Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
838 results about "Energetic material" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
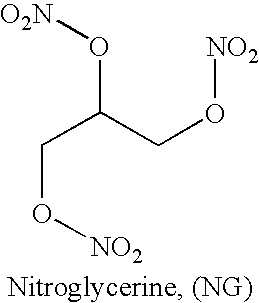
Energetic materials are a class of material with high amount of stored chemical energy that can be released. Typical classes of energetic materials are e.g. explosives, pyrotechnic compositions, propellants (e.g. smokeless gunpowders and rocket fuels), and fuels (e.g. diesel fuel and gasoline).
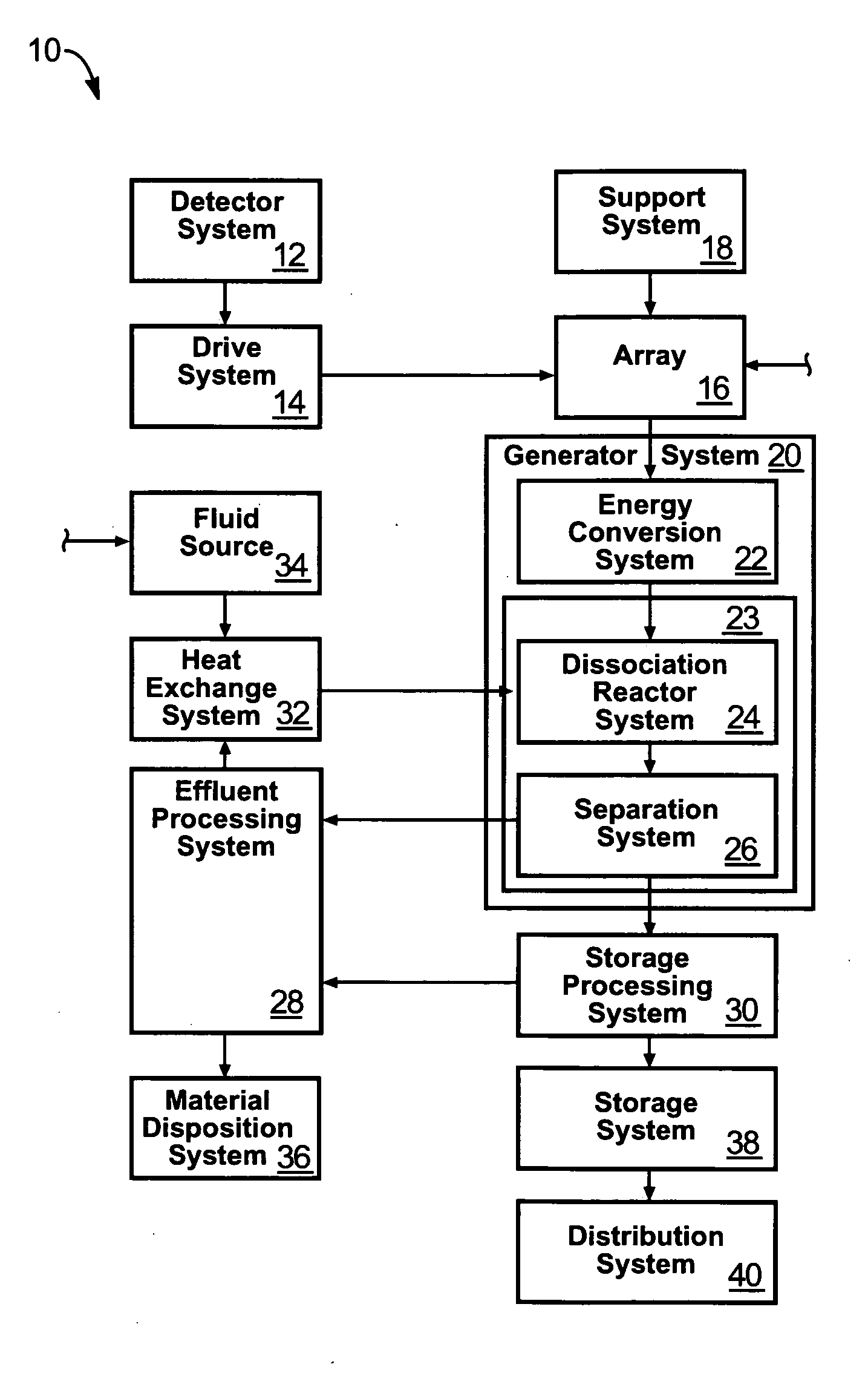
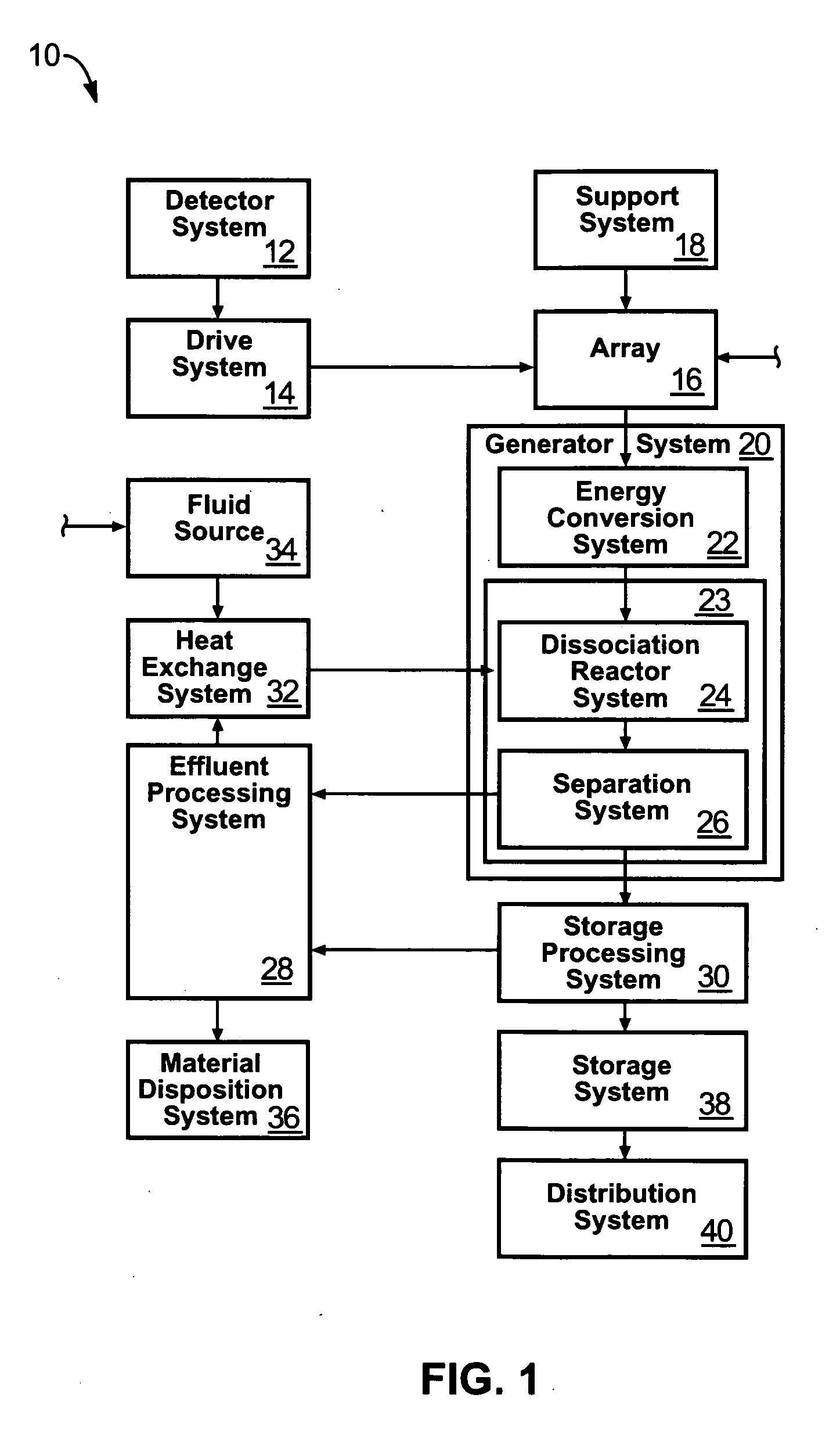
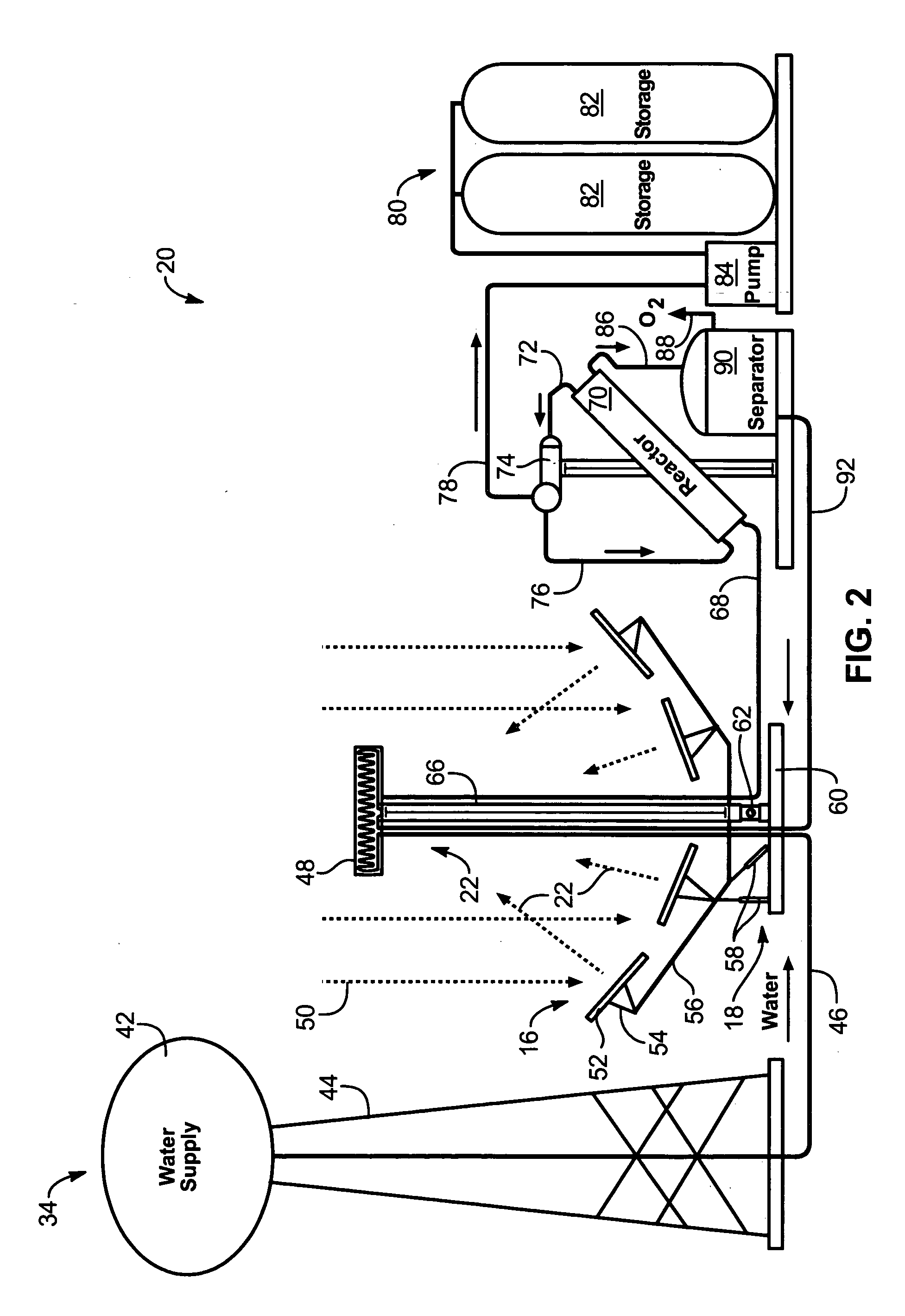
Solar, catalytic, hydrogen generation apparatus and method
InactiveUS20060048808A1High thermodynamic availabilityLoss in efficiencySolar heating energySolar heat collector controllersThermal energyPorosity
An apparatus for producing hydrogen may include a collector of radiation to concentrate solar radiation on a converter having an absorptivity to convert the solar radiation to thermal energy to drive a chemical process using a feedstock to dissociate into an output chemical and a byproduct. A separator separates the output and byproduct, after which a reactor reacts the output to form a storage chemical, reactive to produce energy but sufficiently stable for safe handling outside designation as an energetic material. The separator may have a porosity to substantially pass hydrogen and block oxygen and water. A sweep gas may sweep hydrogen away from the separation barrier to change equilibrium. Catalysts may reduce temperature of dissociation and a subsequent reaction to combine it in a more stable, storable form.
Owner:PURESCI
Convertible downhole devices
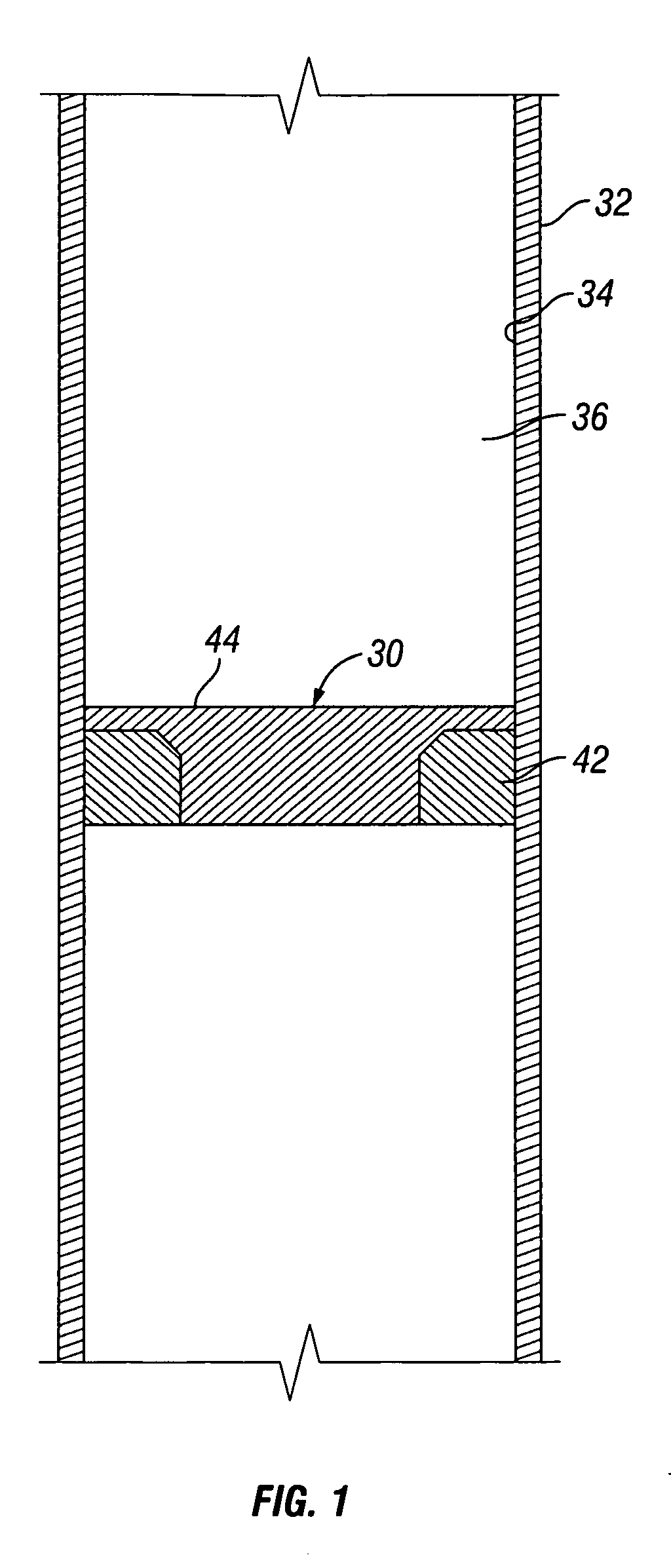
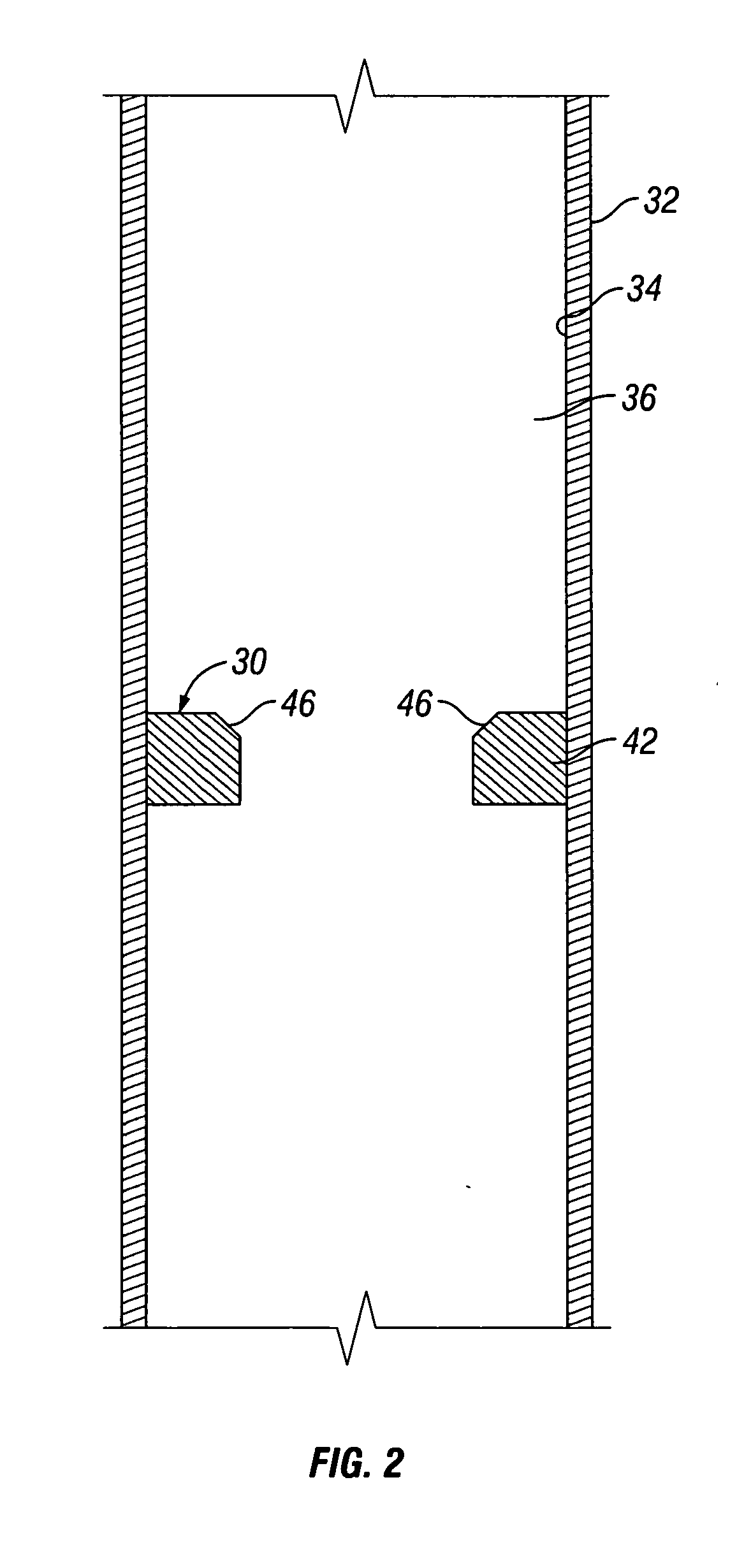
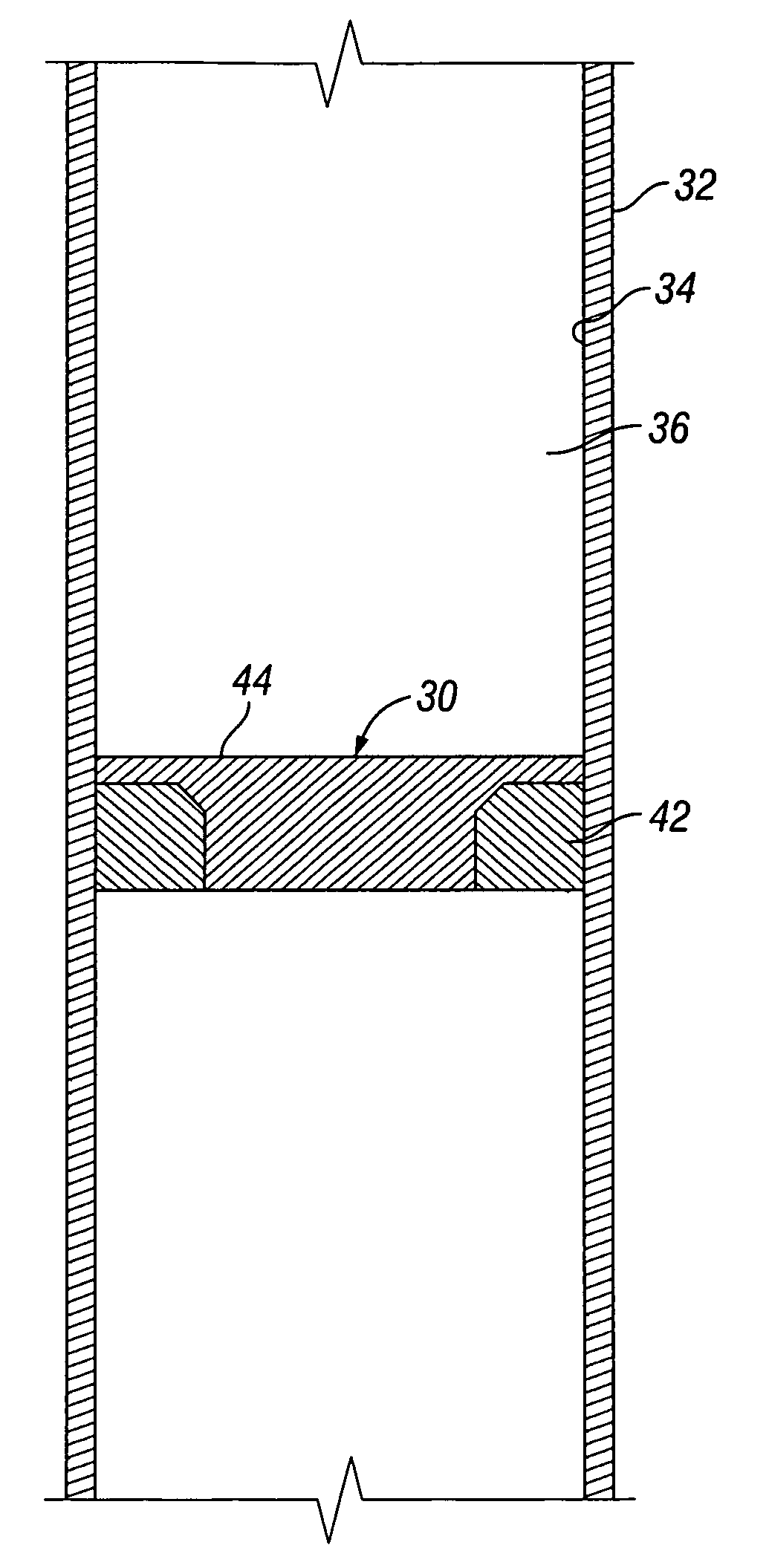
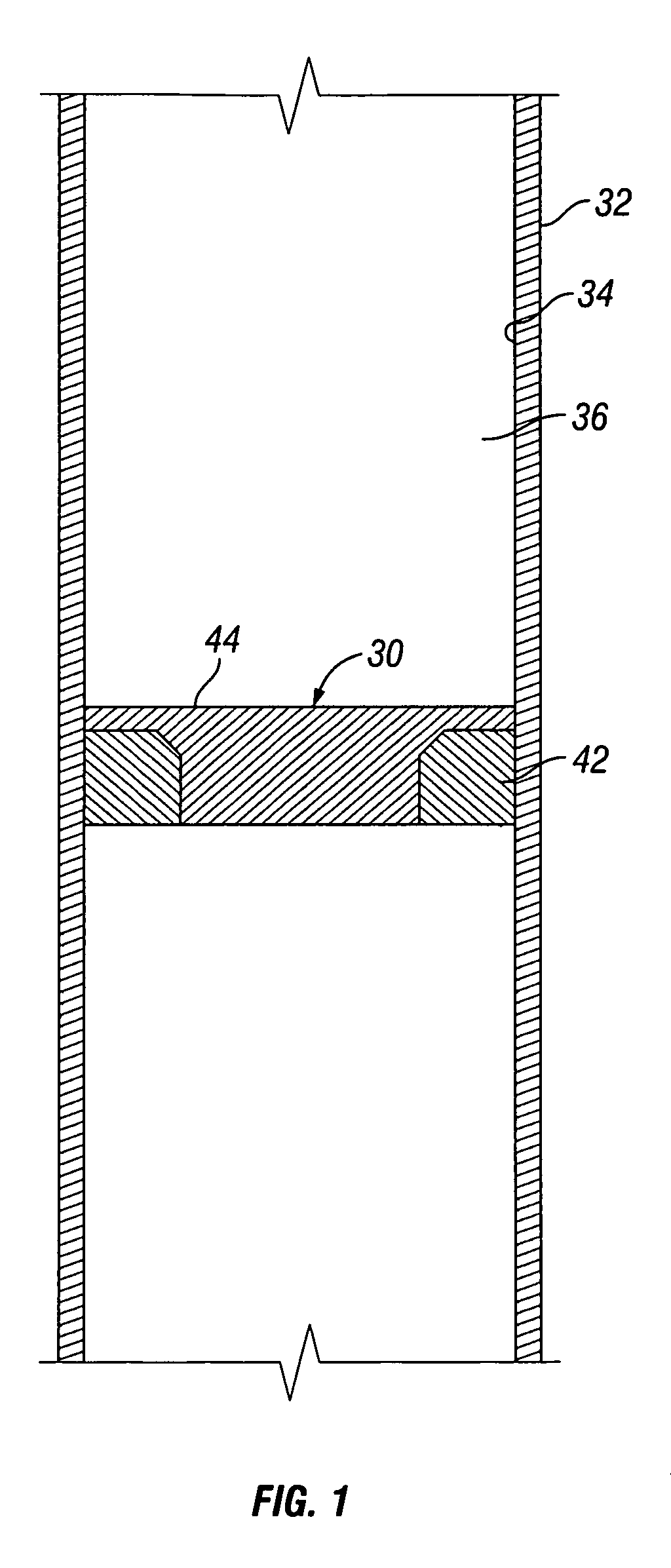
A convertible downhole device comprises at least one sacrificial material to provide two or more configurations so that two or more different operations or functions are performable by the downhole device, one in which the sacrificial material is fully intact and another in which the sacrificial material is at least partially removed or disappeared. The sacrificial material may be removable through any suitable method or device, such as by contacting with a fluid, by temperature, by pressure, or by combustion, ignition, or activation of a fusible or energetic material, or crushing or breaking up of a frangible material. Upon removal of the sacrificial material, the downhole device has at least one additional configuration so that at least a second operation can be performed by the downhole device.
