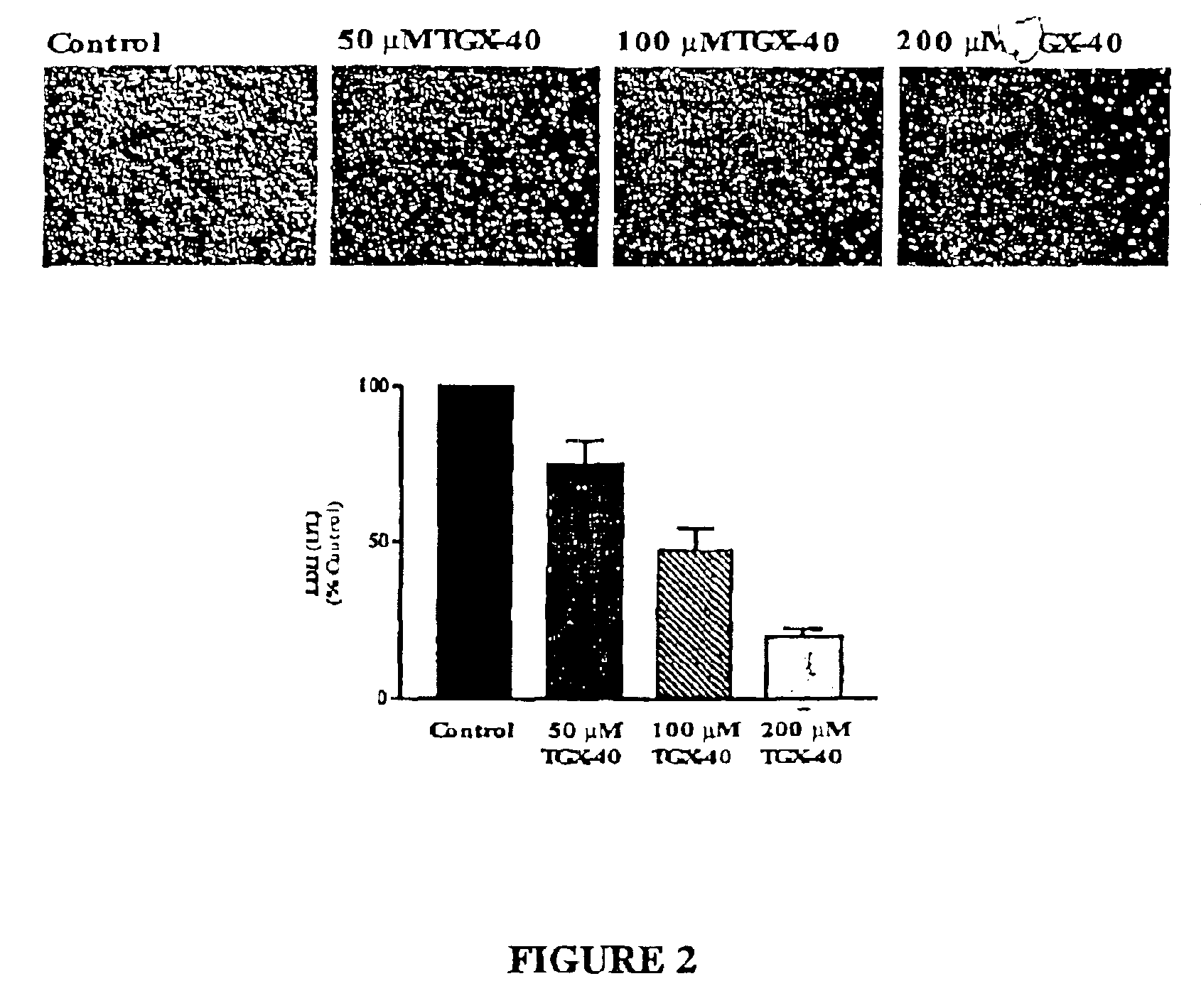
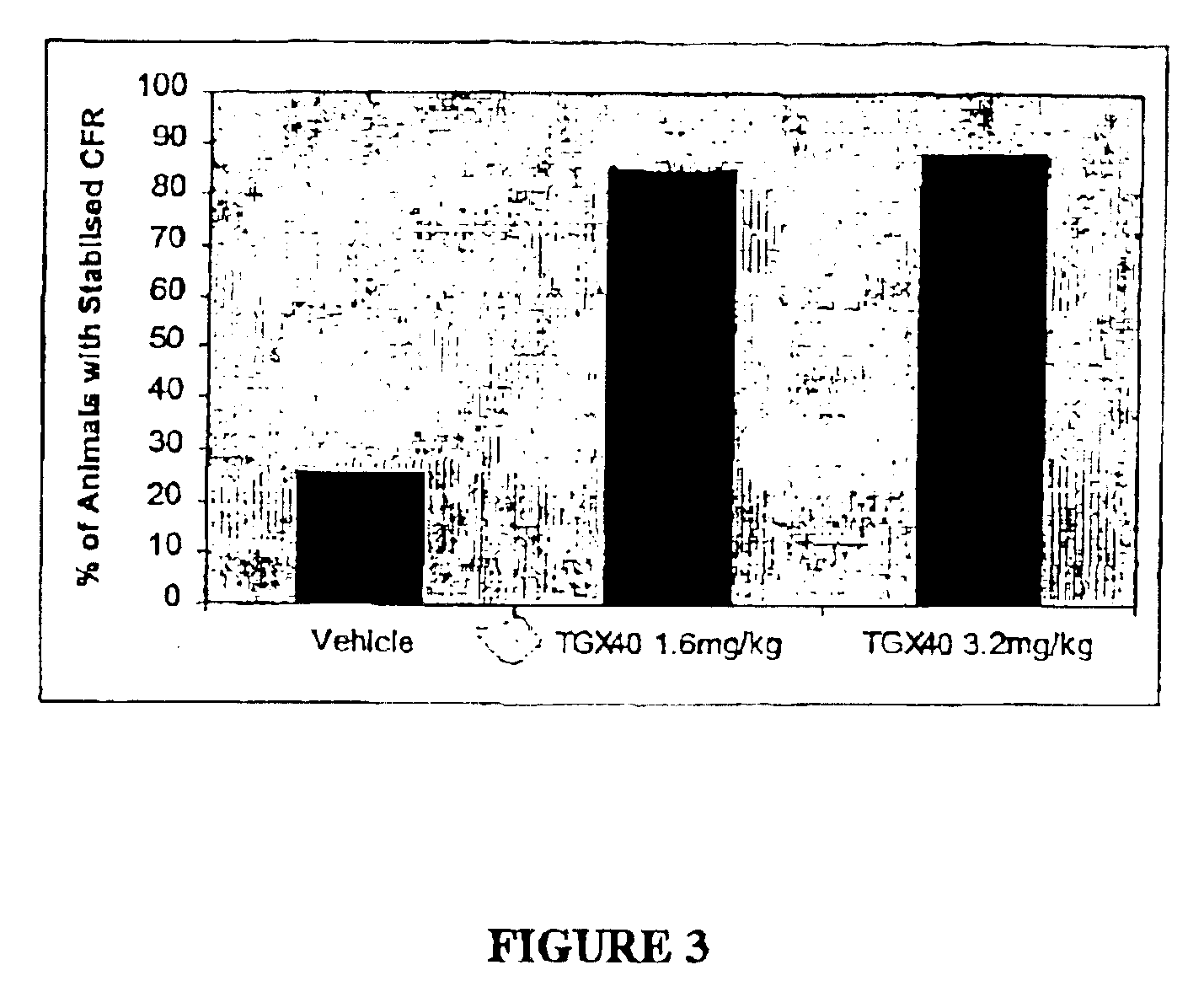
Therapeutic morpholino-substituted compounds
Morpholino-substituted pyridopyrimidine, quinolone, and benzopyranone derivatives inhibit phosphoinositide (PI) 3-kinase, an enzyme that regulates platelet-adhesion processes. As a consequence, the compounds in question have anti-thrombotic activity, as well as other pharmaceutical properties. The compounds claimed are represented by formula (I), (II) and (III). PI 3-kinase generates 3-phosphorylated PI second messengers which stimulate platelet adhesion under blood-flow conditions. Because platelet adhesion is a necessary step in the formation of a thrombus, inhibition by these compounds of PI 3-kinase under such conditions inhibits or prevents thrombus formation. The compounds are useful in treating PI 3-kinase-dependent conditions including cardiovascular diseases such as coronary artery occlusion, stroke, acute coronary syndrome, acute myocardial infarction, vascular restenosis, atherosclerosis, and unstable angina; respiratory diseases such as asthma, chronic obstructive pulmonary diseases (COPD), and bronchitis; inflammatory disorders; neoplasms including cancers such as glioma, prostate cancer, small cell lung cancer, and breast cancer, and diseases linked to disordered white blood cell function, such as autoimmune and inflammatory diseases.
