Synthesis method of drug Tafamidis
A synthesis method and drug technology, applied in the field of synthesis of the drug Tafamidis, can solve the problems of only 43%, potential safety hazards, and high price of diaryliodonium compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the compound shown in preparation formula Ia structure
[0048] The reaction formula is as follows:
[0049]
[0050] Add magneton, compound 1 (1.531 g, 10 mmol) and methanol (31 mL) into a clean reaction flask in sequence, then slowly add concentrated sulfuric acid (3.1 mL) dropwise under stirring at room temperature, and then heat up to reflux for reaction . TLC shows that after the reaction of raw materials is completed (about 4 hours), let it cool down to room temperature, add saturated sodium bicarbonate solution to it until the pH reaches alkaline, filter with diatomaceous earth, concentrate the filtrate and transfer it to a separatory funnel with ethyl acetate , washed with water and saturated sodium chloride solution respectively, the organic phase was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated and vacuum-dried to obtain 1.572 g of a brown solid, with a yield of 94%. 1 H NMR (400 MHz, DMSO-d6) δ 9.44 (s...
Embodiment 2
[0051] Embodiment 2, the compound shown in preparation formula IIIa structure
[0052] The reaction formula is as follows:
[0053]
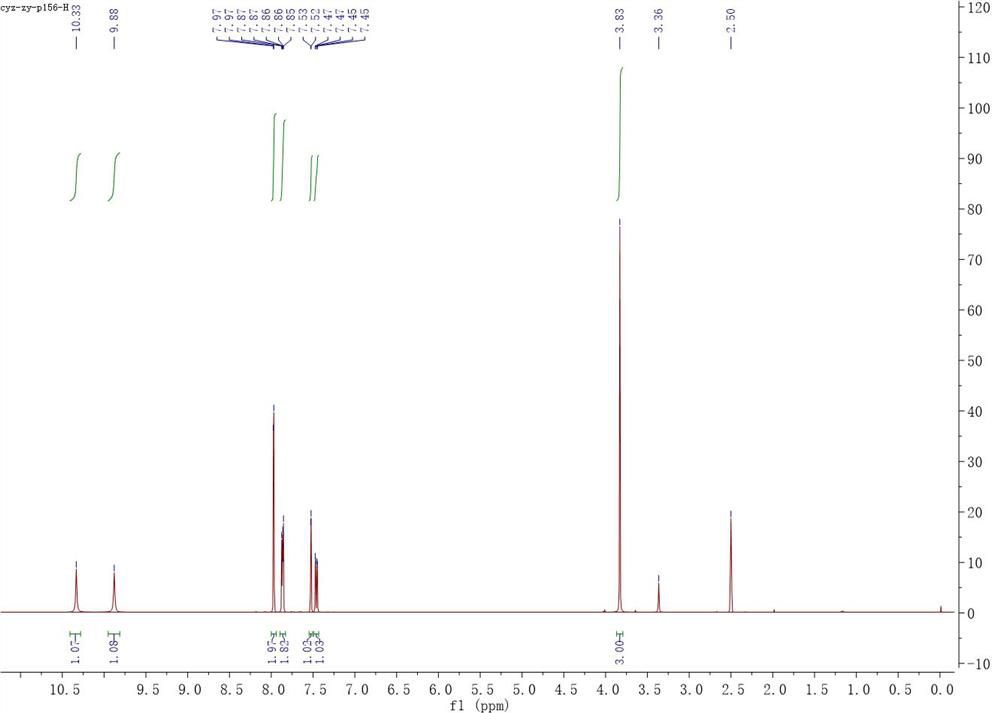
[0054] Into a clean reaction flask, add magnetons, compound of formula Ia (0.836 g, 5 mmol), pyridine (1.187 g, 15 mmol) and tetrahydrofuran (74 mL), and then add compound of formula IIa (1.047 g, 5 mmol), let it react at room temperature after the addition. TLC showed that after the completion of the reaction of the raw materials (about 6 hours), the reaction solution was directly concentrated and dissolved in ethyl acetate, then transferred to a separatory funnel, washed with saturated sodium bicarbonate, 10% hydrochloric acid and saturated sodium chloride, and the organic phase Anhydrous sodium sulfate was dried and filtered, and the filtrate was concentrated and vacuum-dried to obtain 1.680 g of a brown solid with a yield of 99%. 1 H NMR (400 MHz, DMSO-d6) δ 10.33 (brs, 1H), 9.88 (brs, 1H), 7.97 (d, J = 1.8Hz, 2H), 7.89 – 7.83 (m, 2H), ...
Embodiment 3
[0055] Embodiment 3, the compound shown in preparation formula IVa structure
[0056] The reaction formula is as follows:
[0057]
[0058] Into a clean reaction bottle, add magneton, compound of formula IIIa (0.340 g, 1 mmol), p-toluenesulfonic acid monohydrate (0.038 g, 0.2 mmol) and toluene (8 mL), after the addition, heat up to reflux reaction. TLC shows that after the raw material has reacted (about 12 hours), let it cool to room temperature and add 1 mol / L sodium hydroxide solution thereinto until the pH reaches alkalinity, then add an appropriate amount of water and ethyl acetate to the above solution, Then it was transferred to a separatory funnel, the organic phase was separated and then washed with saturated sodium chloride, the organic phase was dried over anhydrous sodium sulfate and filtered, the filtrate was concentrated and vacuum-dried to obtain 0.320 g of light pink solid, collected 99% rate, 1 H NMR (400 MHz, CDCl 3 ) δ 8.27 (s, 1H), 8.14 (s,2H), 8.11 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com