Owner:BAKER HUGHES INC
Convertible downhole devices and method of performing downhole operations using convertible downhole devices
A convertible downhole device comprises at least one sacrificial material to provide two or more configurations so that two or more different operations or functions are performable by the downhole device, one in which the sacrificial material is fully intact and another in which the sacrificial material is at least partially removed or disappeared. The sacrificial material may be removable through any suitable method or device, such as by contacting with a fluid, by temperature, by pressure, or by combustion, ignition, or activation of a fusible or energetic material, or crushing or breaking up of a frangible material. Upon removal of the sacrificial material, the downhole device has at least one additional configuration so that at least a second operation can be performed by the downhole device.
Owner:BAKER HUGHES INC
Use of aluminum in perforating and stimulating a subterranean formation and other engineering applications
InactiveUS7393423B2More energy outputImprove mechanical propertiesExplosive chargesBlasting cartridgesMolten stateThermal energy
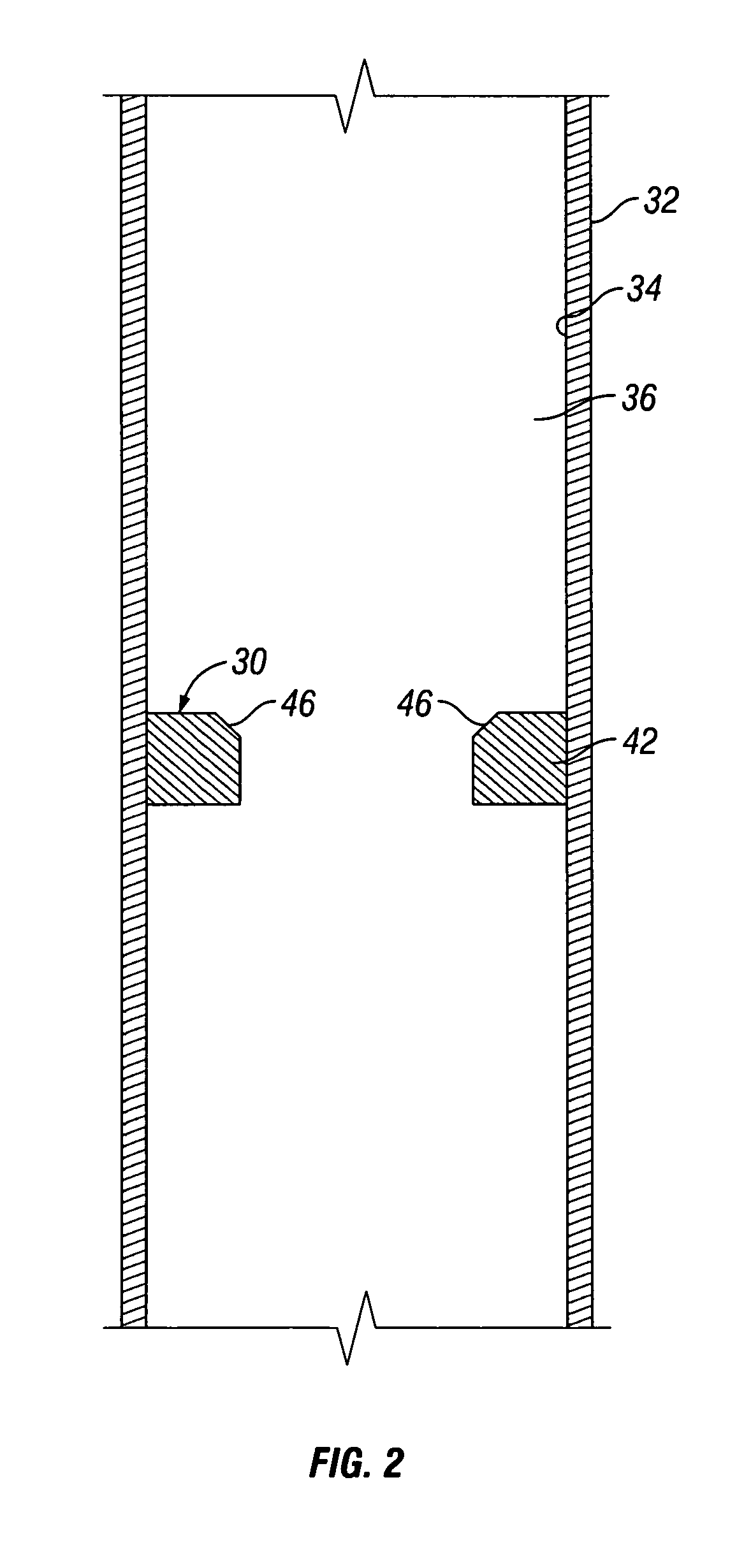
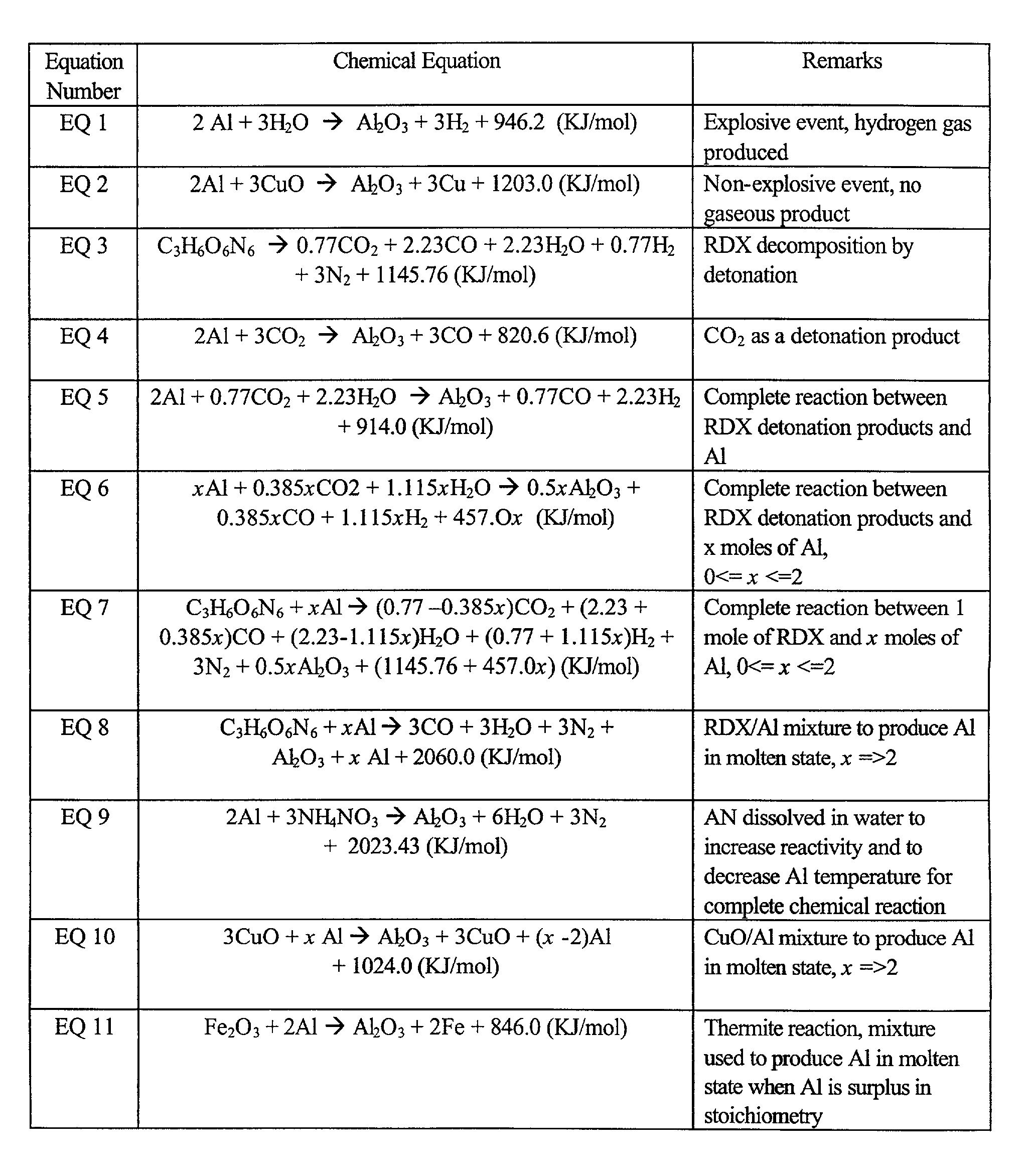
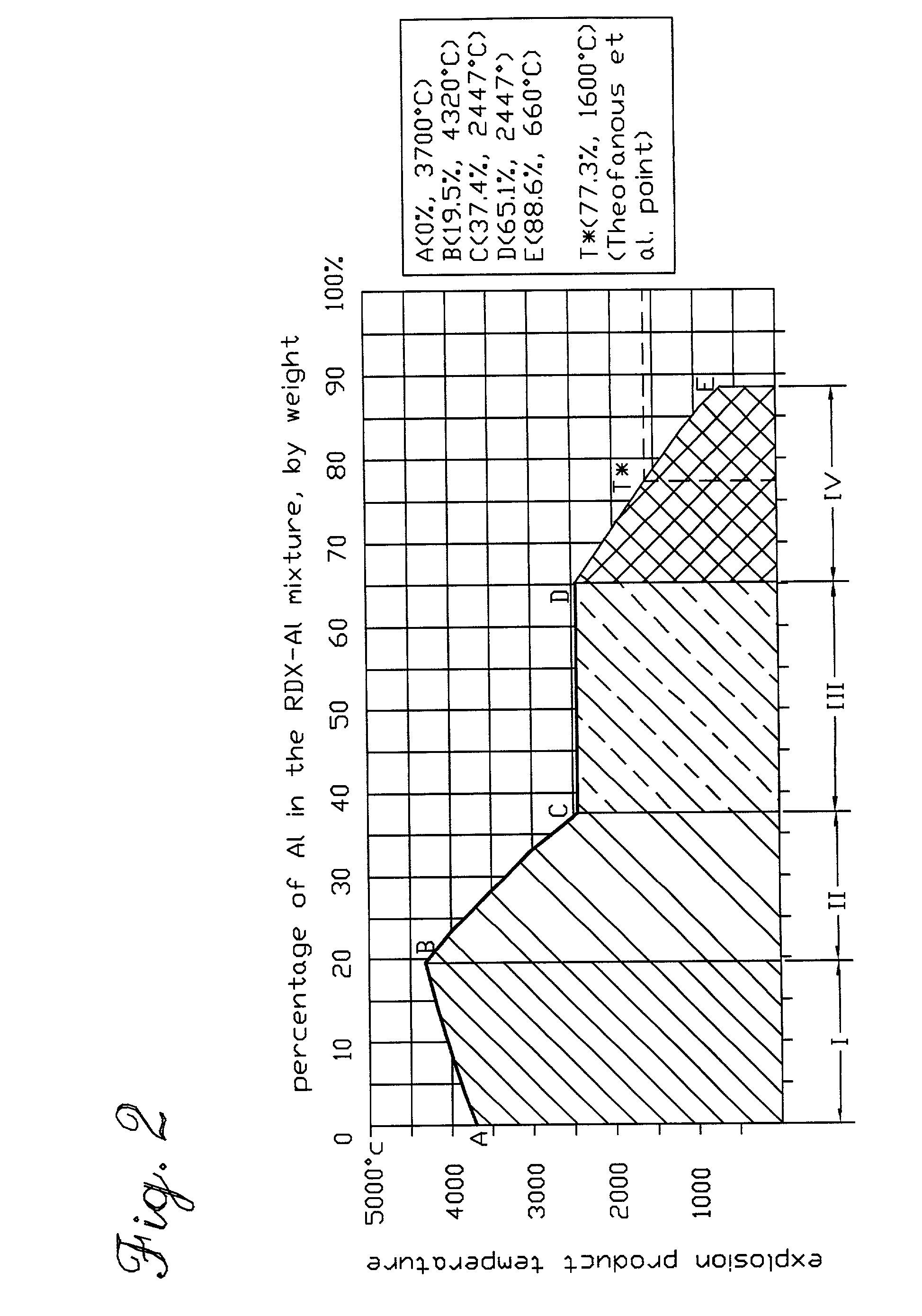
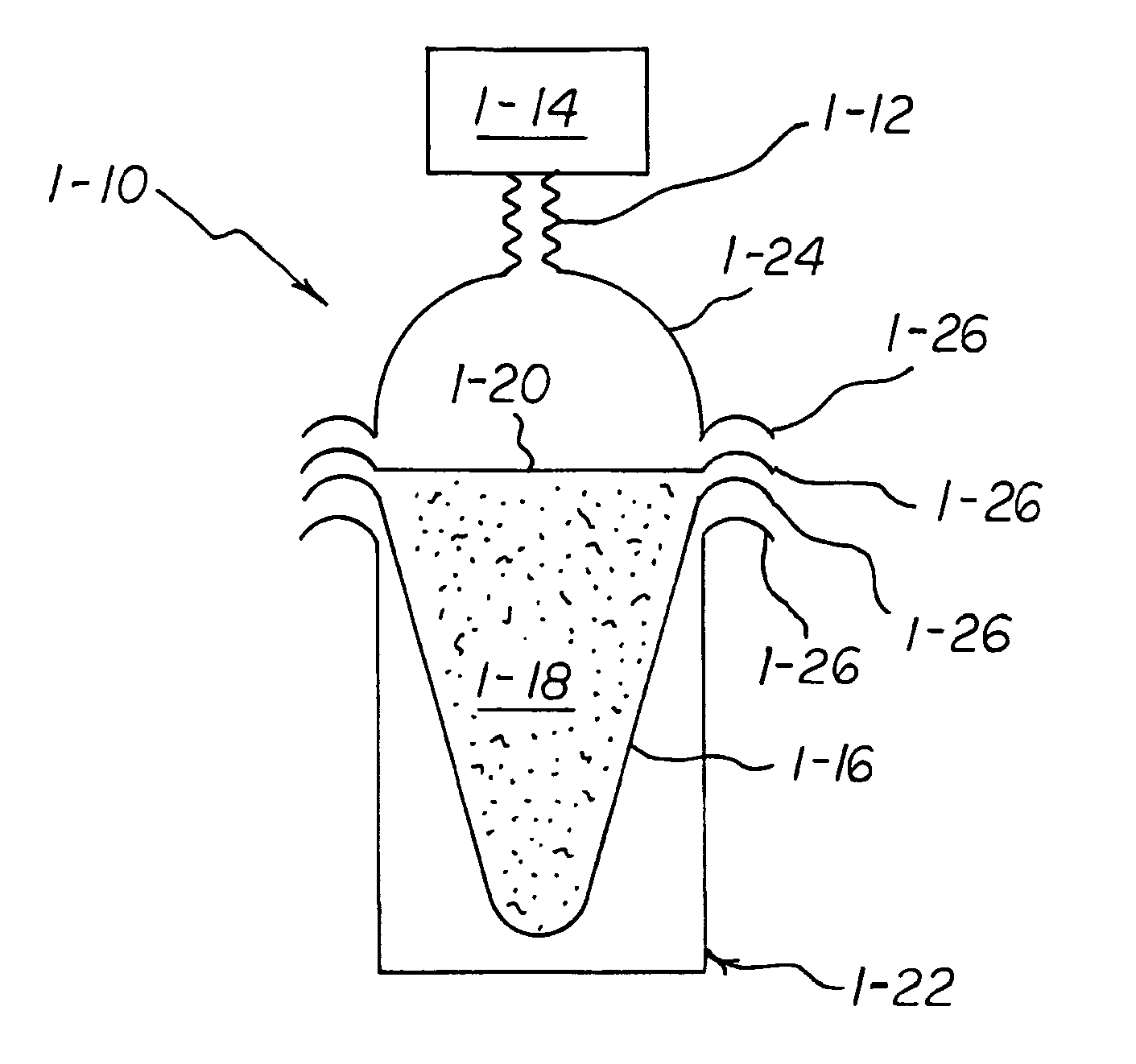
A chemical reaction between molten aluminum and an oxygen carrier such as water to do useful work is disclosed, and in particular two chemical methods to obtain aluminum in its molten state. One is to detonate a HE / Al mixture with surplus Al in stoichiometry, and the other is to use an oxidizer / Al mixture with surplus Al in stoichiometry. Additionally, there is a physical method of shocking and heating Al using high temperature reaction products. The produced Al in its liquid form is forced to react with an oxygen carrying liquid (e.g. water), giving off heat and releasing hydrogen gas or other gaseous material. A water solution of some oxygen-rich chemicals (e.g. ammonium nitrate) can be advantageously used in place of water. A shaped charge is also disclosed having a liner that contains aluminum, propelled by a high explosive such as RDX or its mixture with aluminum powder. Some aluminum in its molten state is projected into the perforation and forced to react with water that also enters the perforation, creating another explosion, fracturing the crushed zone of the perforation and initializing cracks. Another shaped charge is shown having a liner of energetic material such as a mixture of aluminum powder and a metal oxide. Upon detonation, the collapsed liner carries kinetic and thermal energy. Also shown are methods to build and to detonate or fire explosive devices in an oxygen carrying liquid (e.g. water) to perforate and stimulate a hydrocarbon-bearing formation.