Owner:ASTRAZENECA AB
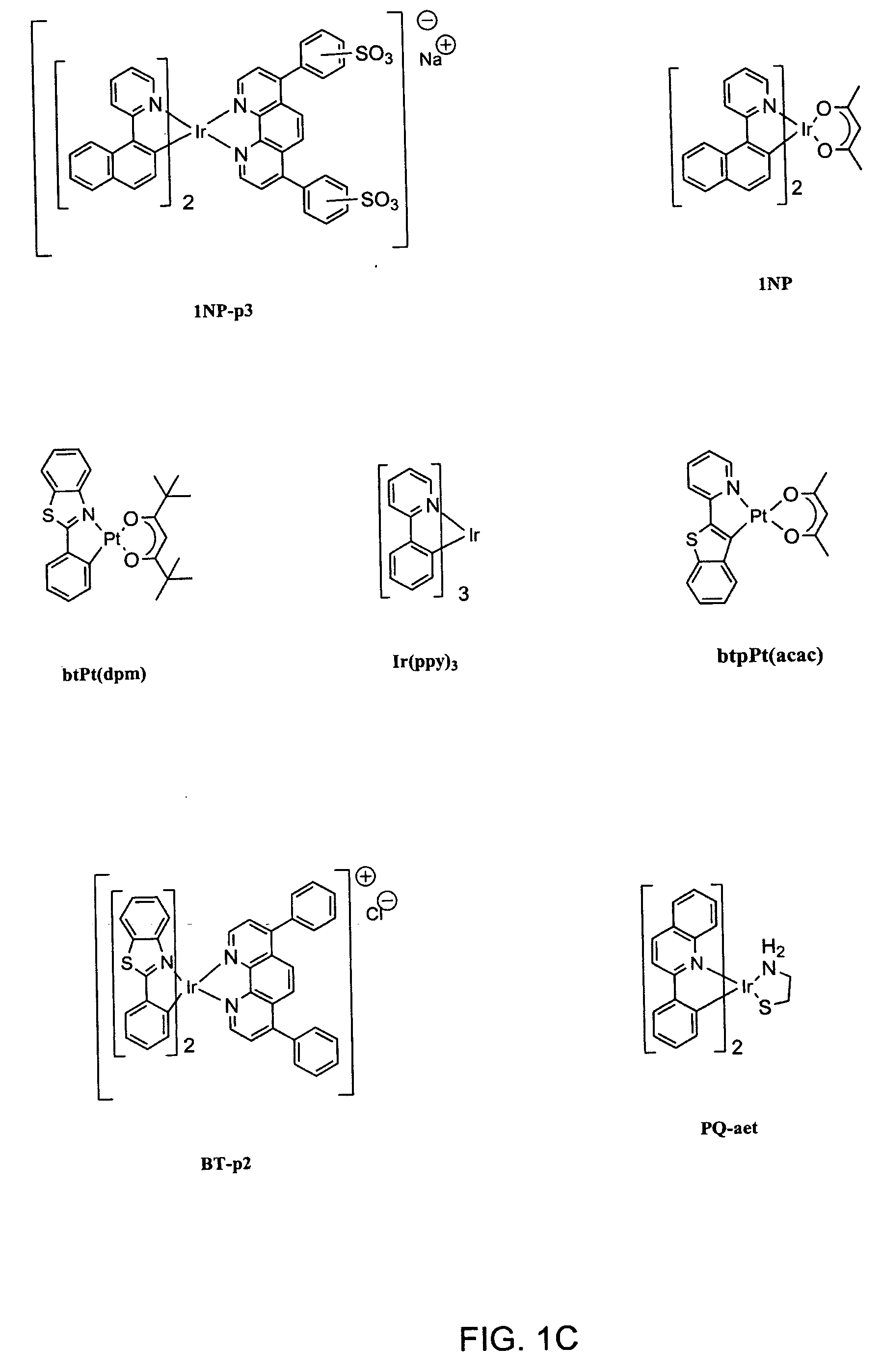
Organometallic complexes as singlet oxygen sensitizers
A series of organometallic complexes and the singlet oxygen sensitization properties of these complexes are provided. Complexes with acetylacetonate ligands give singlet oxygen quantum yields near unity, whether exciting the ligand-based state or the lowest energy excited state (MLCT+3LC). The singlet oxygen quenching rates for these β-diketonate complexes are small, roughly three orders of magnitude slower than the corresponding phosphorescence quenching rate. Similar complexes were prepared with glycine or pyridine tethered to the Ir(III) center (i.e. (bsn)2Ir(gly) and (bt)2Ir(py)Cl, where gly=glycine, and py=pyridine). The glycine and pyridine derivatives give high singlet oxygen yields.