Owner:GEODYNAMICS
Apparatus and Method for Using Tetrazine-Based Energetic Material
InactiveUS20090301601A1Fast coolingInternal combustion piston enginesBlasting cartridgesEngineeringEnergetic material
The present invention comprises apparatus and methods employing a gas produced from a tetrazine-based energetic material such as that known as “BTATz” containing 3;6-BtS(1H-1,2,3,4-Tetrazol-5-ylamino)1-,2,4,5-tetrazine 3;6-BtS(1H-1,2,3,4-Tetrazol-5-ylamino)1-,2,4,5-tetrazine or salts thereof. The tetrazinebased energetic material is ignited through the use of a percussion cap, a piezoelectric crystal or a battery-supplied electric spark or by encapsulating it in a container that is then exposed to a burning flame. The gas produced upon ignition is employed a propellant such as to inflate life rafts, life vests, emergency evacuation slides, tires, air bags and other inflatable devices. The gas produced upon ignition is alternatively employed to power an engine and many other applications such as a fire suppressant.
Owner:ENERSON JON R +4
Bead pack brazing with energetics
InactiveUS7644854B1Reduce energy consumptionPrevent movementFluid removalWelding/cutting media/materialsEnergeticsUnit structure
A method of making porous shapes from unit structures such as beads involves coating the beads with two or more layers of material deposited such that it forms an energetic material. These bi-layer energetic materials are formed from a variety of materials including, but not limited to: Ti & B, Zr & B, Hf & B, Ti & C, Zr & C, Hf & C, Ti & Si, Zr & Si, Nb & Si, Ni & Al, Zr & Al, or Pd & Al, all of which can be deposited from vapor. Pressure is applied to prevent the components from moving and the solid-state reaction between the alternating layers produces exothermic heat. Heat from the reaction alone or in conjunction of an applied brazing compound joins the beads forming a porous shape that is desired. The reaction in the materials may be activated with a small pulse of local energy that can be applied using optical, electrical, or thermal sources. Common examples include an electrical pulse, spark, hot filament, a laser beam, etc. The method reduces energy consumption and the need for specialized equipment. The reactive materials and optional brazing material are preferably applied in a fluidized CVD furnace.
Owner:BAKER HUGHES INC
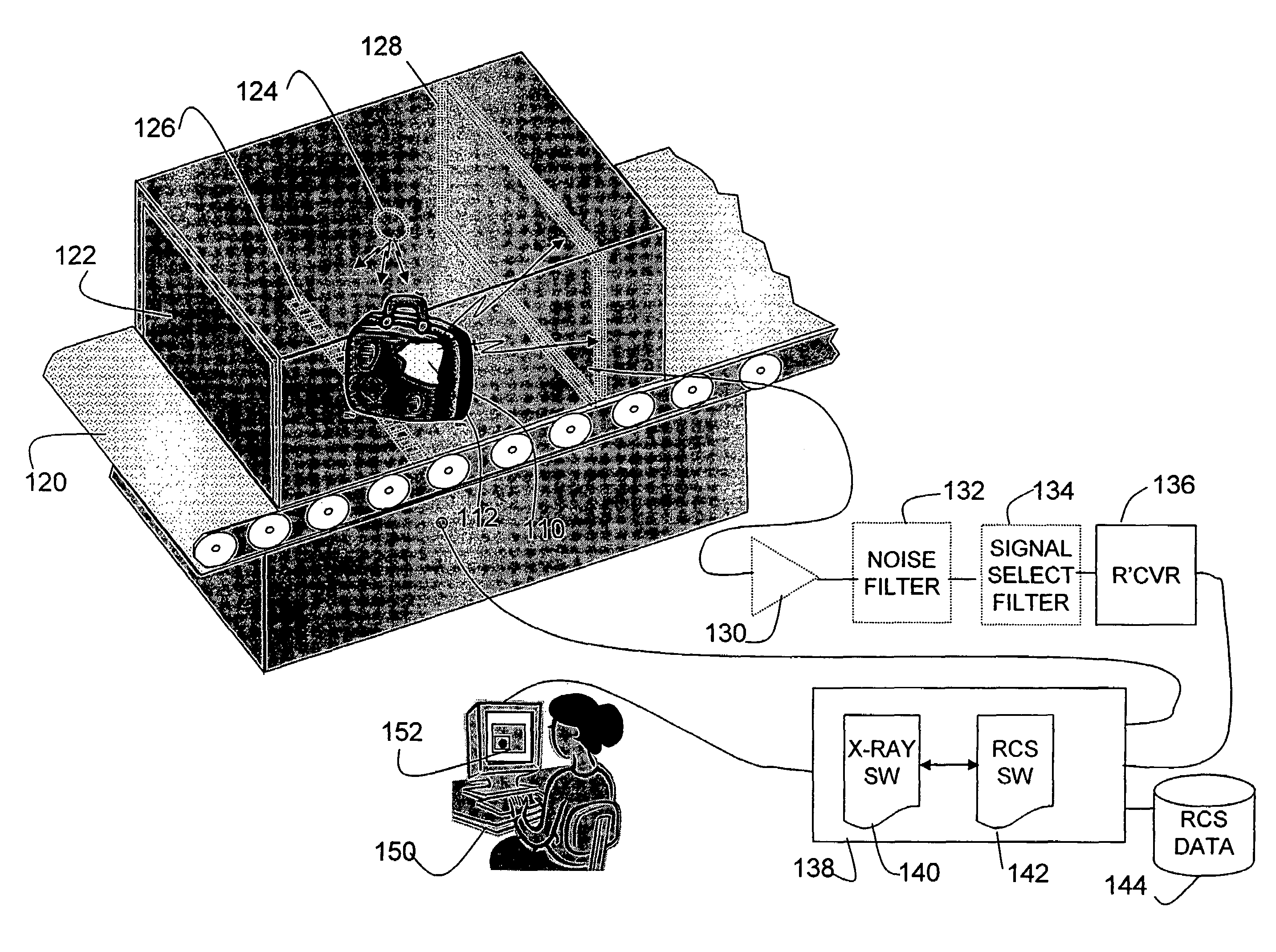
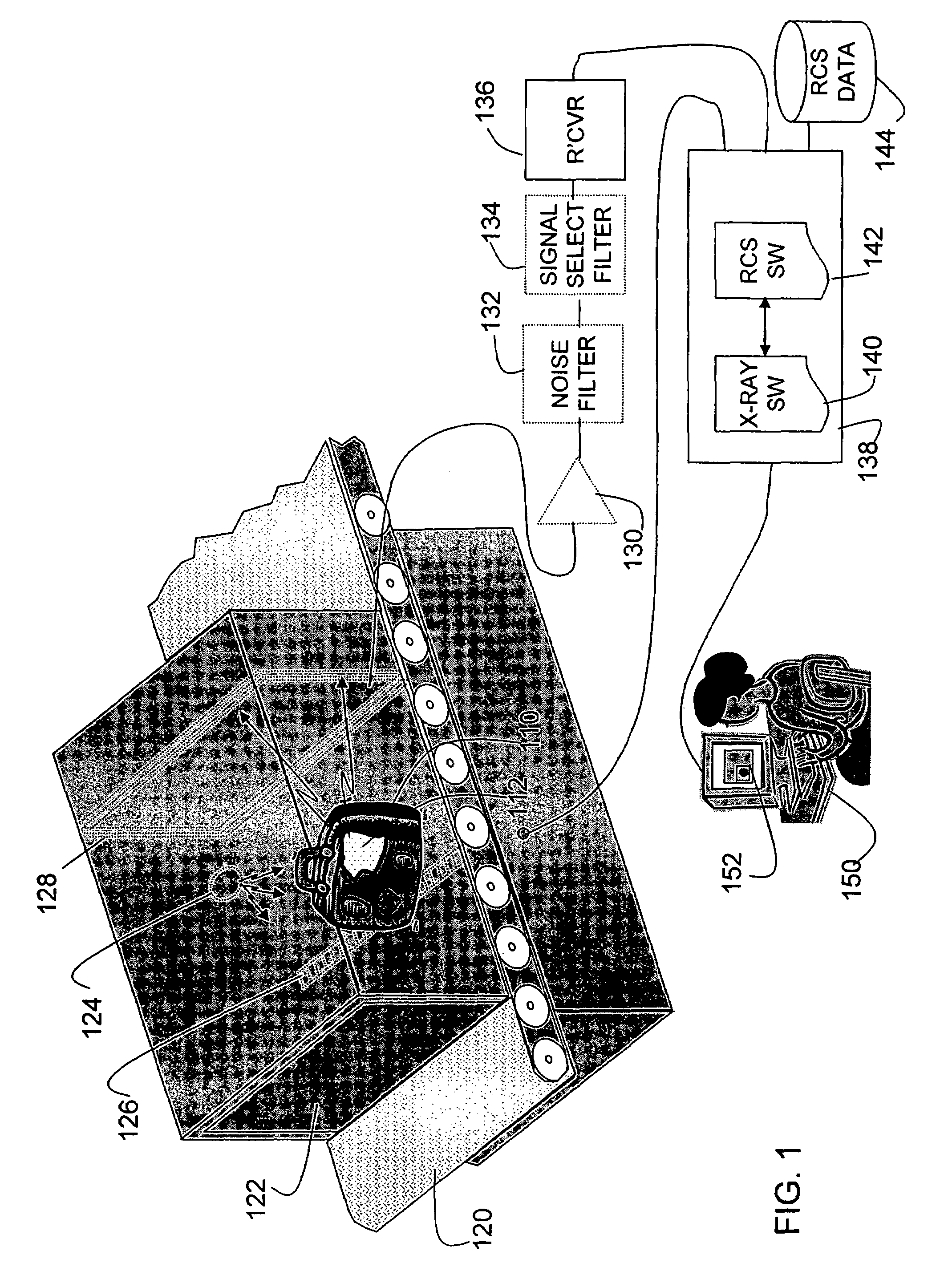
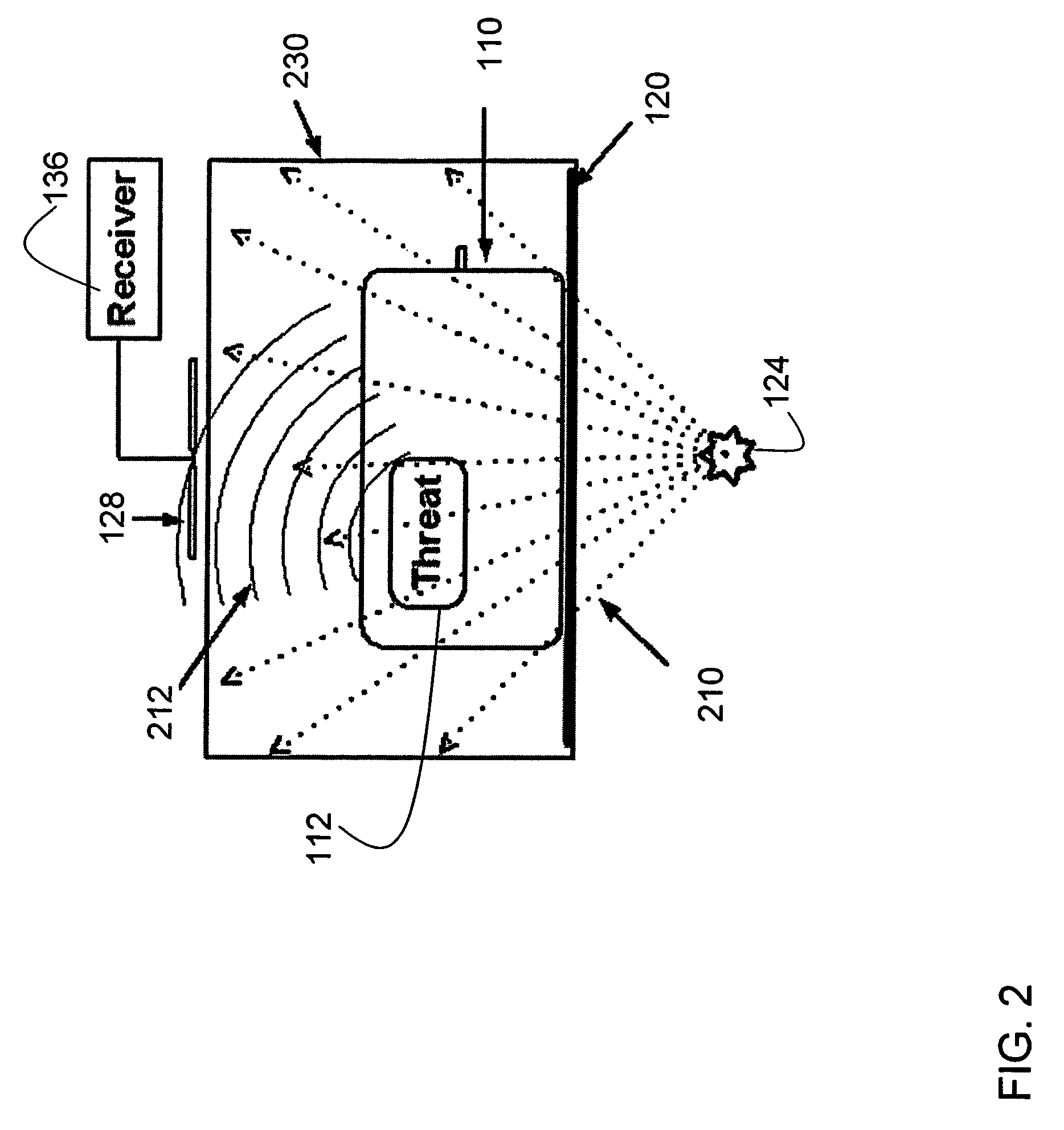
Method and apparatus for detecting contraband using radiated compound signatures
InactiveUS7251310B2Electric/magnetic detectionMaterial analysis by transmitting radiationSecondary analysisIonization
An system for detecting contraband, particularly explosives or other energetic materials. The system includes a source of ionizing radiation that irradiates an item under inspection. The radiation stimulates the emission of RF energy from objects within the item under inspection. The characteristics of the emitted RF energy reveals information about the material composition of the objects. The system detects this emitted RF energy and comparers it to a signature of RF emissions from contraband objects. Apparatus to detect and analyze RF emissions may be constructed as a stand-alone unit or may be incorporated into an imaging system in which the ionizing radiation is used to form an image of the item under inspection. Similarly, the RF analysis may be used, in the first instance, to determine whether an object contains a contraband item or may be used as a second level analysis to clear alarms generated by analysis of images formed by the imaging system.
Owner:L3 COMMUNICATIONS SECURITY & DETECTION SYSTEMS CORPORATION
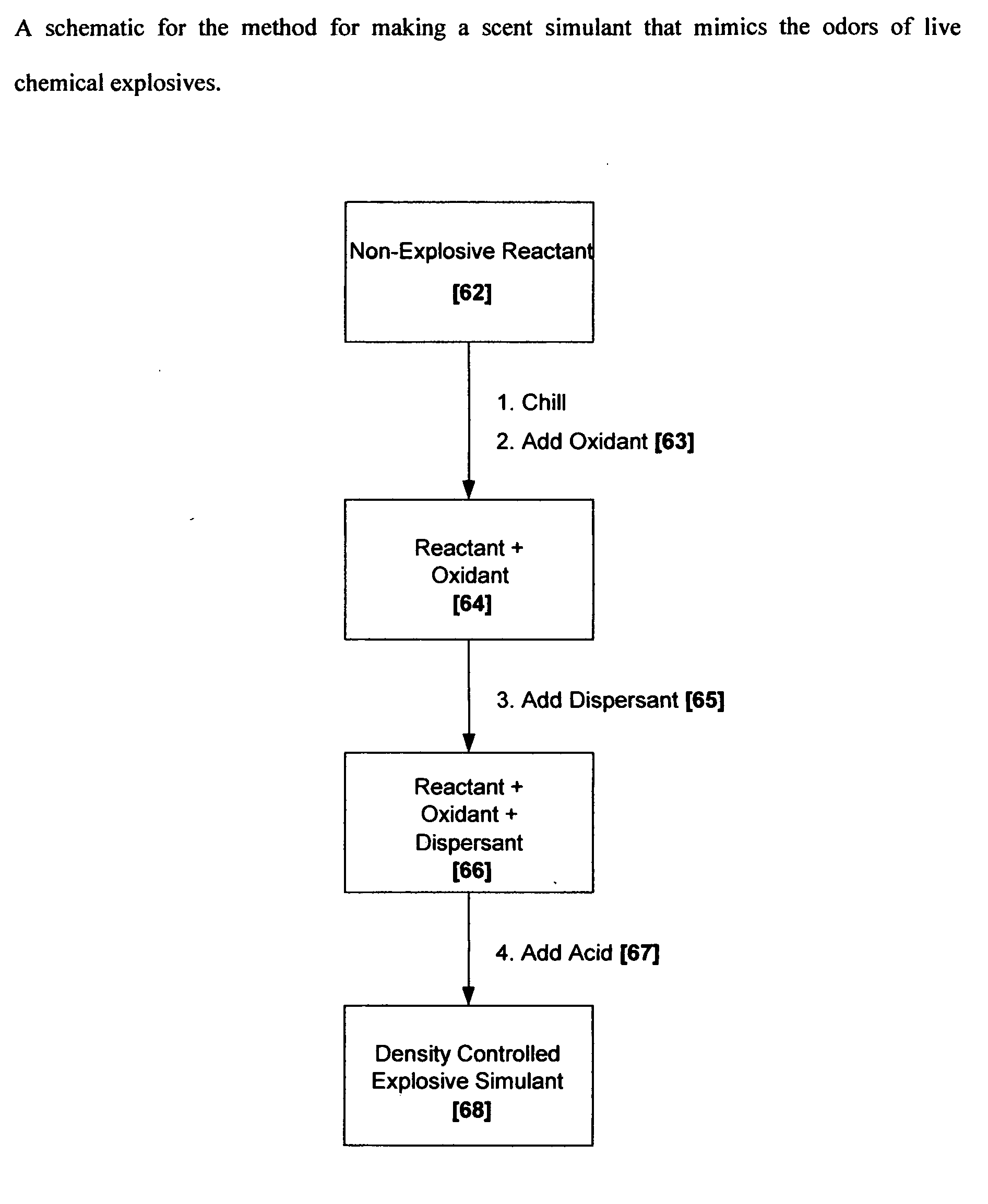
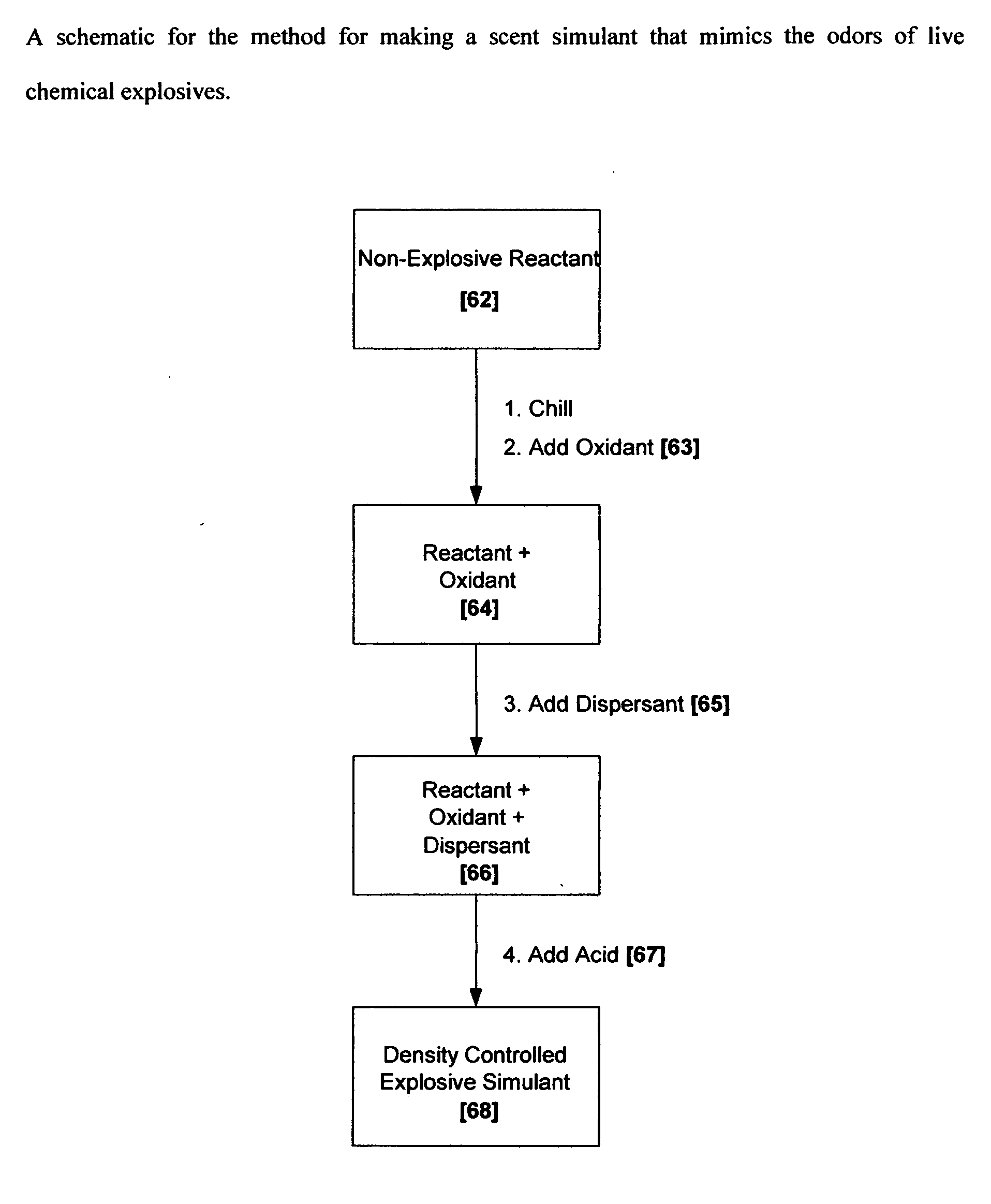
Methods for making scent simulants of chemical explosives, and compositions thereof
ActiveUS20090194744A1Increasing degree of heterogeneosityDefence devicesOther chemical processesChemical explosiveDetonation
The present invention relates to methods for producing non-detonable and non-explosive parent-odor scent simulants of both detonable and entropy-burst chemical explosive materials. A detonable explosive material is a material that explosives with the aid of detonation while an entropy burst explosive material is a very sensitive energetic material that does not require detonation, but explodes through a spontaneous decomposition of its molecules into gaseous products. The invention also presents representative non-detonable, non-hazardous compositions of such simulants that can be safely and effectively utilized within a broad spectrum of biological and non-biological explosives detection programs, non-limiting examples being the training of biological search-and-detect creatures such as explosive detecting dogs and the calibration of electronic explosive detecting instruments that rely on the principles of vapor sampling for their operations.
Owner:ADEBIMPE DAVID O B A
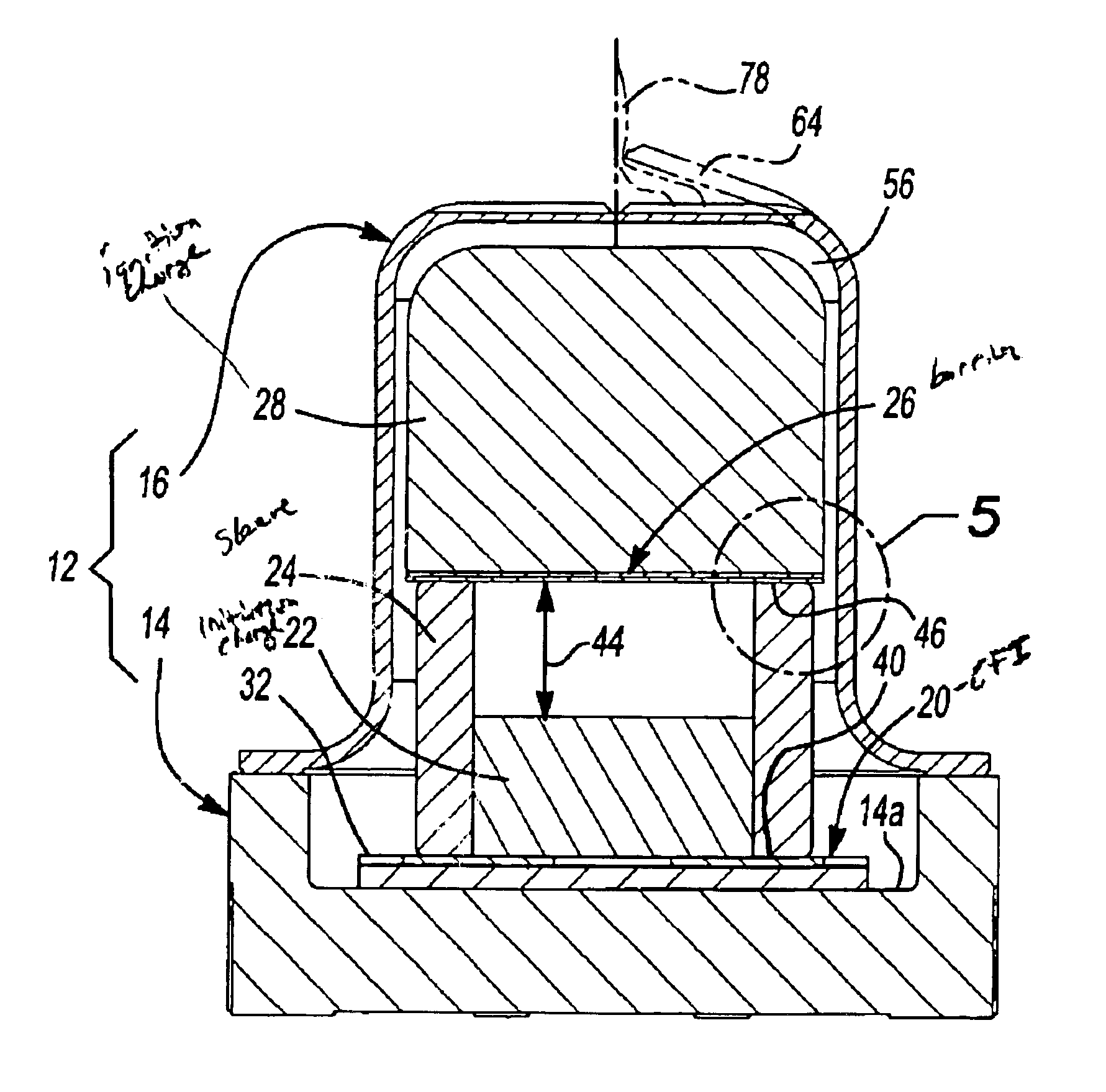
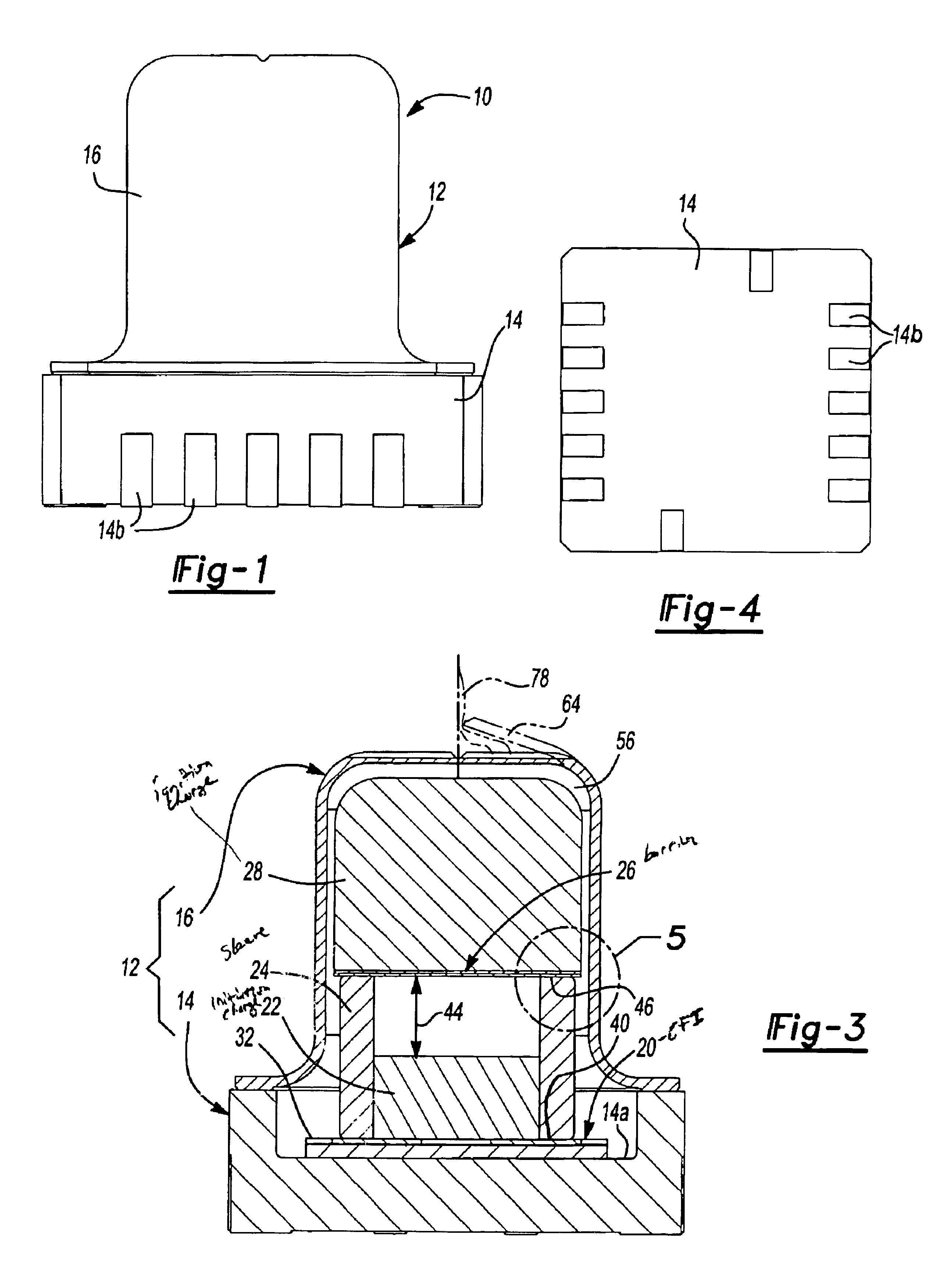
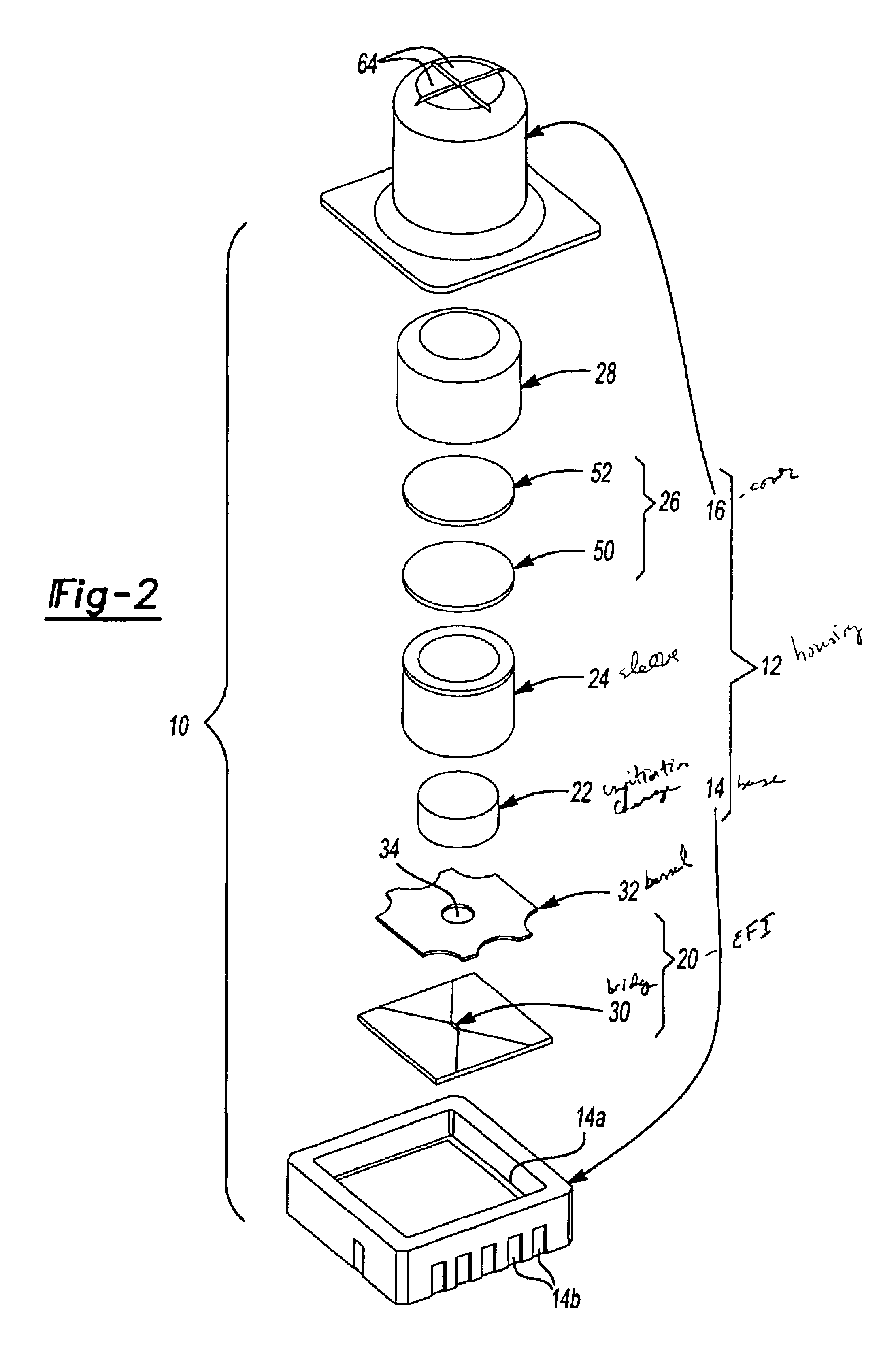
Energetic material initiation device utilizing exploding foil initiated ignition system with secondary explosive material
A device and method that employs a detonation event as an energy source for producing a pyrotechnic output. The device includes an initiation charge that is selectively detonated by an exploding foil initiator. Byproducts from the detonation event are attenuated by a barrier that is disposed between the initiation charge and an ignition charge. The barrier participates in a chemical reaction with the detonation byproducts to oxidize and burn. In this regard, the barrier serves as a fuel that is employed to ignite the ignition charge. The device includes a sealed housing in which the initiator, initiation charge and ignition charges are housed. The housing ruptures in response to the heat and pressure generated by the ignited ignition charge to release the pyrotechnic output.
Owner:REYNOLDS SYST
Wet processing and loading of percussion primers based on metastable nanoenergetic composites
InactiveUS20060113014A1Reduce degradationExtended shelf lifeLoomsWoven fabricsLiquid waterCompound (substance)
A method is disclosed for preparing metastable nanoenergetic composites (MNC) and for wet loading those MNCs into percussion primer cups. The method involves dispersing nanosize reactants in an inert liquid or, alternatively, making a nanosize reactant surface modification for improvement of reactant's chemical inertness towards water, followed by application of additives supporting a solid reactant particle dispersion in water or water solution prior to mixing. After mixing of the reactants, one maintains the presence of liquid water together within an energetic material in order to enhance safety during pre-loading of the primer mixture into the primer cups and during the final drying.
Owner:INNOVATIVE MATERIALS & PROCESSES LLC +1
Method for destroying energetic materials
InactiveUS6121506AAffect environmentSafely, simply and economicallyAmmunitionHalogenated hydrocarbon separation/purificationChemical reactionSolvation
Energetic materials, such as nitrocellulose, TNT, RDX, and combinations thereof, optionally in combination with chemical warfare agents, such as mustard gas, Lewisite, Tabun, Sarin, Toman, VX, and combinations thereof, are destroyed when chemically reacted according to the method of the invention. The method comprises reacting the energetic materials and chemical warfare agents, of present, with solvated electrons which are preferably produced by dissolving an active metal such as sodium in a nitrogenous base such as anhydrous liquid ammonia.
Owner:MILFORD CAPITAL & MANAGEMENT
Energetic material initiation device utilizing exploding foil initiated ignition system with secondary explosive material
An igniter assembly with a housing, an initiator, an input charge, a first barrier, a second barrier, and an output charge. The input charge is formed of a secondary explosive and disposed adjacent the initiator. The first barrier cooperates with the housing to form a chamber into which the input charge is received. The second barrier is disposed on a side of the first barrier opposite the input charge and combusts in response to energy released during detonation of the input charge. The output charge is formed of a pyrotechnic material and disposed on a side of the second barrier opposite the first barrier. The output charge can combust in response to energy released during combustion of the second barrier. The housing and the first barrier cooperate to isolate the output charge from heat and pressure generated by the input charge if the input charge is cooked-off.