Owner:UNIV OF SOUTHERN CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
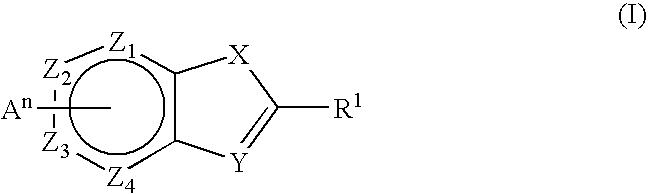
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00001.png)
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00002.png)
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00003.png)

![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a208a788-d164-4d46-bdfe-b503be2f9d03/US08450340-20130528-C00001.png)
![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a208a788-d164-4d46-bdfe-b503be2f9d03/US08450340-20130528-C00002.png)
![1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof 1H-pyrazolo[3,4-<i>b</i>]pyridines and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a208a788-d164-4d46-bdfe-b503be2f9d03/US08450340-20130528-C00003.png)
![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-D00001.png)
![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-C00001.png)
![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-C00002.png)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00001.png)
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00002.png)
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00003.png)


![Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98a75e2d-75e1-48ce-aec0-985d9e07e9a0/US09708322-20170718-C00001.png)
![Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98a75e2d-75e1-48ce-aec0-985d9e07e9a0/US09708322-20170718-C00002.png)
![Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity Substituted pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalines for inhibiting serotonin reuptake transporter activity](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98a75e2d-75e1-48ce-aec0-985d9e07e9a0/US09708322-20170718-C00003.png)
![Substituted pyrrolo[2.3-B]pyridines Substituted pyrrolo[2.3-B]pyridines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/731b3571-2c88-4f7a-b2a2-7e61bc0eb242/US07465726-20081216-C00001.png)
![Substituted pyrrolo[2.3-B]pyridines Substituted pyrrolo[2.3-B]pyridines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/731b3571-2c88-4f7a-b2a2-7e61bc0eb242/US07465726-20081216-C00002.png)
![Substituted pyrrolo[2.3-B]pyridines Substituted pyrrolo[2.3-B]pyridines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/731b3571-2c88-4f7a-b2a2-7e61bc0eb242/US07465726-20081216-C00003.png)







![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00001.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00002.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00003.png)
![Substituted 1H-pyrrolo[3,2-b, 3,2-c, and 2,3-c]pyridine-2-carboxamides and related analogs as inhibitors of casein kinase lepsilon Substituted 1H-pyrrolo[3,2-b, 3,2-c, and 2,3-c]pyridine-2-carboxamides and related analogs as inhibitors of casein kinase lepsilon](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af157274-eeae-48f2-87f4-f38e08b2afcc/US20050131012A1-20050616-C00001.png)
![Substituted 1H-pyrrolo[3,2-b, 3,2-c, and 2,3-c]pyridine-2-carboxamides and related analogs as inhibitors of casein kinase lepsilon Substituted 1H-pyrrolo[3,2-b, 3,2-c, and 2,3-c]pyridine-2-carboxamides and related analogs as inhibitors of casein kinase lepsilon](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af157274-eeae-48f2-87f4-f38e08b2afcc/US20050131012A1-20050616-C00002.png)
![Substituted 1H-pyrrolo[3,2-b, 3,2-c, and 2,3-c]pyridine-2-carboxamides and related analogs as inhibitors of casein kinase lepsilon Substituted 1H-pyrrolo[3,2-b, 3,2-c, and 2,3-c]pyridine-2-carboxamides and related analogs as inhibitors of casein kinase lepsilon](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/af157274-eeae-48f2-87f4-f38e08b2afcc/US20050131012A1-20050616-C00003.png)