Owner:REYNOLDS SYST
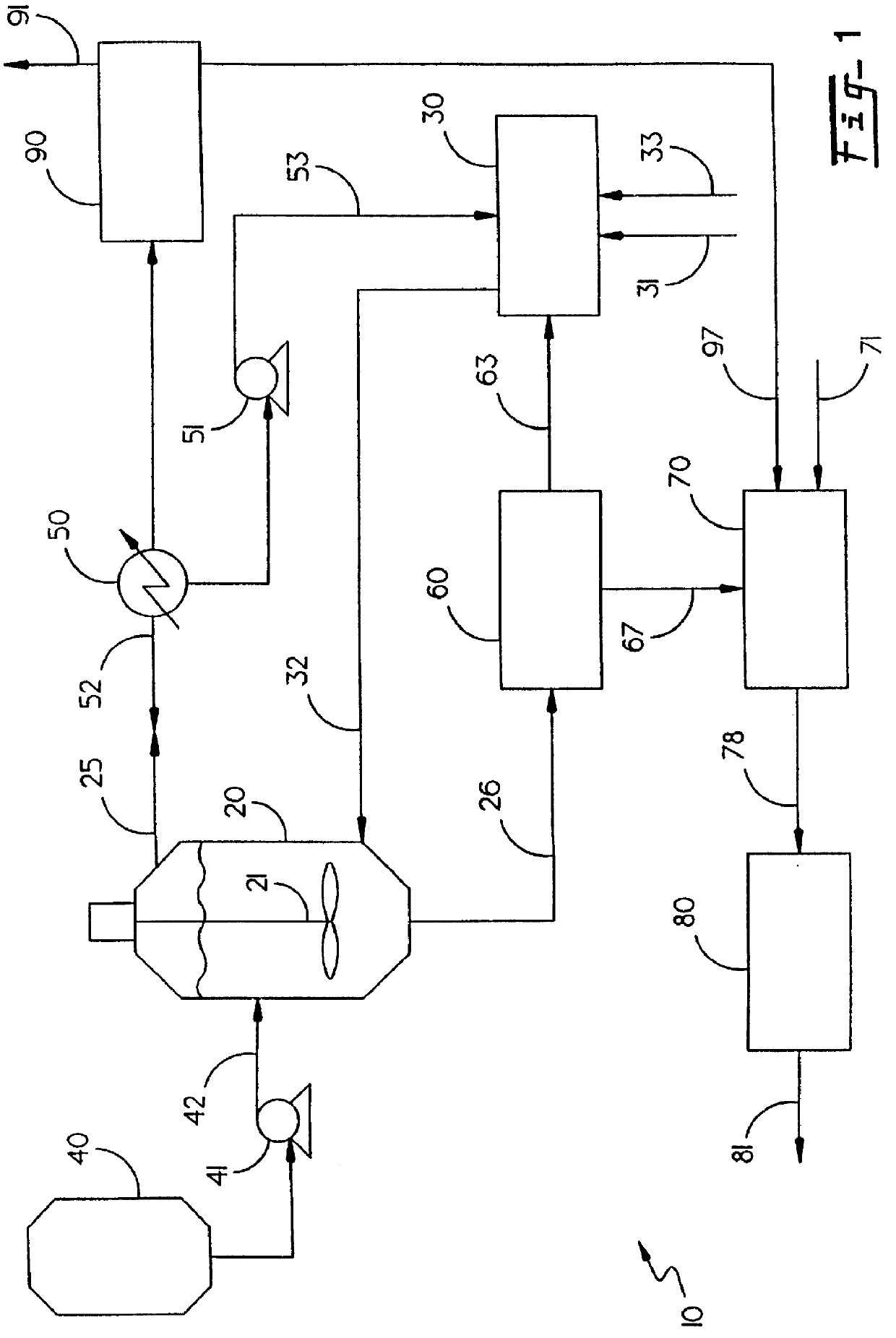
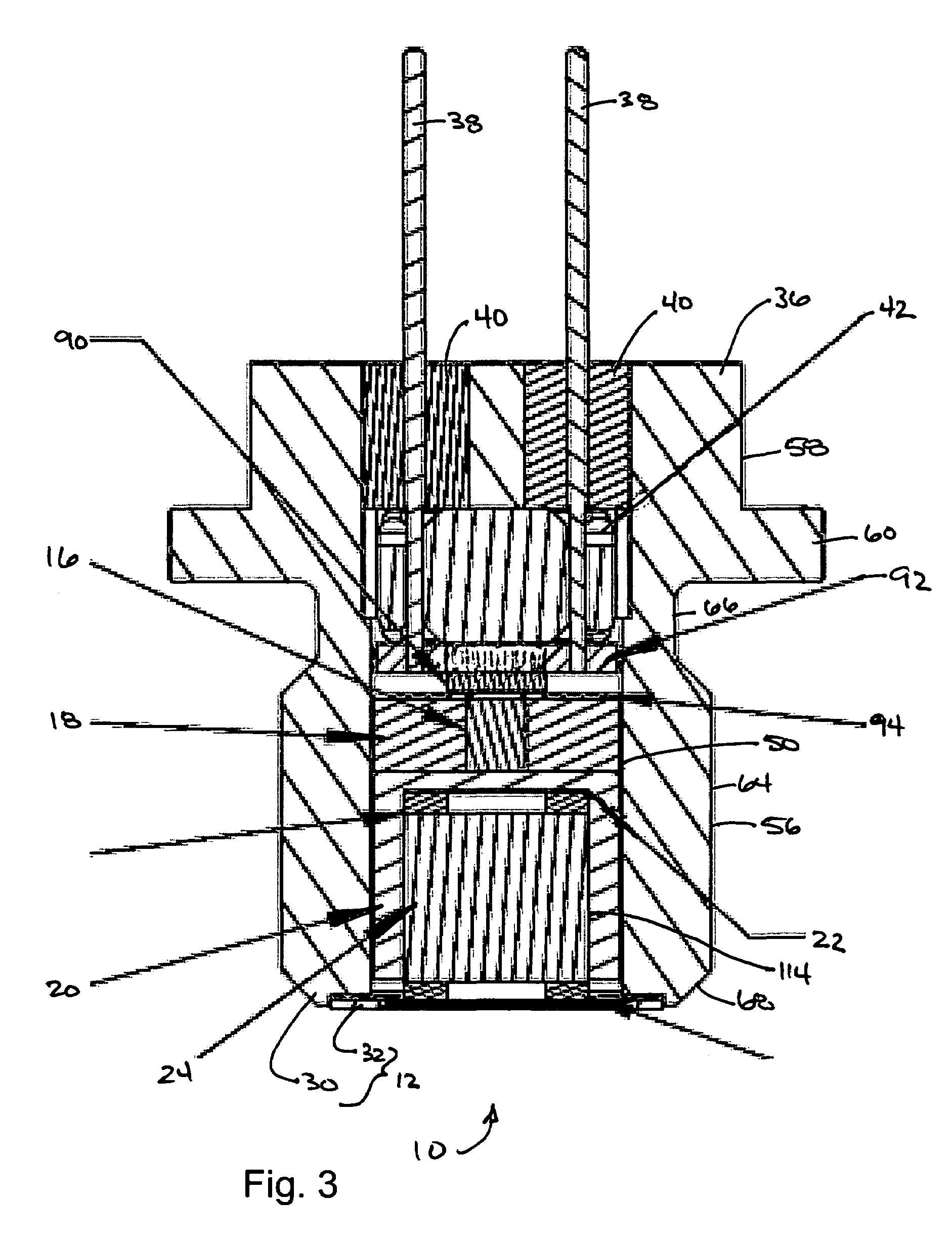
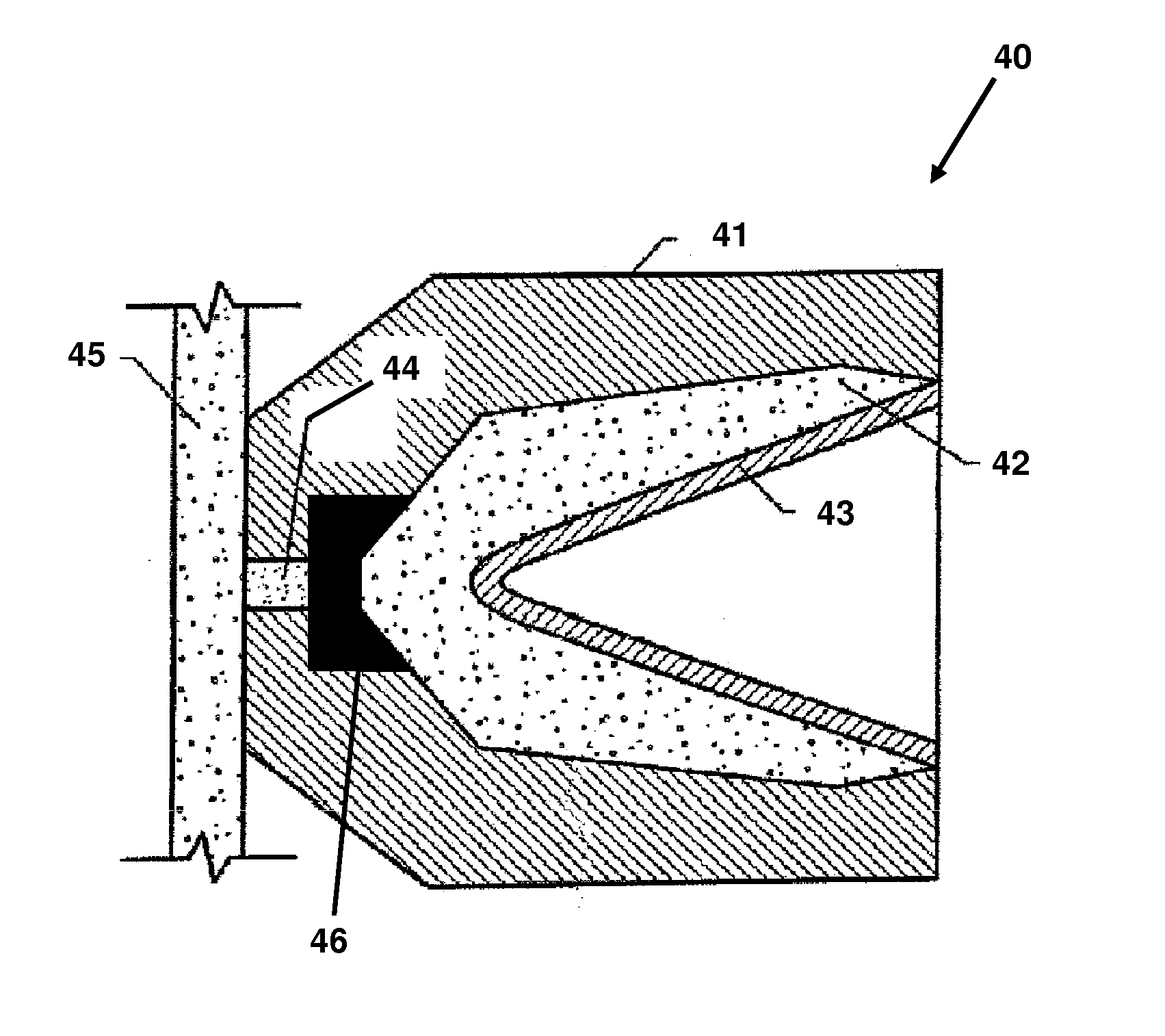
Energetic material applications in shaped charges for perforation operations
A shaped charge includes a cup-shaped casing defining an interior volume; a liner located within the interior volume; an explosive disposed between the liner and the casing; and a reactive material disposed between the liner and the casing. A method for generating a dynamic overbalance inside a wellbore includes disposing a perforation gun in the wellbore; and detonating a shaped charge in the perforation gun, wherein the shaped charge includes a cup-shaped casing defining an interior volume, a liner located within the interior volume, an explosive disposed between the liner and the casing, and a reactive material disposed between the liner and the casing.
Owner:SCHLUMBERGER TECH CORP
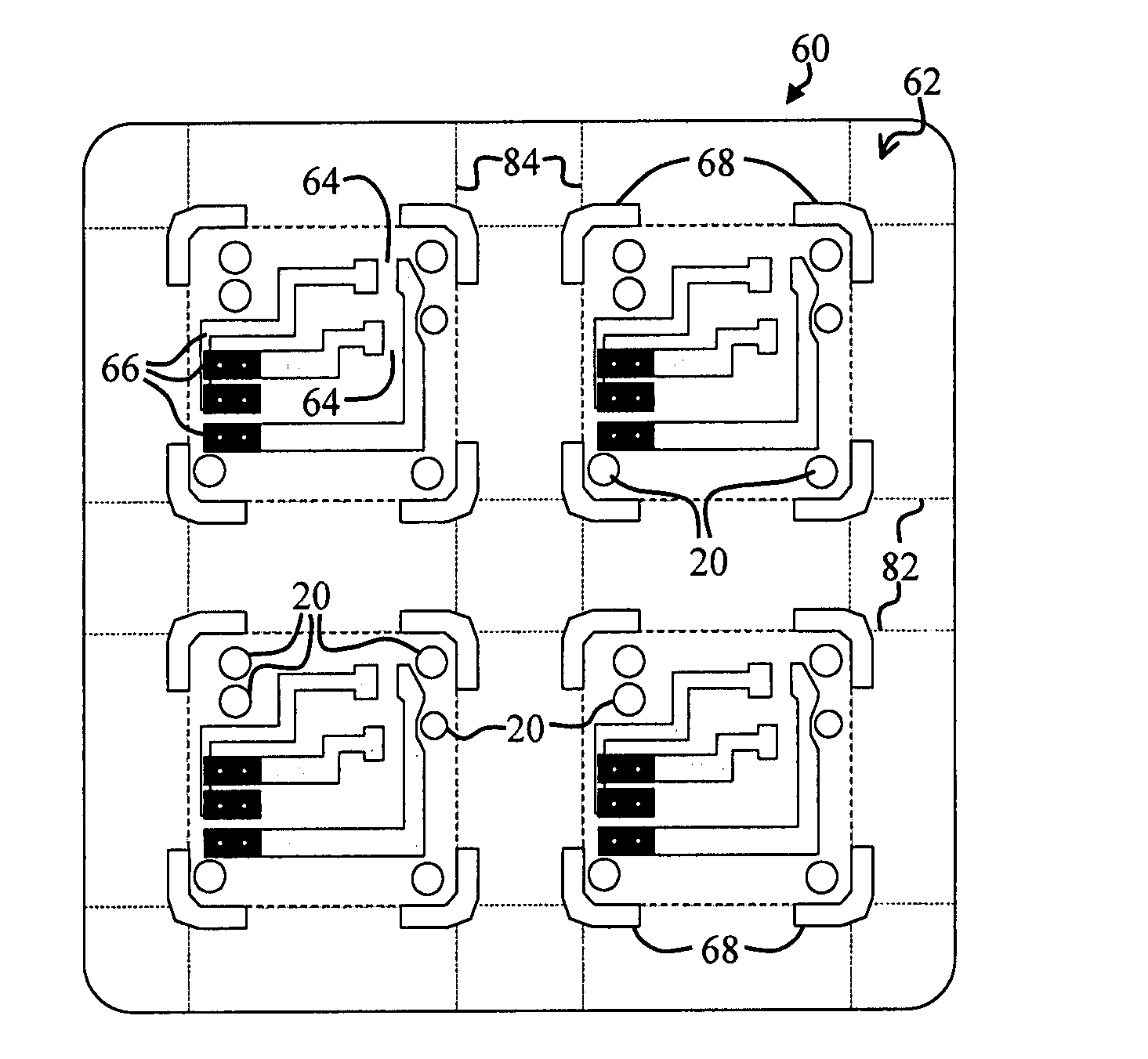
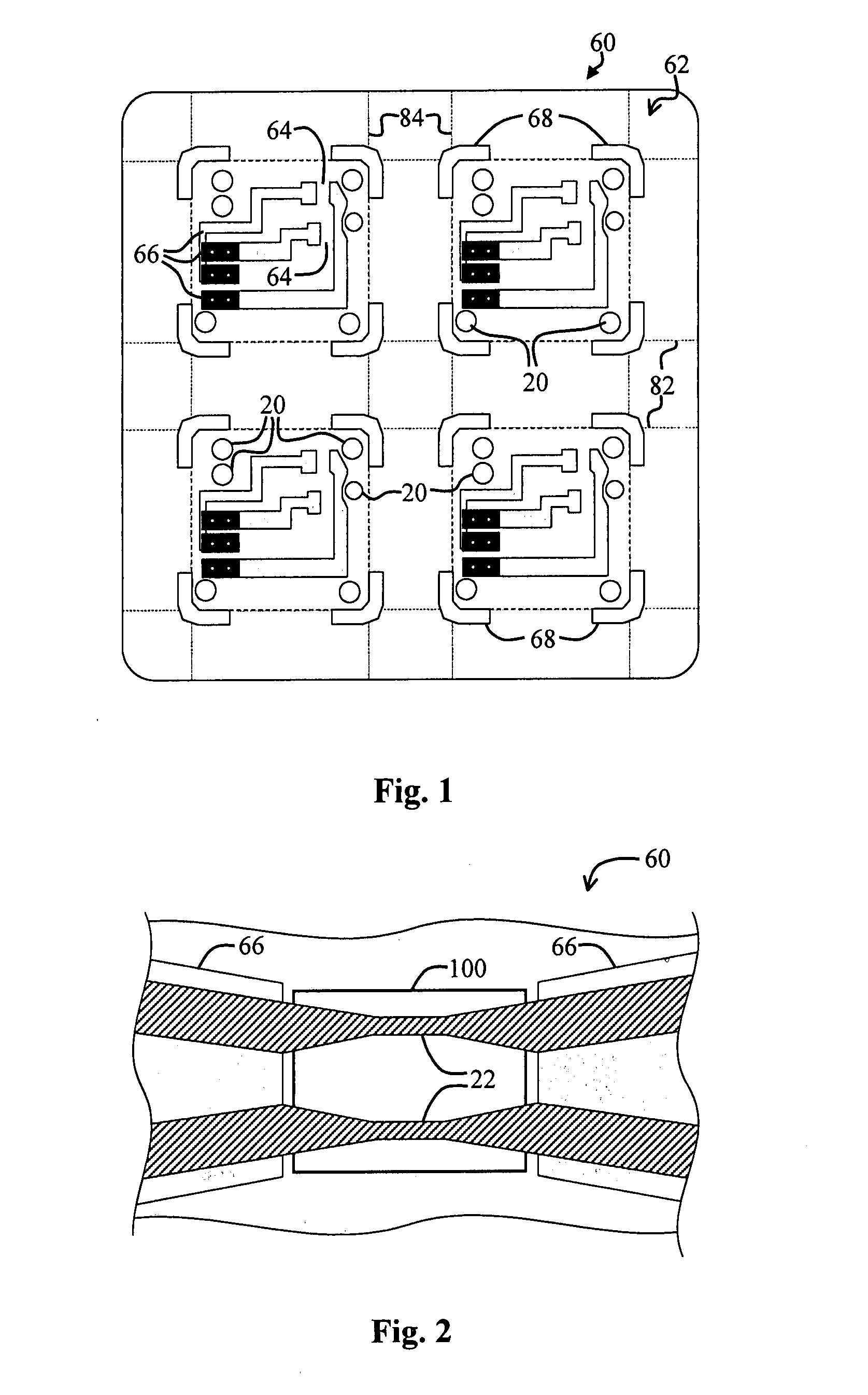
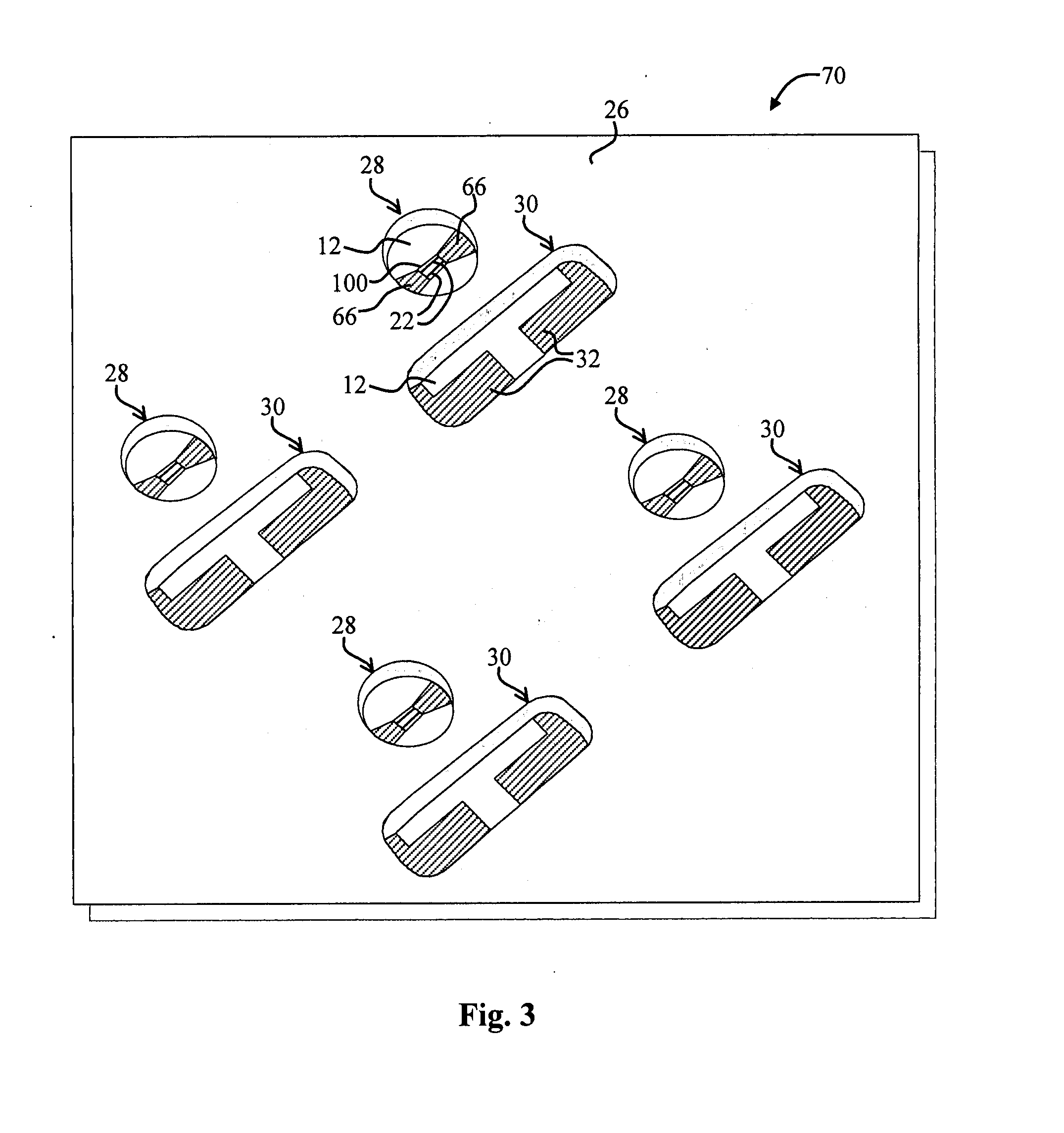
Perforating system comprising an energetic material
A perforating system, including a shaped charge assembly comprising a charge case, a liner, and a main body of explosive. The material of the perforating system components, including the gun body, the charge case and the liner may be comprised of an energetic material that conflagrates upon detonation of the shaped charge. The material may be an oxidizer, tungsten, cement particles, rubber compounds, compound fibers, KEVLAR®, steel, steel alloys, zinc, and combinations thereof.
Owner:BAKER HUGHES INC
Microwave heating of energetic materials
InactiveUS20060011083A1Material nanotechnologyNon-explosive fillers/gelling/thickening agentsGramDegree Celsius
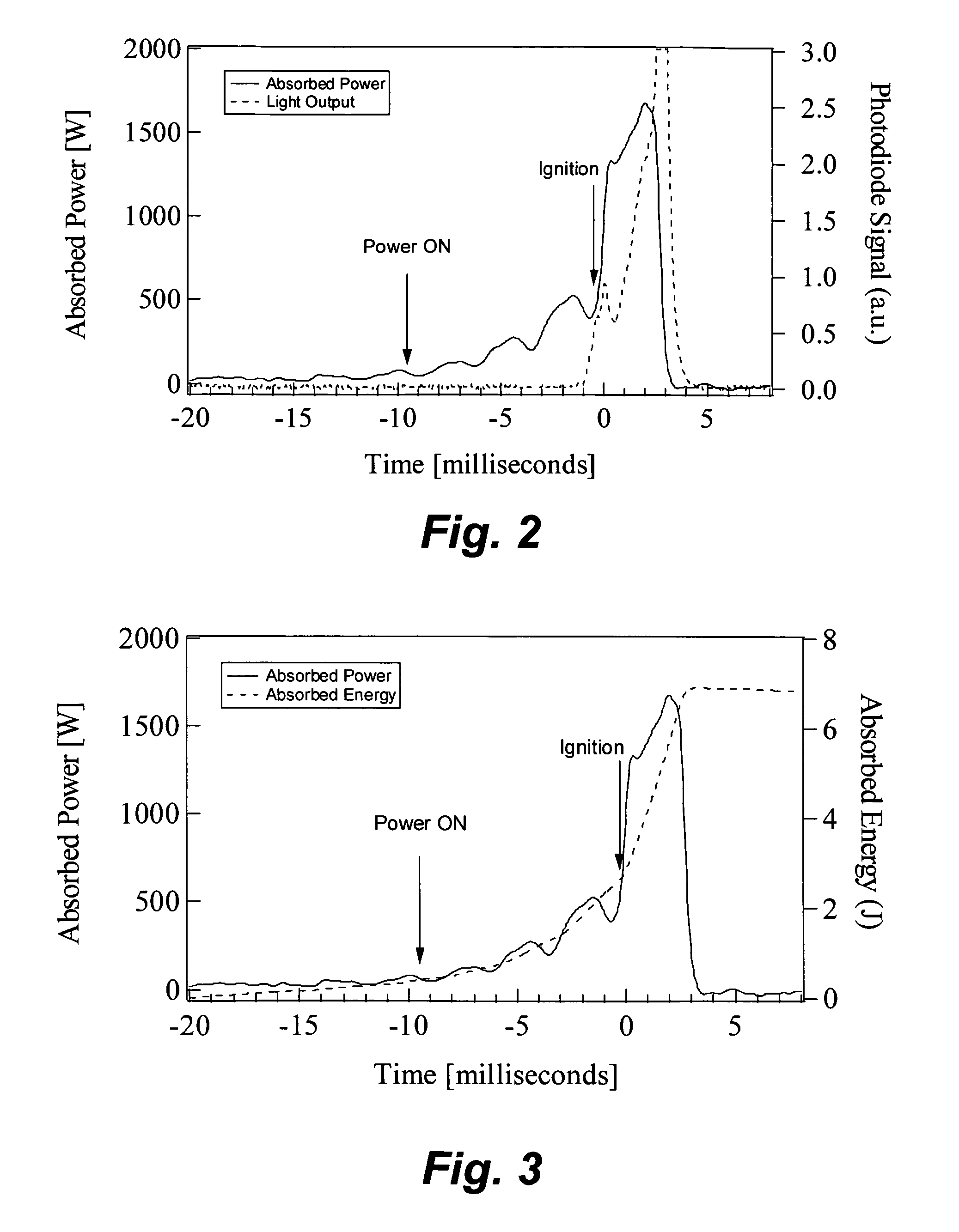
Mixtures of high explosives with materials that readily absorb microwaves ignite more readily when exposed to microwave energy than the corresponding neat explosives. A charge of HMX (0.5 gram) mixed with carbon nanotubes (1 percent by mass) ignited with 7.5 joules at an average rate of 750 W for 10 milliseconds. To raise a charge of the same mass of neat HMX to an autoignition temperature of 200 degrees Celsius would require much more energy (about 110 joules) for a longer duration (about 150 milliseconds).
Owner:LOS ALAMOS NATIONAL SECURITY
Muzzleloader ammunition
ActiveUS7726245B2Accurate CalibrationAmmunition projectilesFiring/trigger mechanismsBurn rateEnergetic material
A fixed round of ammunition for a muzzle loader firearm. The round has a bullet within a sabot that is engaged to a consumable cartridge case. The case is filled with propellant that is precisely calibrated to provide optimal ballistic properties with the particular bullet it is engaged to. The consumable cartridge case can be more tailored to respectively increase or decrease the burn rate of the consumable cartridge case. The consumable cartridge case can be constructed out of nitrocellulose or other energetic materials. A wide range of propellant formulas can be used that are safe because the burn rate is precisely calibrated for the bullet used. The propellant can contain pyrotechnic material and / or other ingredients to reduce the burn rate of a smokeless propellant and can contain a stabilizer to increase the shelf life of the ammunition round.
Owner:FEDERAL CARTRIDGE
Ionic liquid, a method of synthesizing an ionic liquid, a precursor of an explosive composition including at least one ionic liquid, and a method of desensitizing an explosive composition
InactiveUS20080251169A1Sure easyUrea derivatives preparationOrganic compound preparationIonic liquidEnergetic material
An ionic liquid is disclosed A precursor composition that comprises at least one ionic liquid and at least one energetic material is also disclosed, as is a method of synthesizing an ionic liquid and a method of desensitizing an explosive composition.
Owner:NORTHROP GRUMMAN SYST CORP
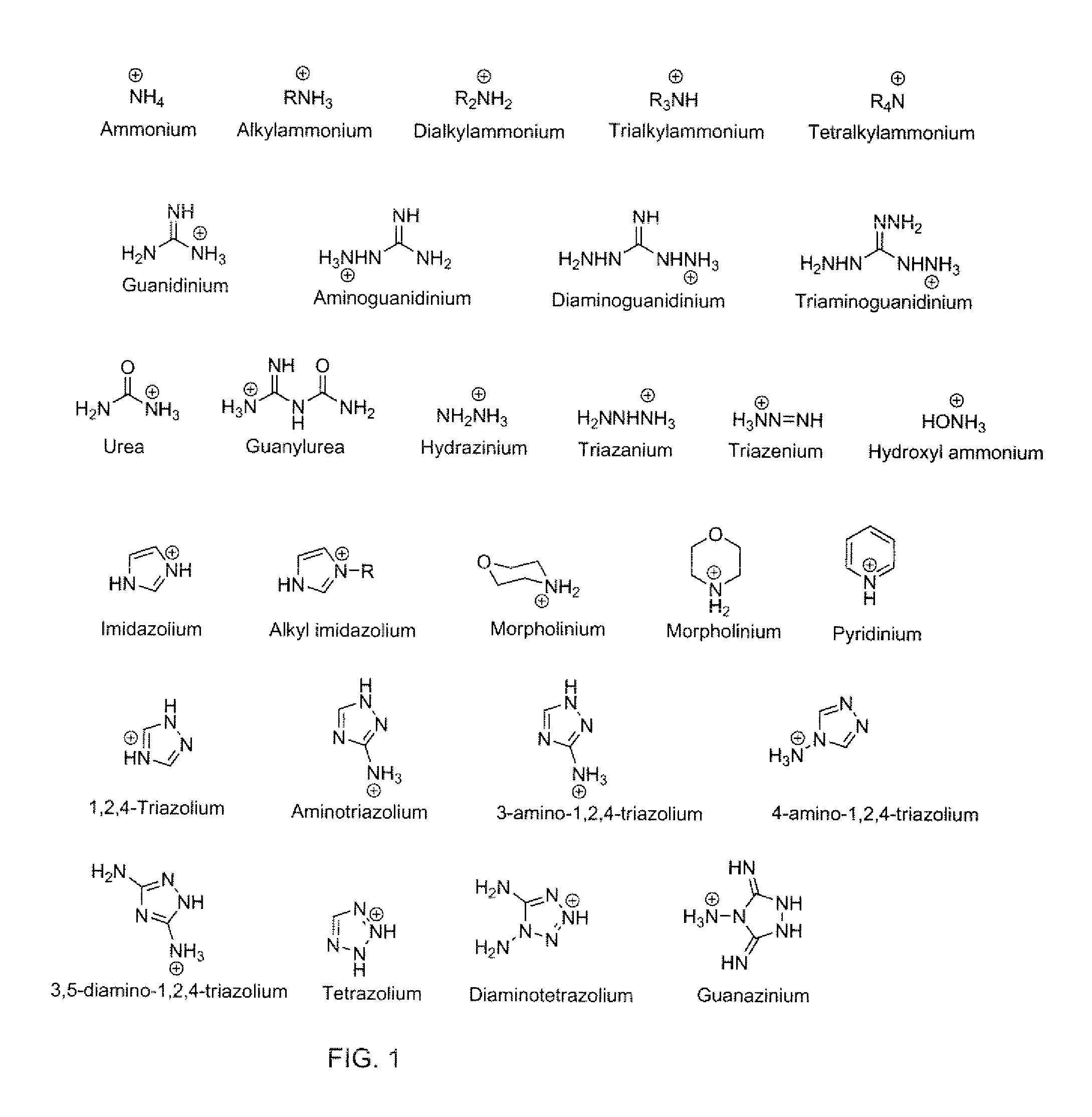
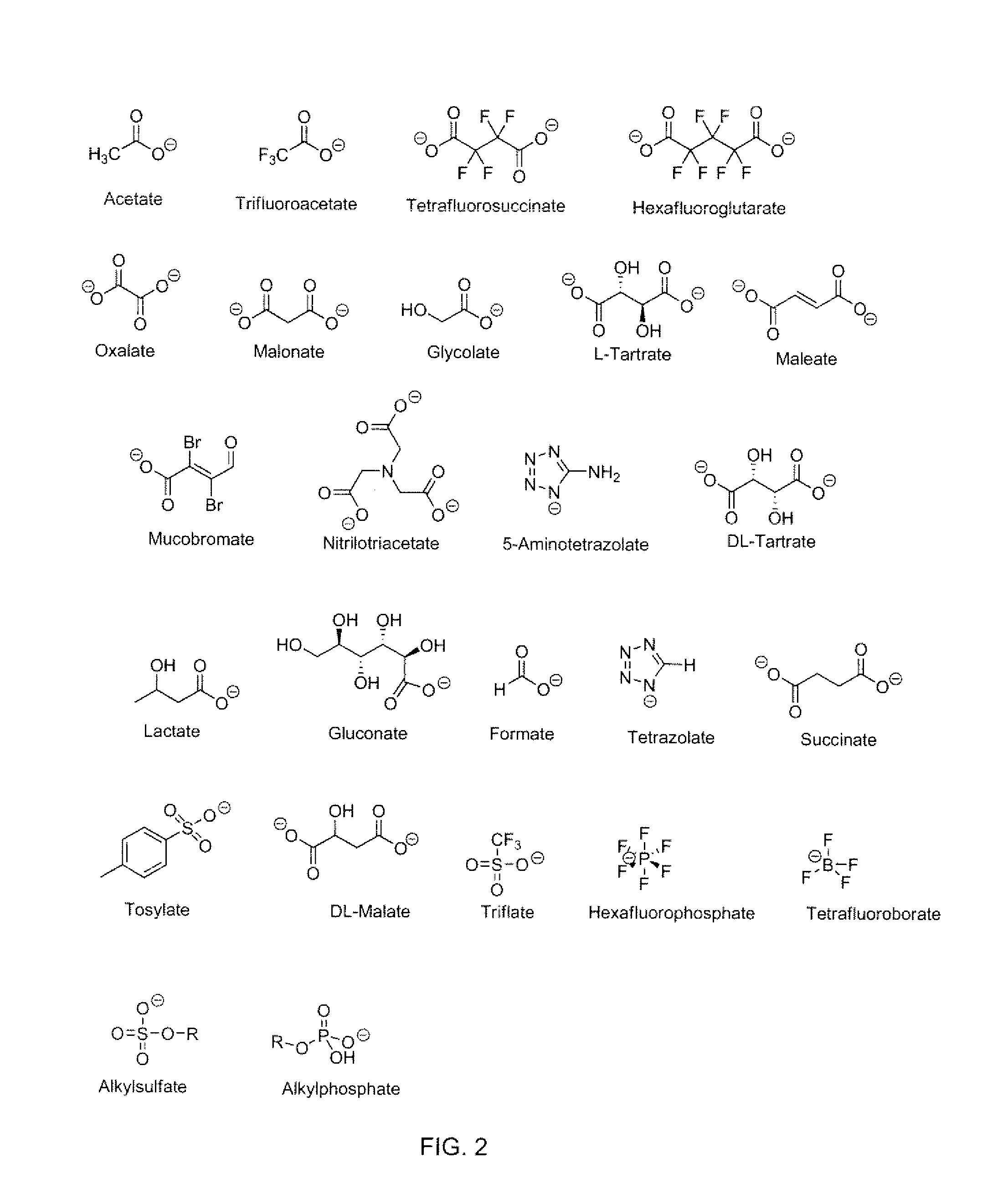
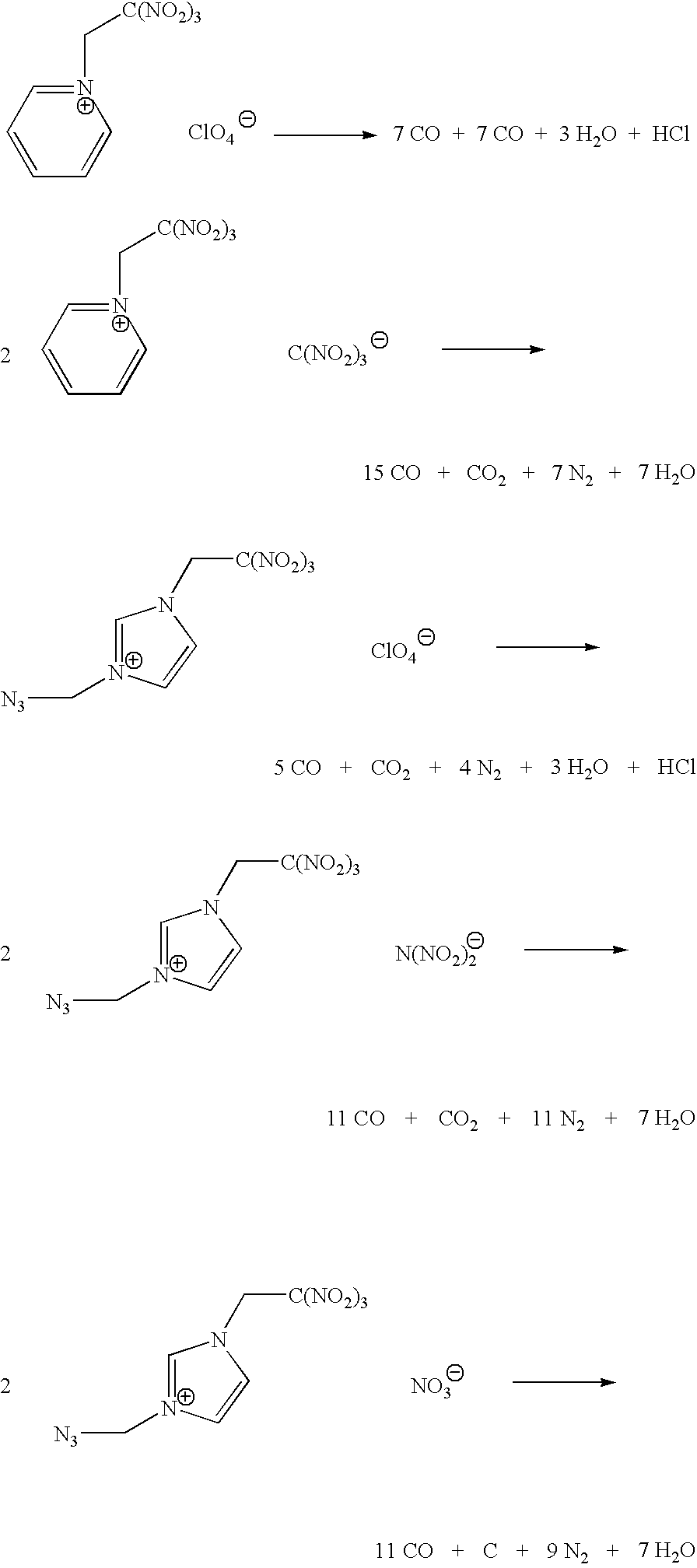
Energetic ionic liquids
Provided are energetic materials of low vapor pressure in the form of ionic liquids having fuel and oxidizer ions including, substituted pyridinium or imidazolium cations paired with nitrato-, perchlorato-, or nitramido-based anions, to form such ionic liquids or salts. The salts of the present invention are low melting and have essentially little or no vapor pressure over a wide temperature range. The salts of this invention are thus an important breakthrough since they can serve as high-performing monopropellants which are not complex mixtures and have no vapor toxicity. Such salts also find use as munitions, liquid explosives, reaction media for the synthesis of other high-energy materials, and as plasticizers.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERCIA AS REPRESENTED BY THE SEC OF THE AIR FORCE
Sealing material to metal bonding compositions and methods for bonding a sealing material to a metal surface
A downhole well tool having a component with a metal surface to which a sealing material is adhered is disclosed. The sealing material is bonded to the metal surface through the use of an energetic material disposed between the sealing material and the metal surface. Upon activation or initiation of the energetic material, the sealing material becomes bonded to the metal surface. A plastic layer may be disposed between the sealing material and the metal surface to facilitate bonding sealing material to the metal surface. The energetic material is used to bond the plastic layer to the metal surface and may be used to bond the plastic layer to the sealing material.
Owner:BAKER HUGHES INC
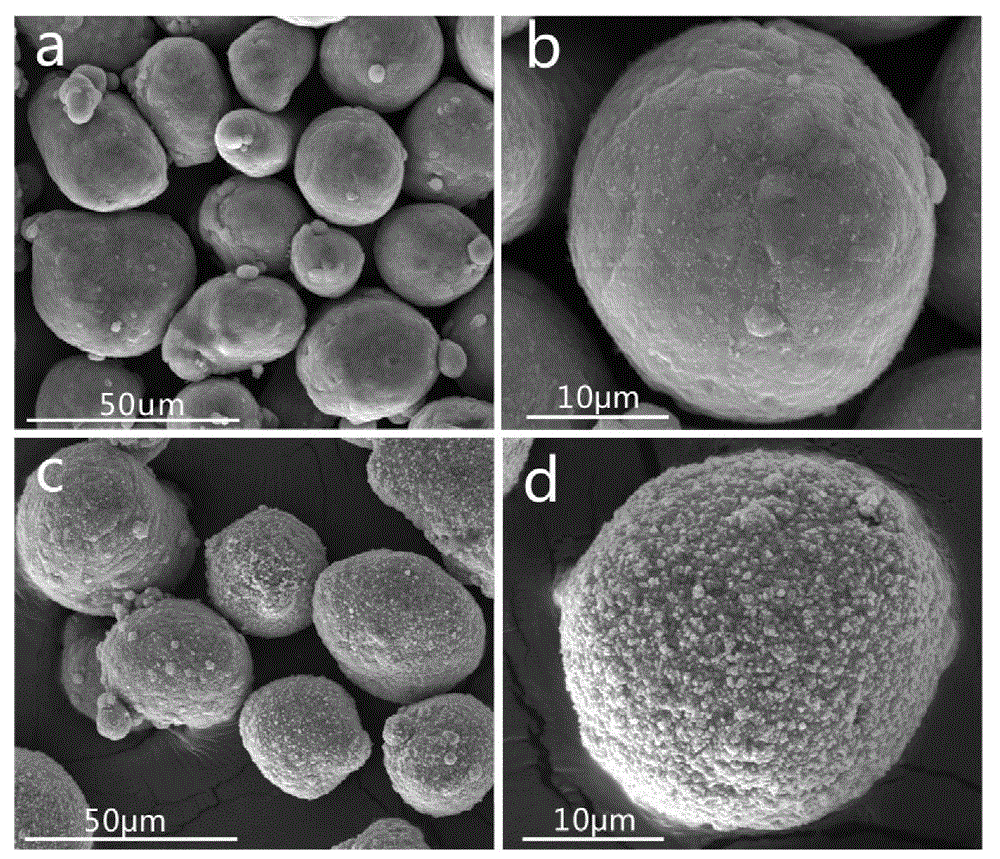
Polydopamine interface regulation-based nitramine explosive modified aluminum powder and preparation method thereof
ActiveCN109704896AEvenly distributedCompact structureExplosive working-up apparatusHigh energyAdhesive
The invention relates to a polydopamine interface regulation-based nitramine explosive modified aluminum powder and a preparation method thereof. Micron-sized crystals containing an aluminum powder and a nitramine explosive are prepared by an anti-solvent process, uniform distribution and close contact of the aluminum powder and the nitramine explosive are achieved, and the crystals have the advantages of compact structure, high thermal stability and high density. The adoption of the polydopamine interface regulation-based nitramine explosive modified aluminum powder in an aluminum-containingexplosive reduces the content of an adhesive and additives in a formula, increases the mass fraction of the main explosive, and achieves the purpose of developing high-energy explosives. In addition,a part of the energetic material adopted to replace RDX, HMX, CL-20 and other nitramine explosives in traditional solid propellants improves the energy level of the propellants, improves the problem of too large viscosity of a propellant slurry, caused by the addition of nano-aluminum powder or high-aluminum powder, and optimizes the preparation technology of the propellants.
Owner:NORTHWESTERN POLYTECHNICAL UNIV +1
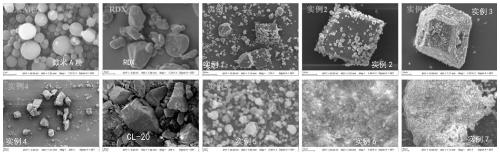
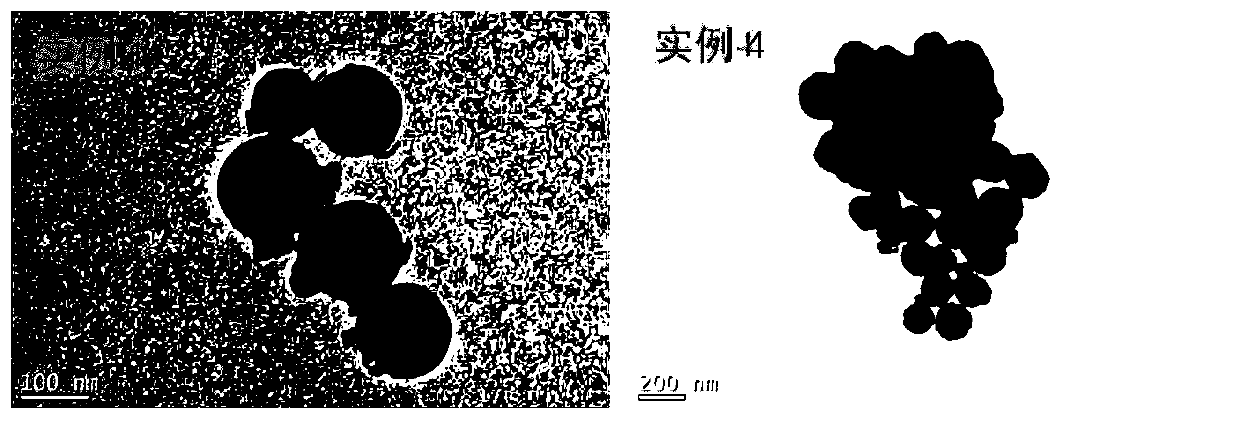
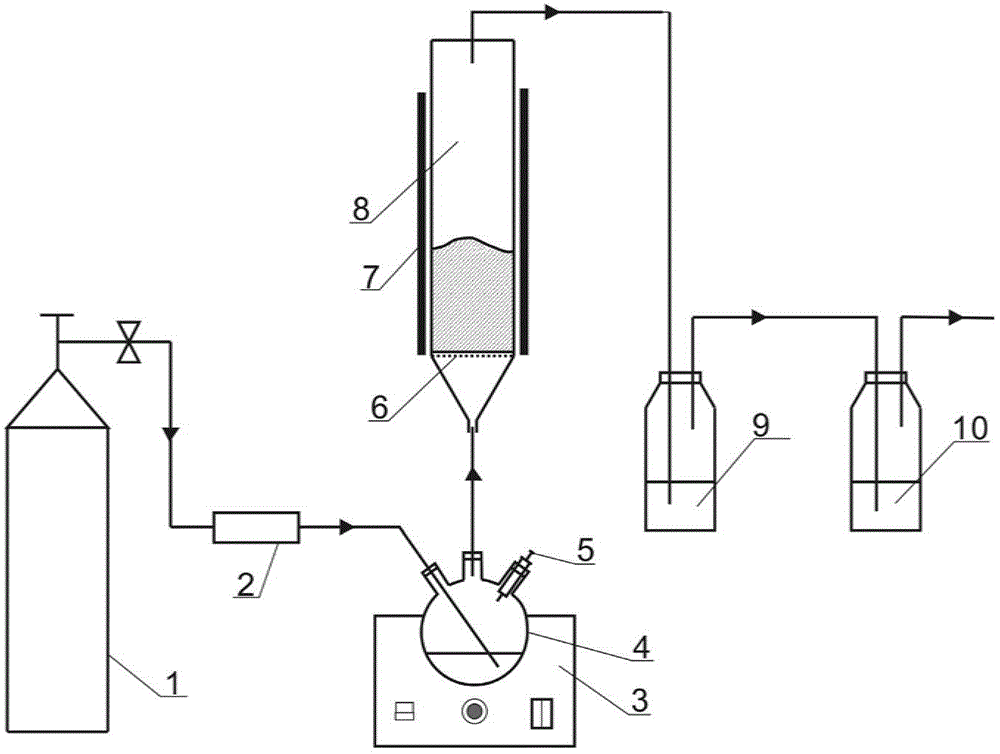
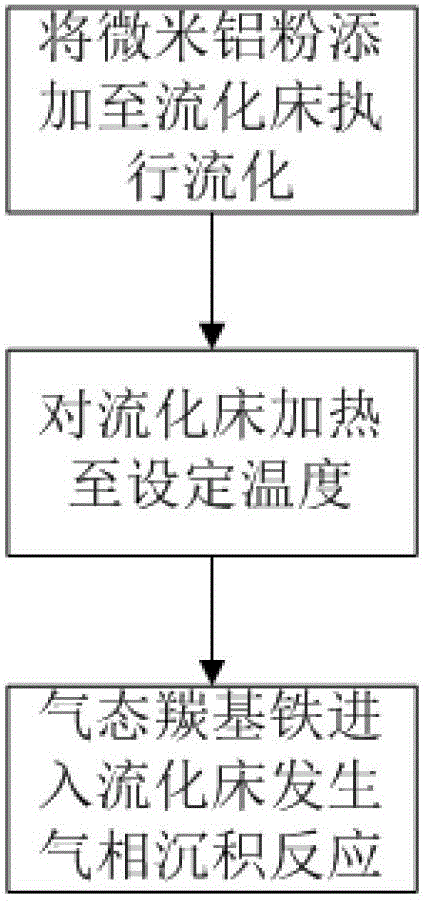
Preparation method of iron-clad aluminum type composite powder and product thereof
InactiveCN103060770AIncrease contact areaHigh mass/heat conversion rateExplosivesChemical vapor deposition coatingSolventAluminum composites
The invention discloses a preparation method of iron-clad aluminum type composite powder. The method comprises the following steps of: (a) adding micron aluminum powder to a fluidized bed and introducing a carrier gas, thereby performing a fluidization process of aluminum powder; (b) continuously introducing the carrier gas to empty air in the fluidized bed, and heating the fluidized bed so as to reach a predetermined temperature; and (c) adding carbonyl iron to a bubbler and heating to volatile carbonyl iron to be brought into the fluidized bed by the carrier gas to generate a vapor deposition reaction, thereby producing an iron-clad aluminum composite powder product. The invention further discloses the corresponding composite powder product and use of the composite powder product. Through the invention, the iron-clad aluminum type composite powder can be prepared without requiring a solvent, later separating and drying steps are saved, the prepared product has a uniform and dense coating layer, excellent thermal properties and good heat release concentration, and is especially applicable to the uses of the aspects in processing energetic materials, laser surface cladding and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
Multilayered microcavities and actuators incorporating same
ActiveUS20080276819A1Innovative designCost-effective manufacturingPrinted electric component incorporationBlasting cartridgesActuatorBridgewire
A microcavity structure. In an illustrative embodiment, the microcavity structure includes a first substrate, which has a region of interest. A second substrate with a perforation therein is bonded to the first substrate. The perforation coincides with the region of interest. In a specific embodiment, the first substrate is implemented via a Printed Circuit Board (PCB). The region of interest includes one or more circuit components, including an actuator, such as a bridgewire, thereon or therein. A smoothing layer is included between the PCB and the actuator. A bonding gasket adheres the first substrate to the second substrate. The perforation accommodates energetic material that is selectively ignited via the actuator.
Owner:DESAI AMISH +4
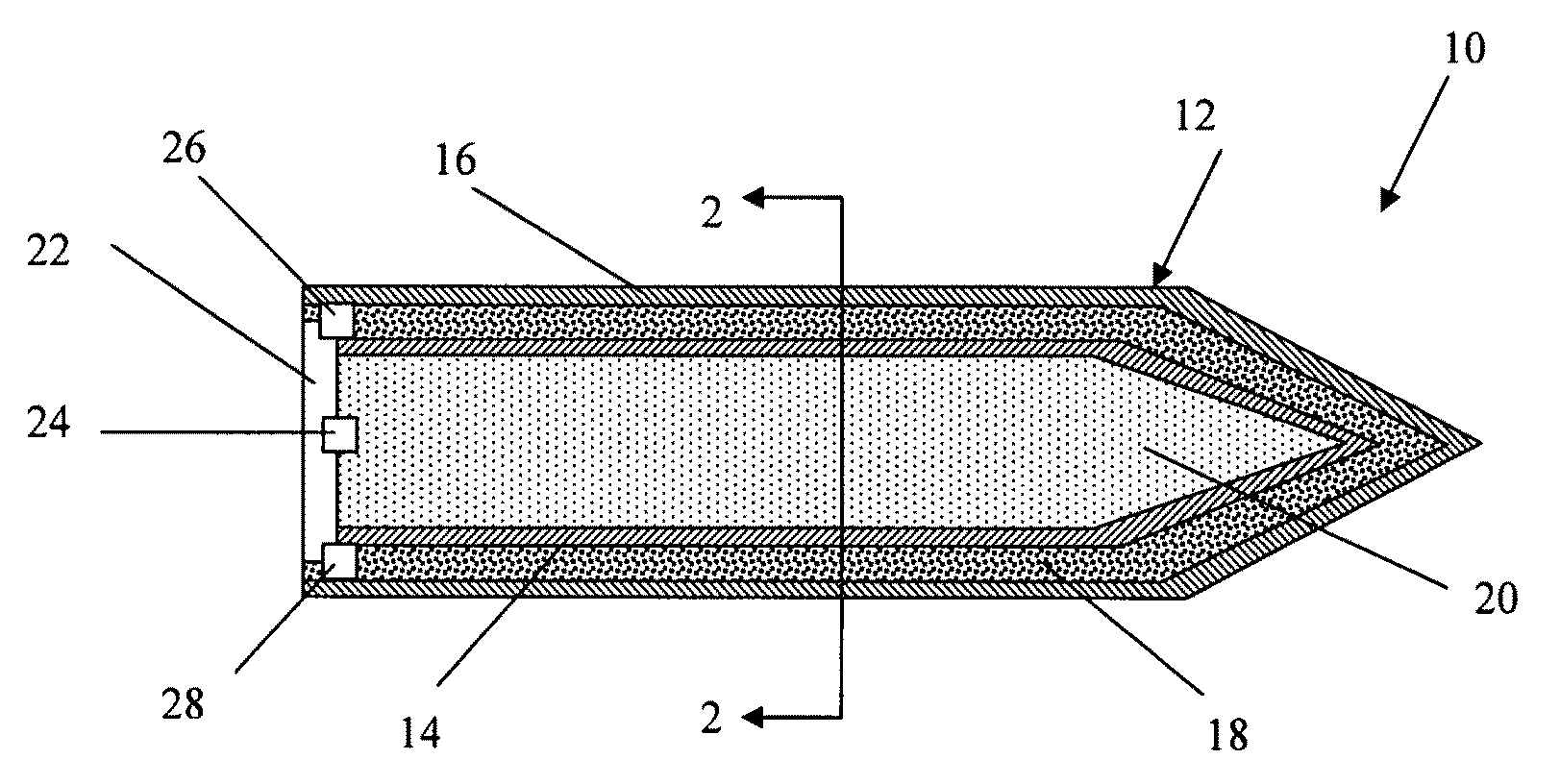
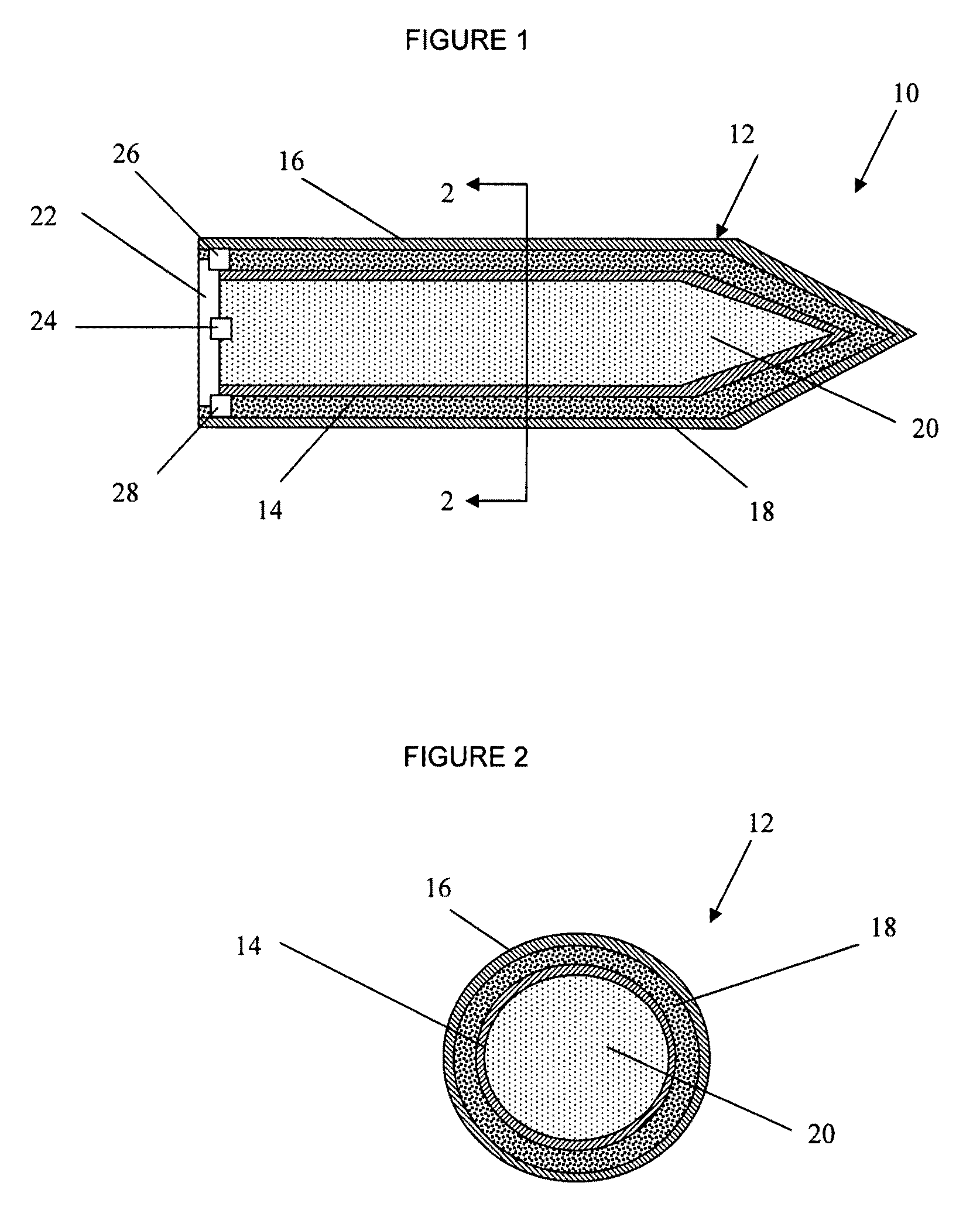
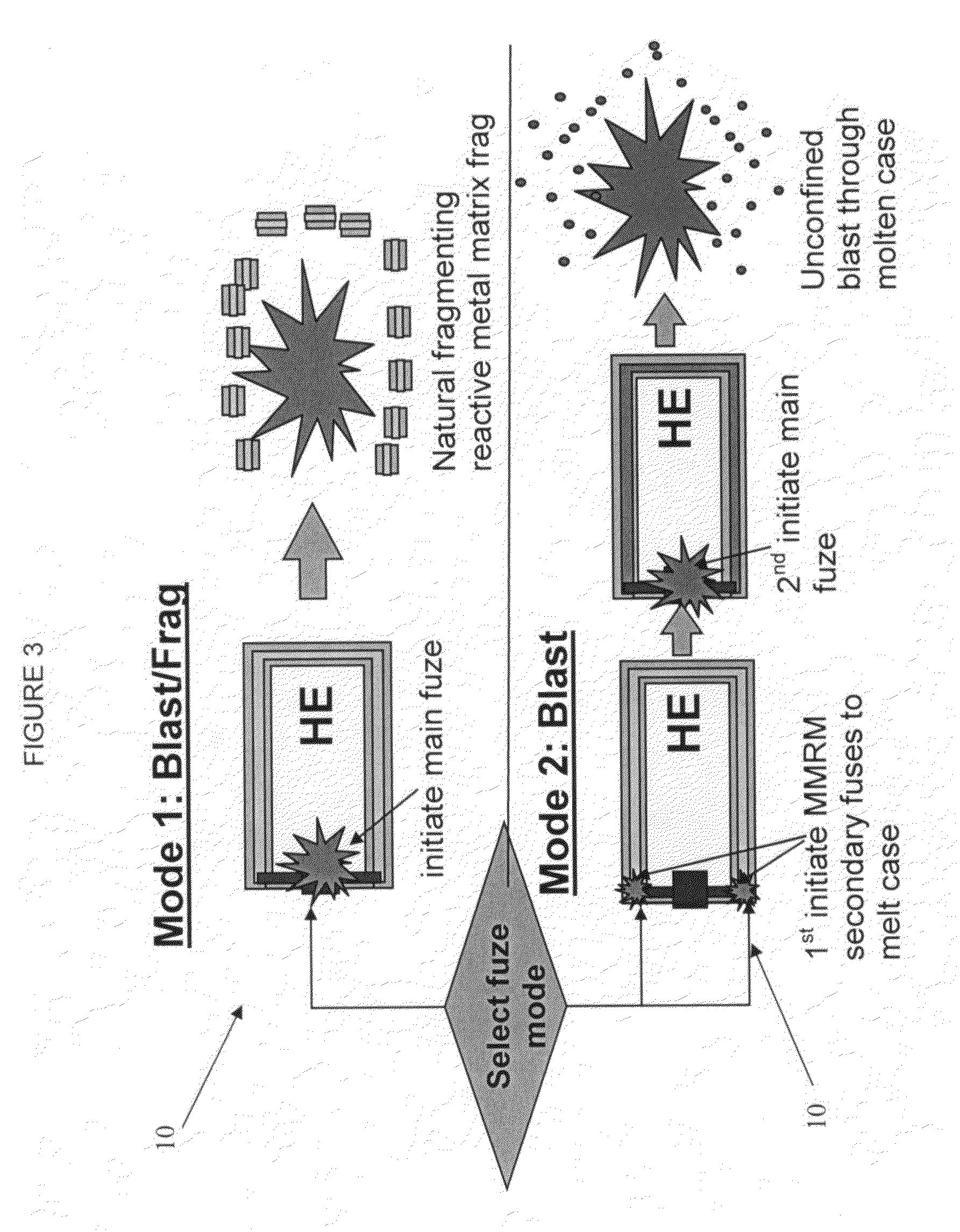
Selectable effect warhead
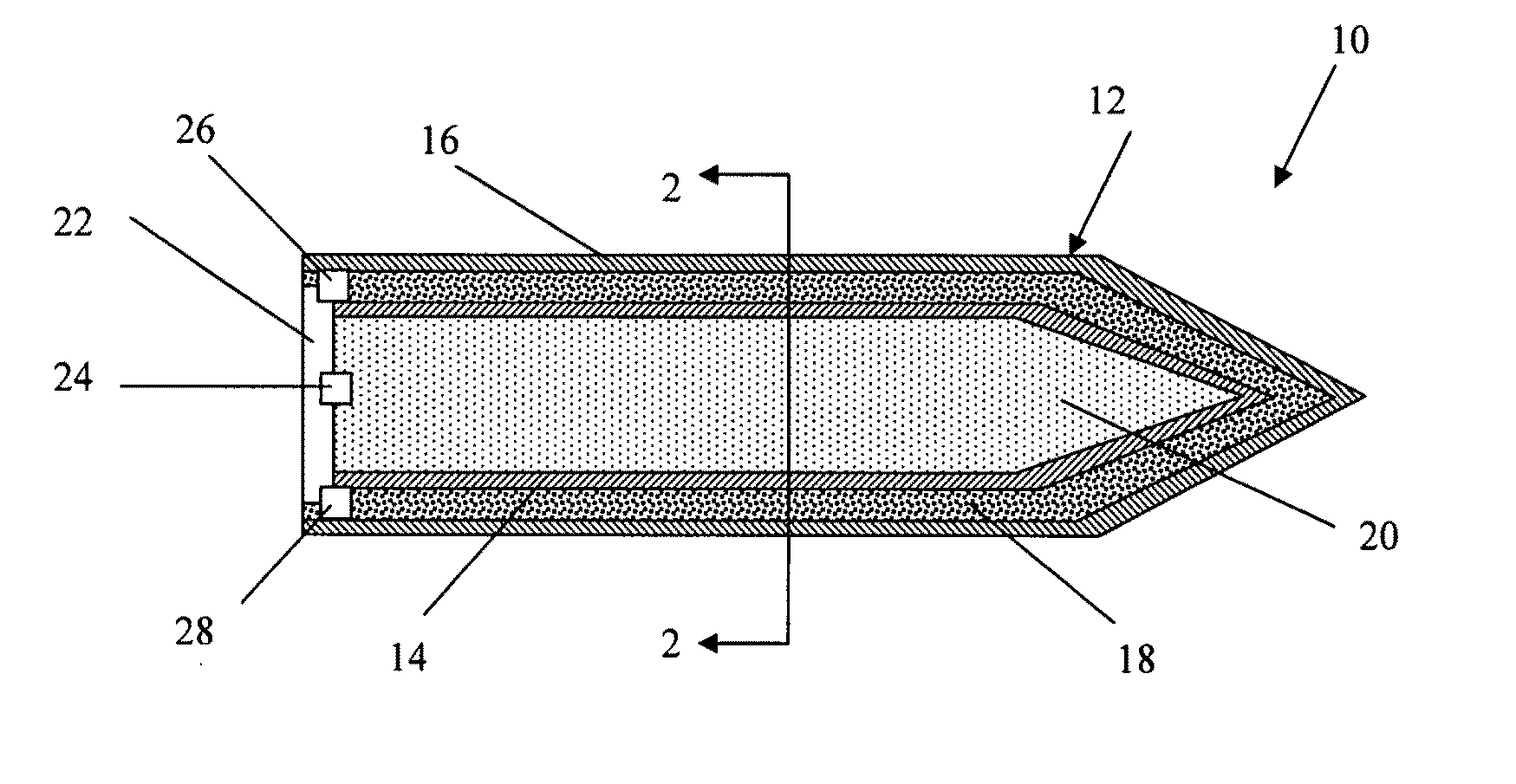
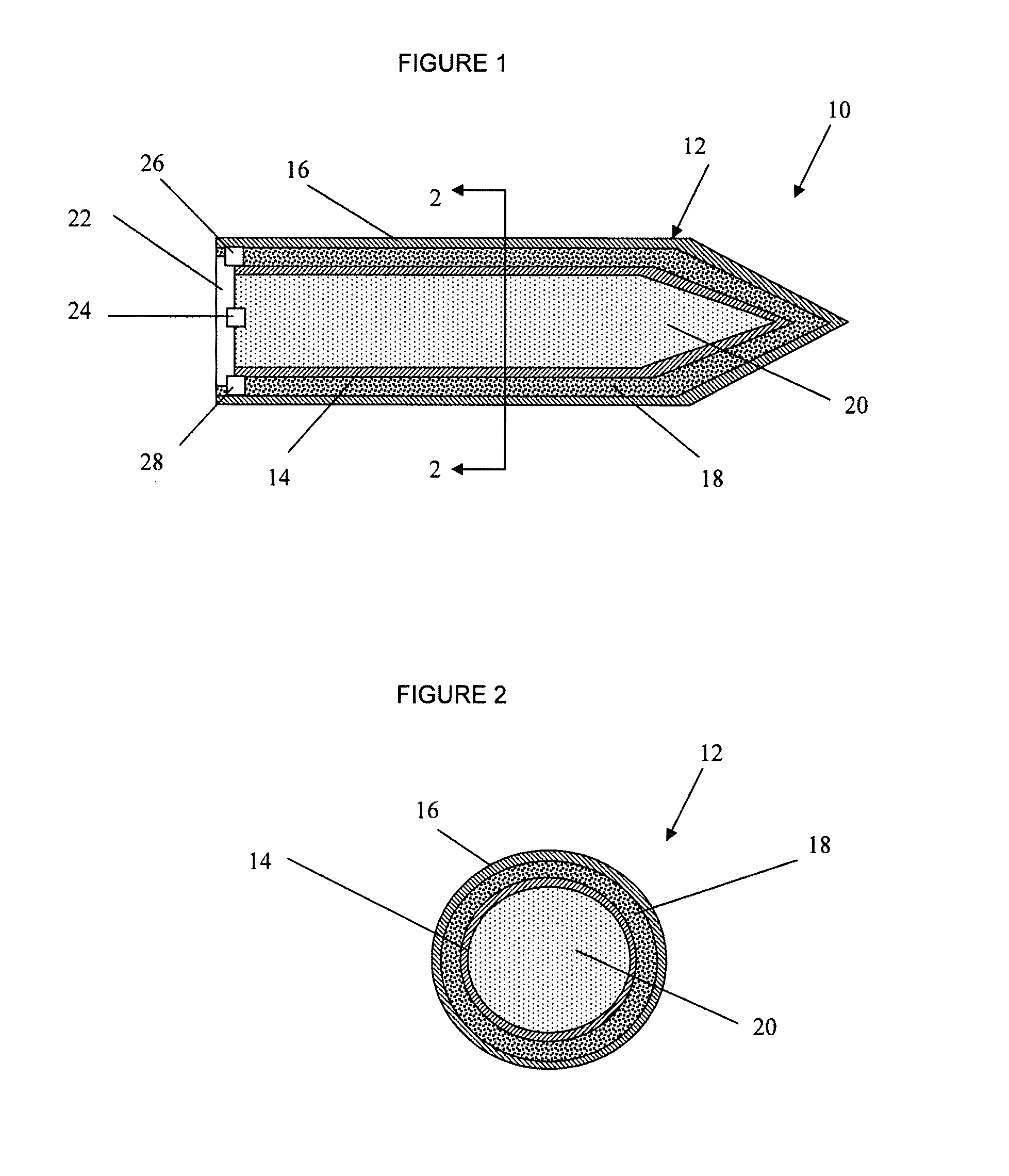
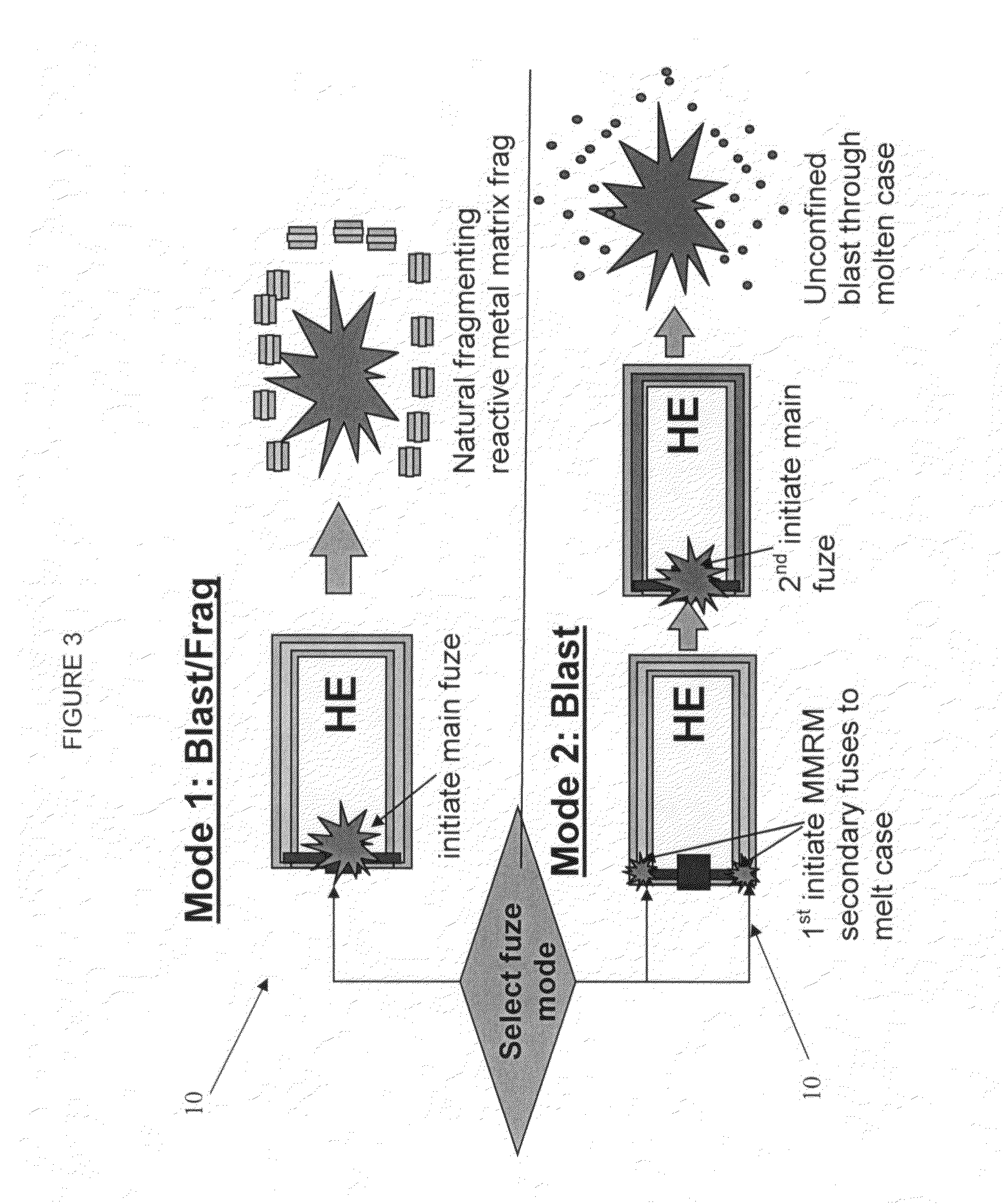
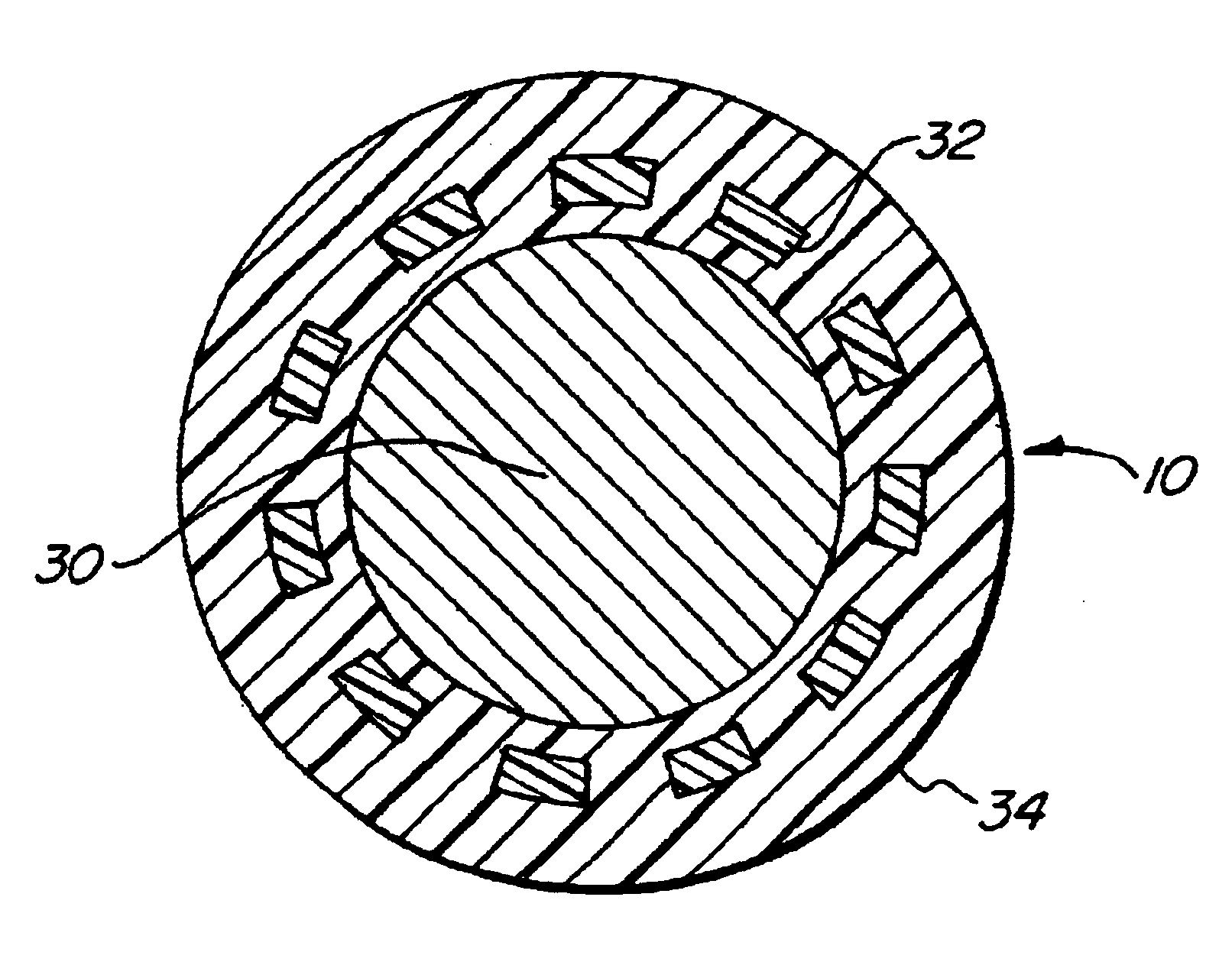
ActiveUS7845282B2Reduce impactSelectively eliminatedAmmunition projectilesProjectilesBlast effectsOperation mode
A munition includes a casing, the casing formed at least in part from a material comprising (i) a meltable or phase-changing material, and (ii) an energetic material; an explosive payload contained within the casing; and a fuze arrangement, the fuze arrangement comprising a main fuze configured and arranged to ignite the high explosive, and at least one secondary fuze configured and arranged to cause the casing material to melt or undergo a phase change. A method of selectively altering the mode of operation of a munition includes: forming a casing, the casing comprising a material comprising (i) a meltable or phase-changing material, and (ii) an energetic material; introducing an explosive payload into the casing; providing a fuze arrangement comprising a main fuse and at least one secondary fuze configured and arranged to cause the casing material to melt or undergo a phase change; and selectively activating the main fuze and the at least one secondary fuze in a manner that provided at least a first and a second mode of operation, the first mode of operation comprising blast coupled with fragmentation effects, and the second mode of operation comprising mainly blast effects.
Owner:LOCKHEED MARTIN CORP
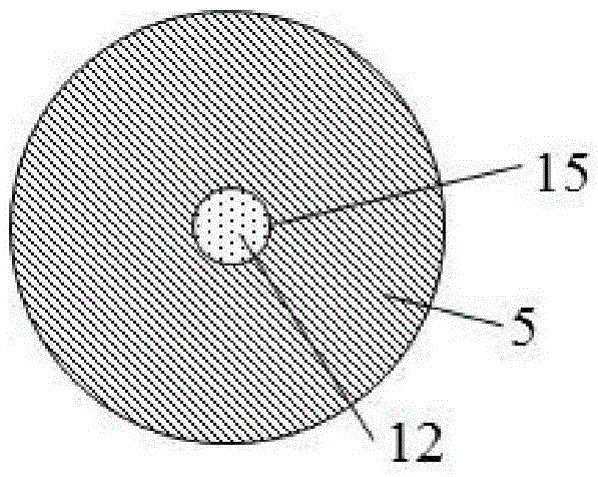
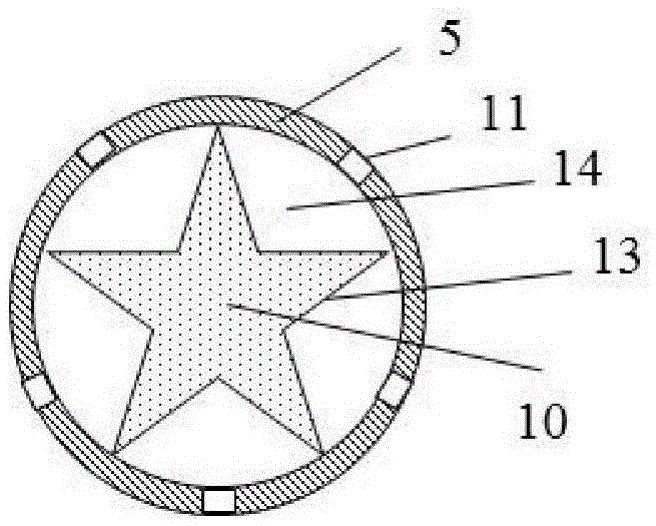
Energetic material for cigarette and low-temperature heated cigarette
ActiveCN105533800AAvoid the problem of high generation volumeThere will be no problem of collapsing and falling offCigar manufactureSmoke/mist generationCombustionSource area
The invention discloses an energetic material for a cigarette and a low-temperature heated cigarette, wherein the energetic material for the cigarette is processed by mixing the following raw materials in percentage by mass: 40-55% of an oxidizing agent, 25-35% of combustible agent, 5-20% of a binding agent and 3.5-20% of other adjuvant materials; a pentagram-shaped copper barrel (13) is arranged in a heat source area (1) of the low-temperature heated cigarette; five points of the pentagram-shaped copper barrel (13) are connected to the wall of an insulating ceramic sleeve (5); and the energetic material (10) fills the pentagram-shaped copper barrel (13). The energetic material disclosed by the invention is relatively easily combustible, and the combustion speed and temperature are controllable; heat energy released from the combustion is effectively transmitted to air in the modes of transfer and radiation only; and formed hot air flows to an aerosol generating agent and tobacco shreds and is capable of heating the aerosol generating agent and the tobacco shreds, so that smoke is generated.
Owner:CHINA TOBACCO ANHUI IND CO LTD
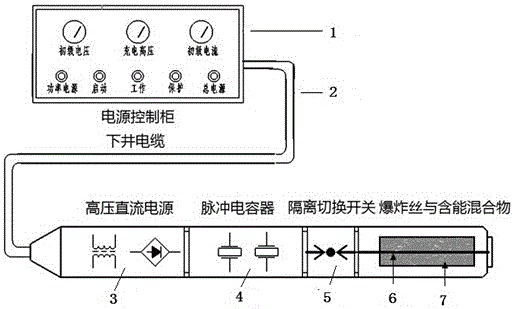
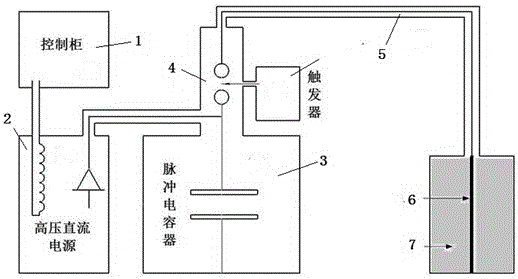
Method driving energetic electrode to release energy and produce shock waves by high-voltage discharge
The invention provides a method driving an energetic material to release energy and produce shock waves by high-voltage pulse discharge plasmas underground. A cylinder (an energetic electrode for short), clamped between a high-voltage electrode and a ground electrode of a device and made by a central metal wire and a composite energetic material, is triggered by plasmas produced by high-power pulse discharge to rapidly react. Within 50 microseconds, energy is instantly released, and a large quantity of gas is produced. High-energy shock waves are produced, the high-energy shock waves act on the stratum with oscillation caused by air bubble compression and expansion, and in this way, the purposes of relieving blockage, removing scale, forming cracks, improving seepage conditions, improving permeability and communicating microchannels underground are achieved.
Owner:XIAN GUANTONG ENERGY TECH
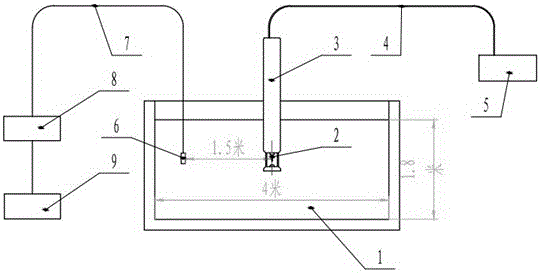
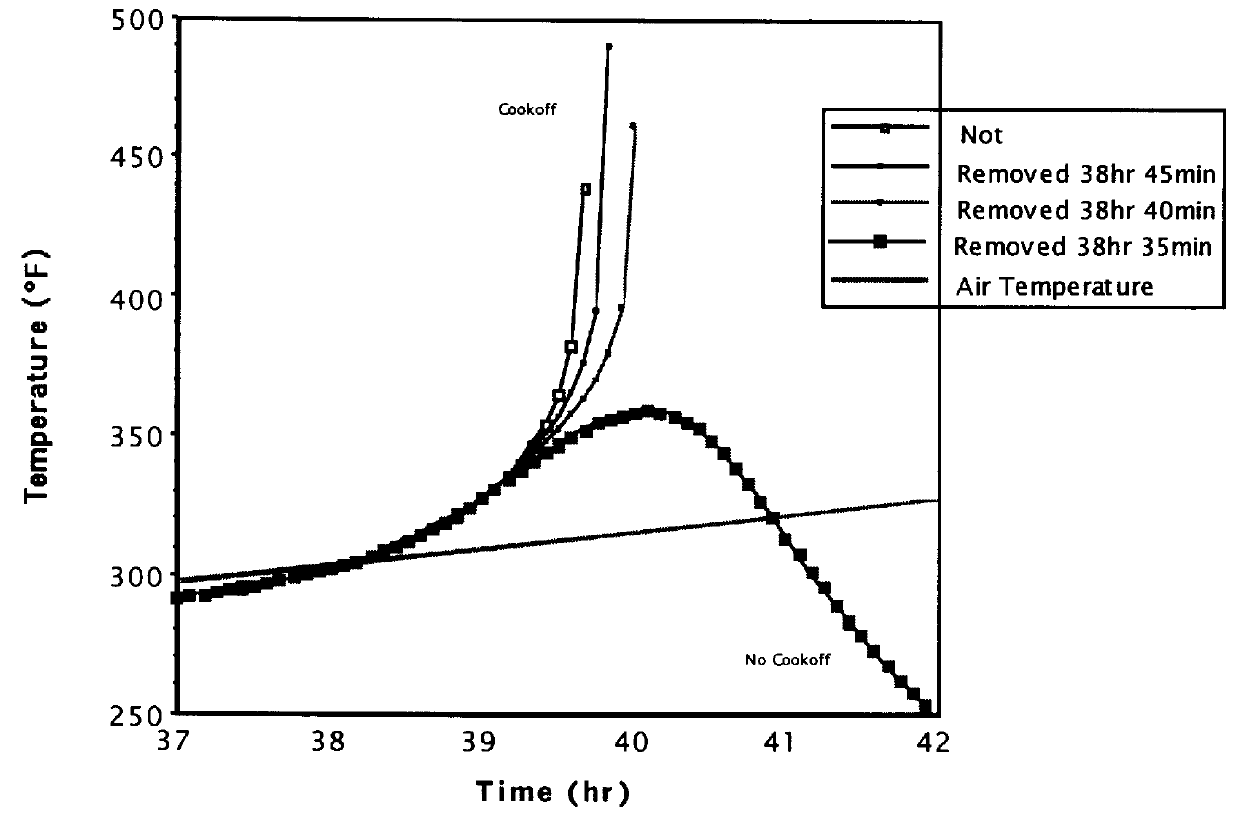
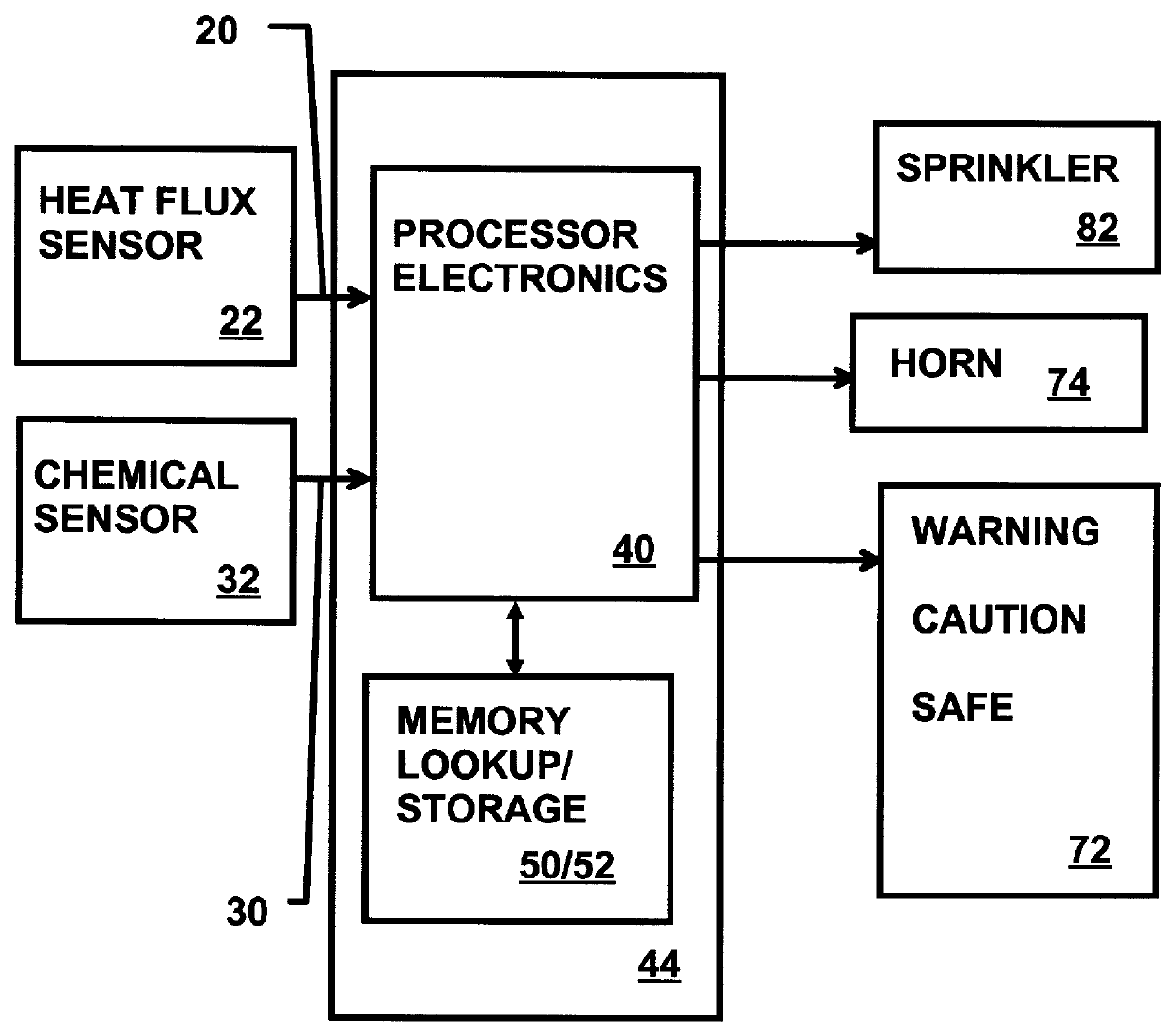
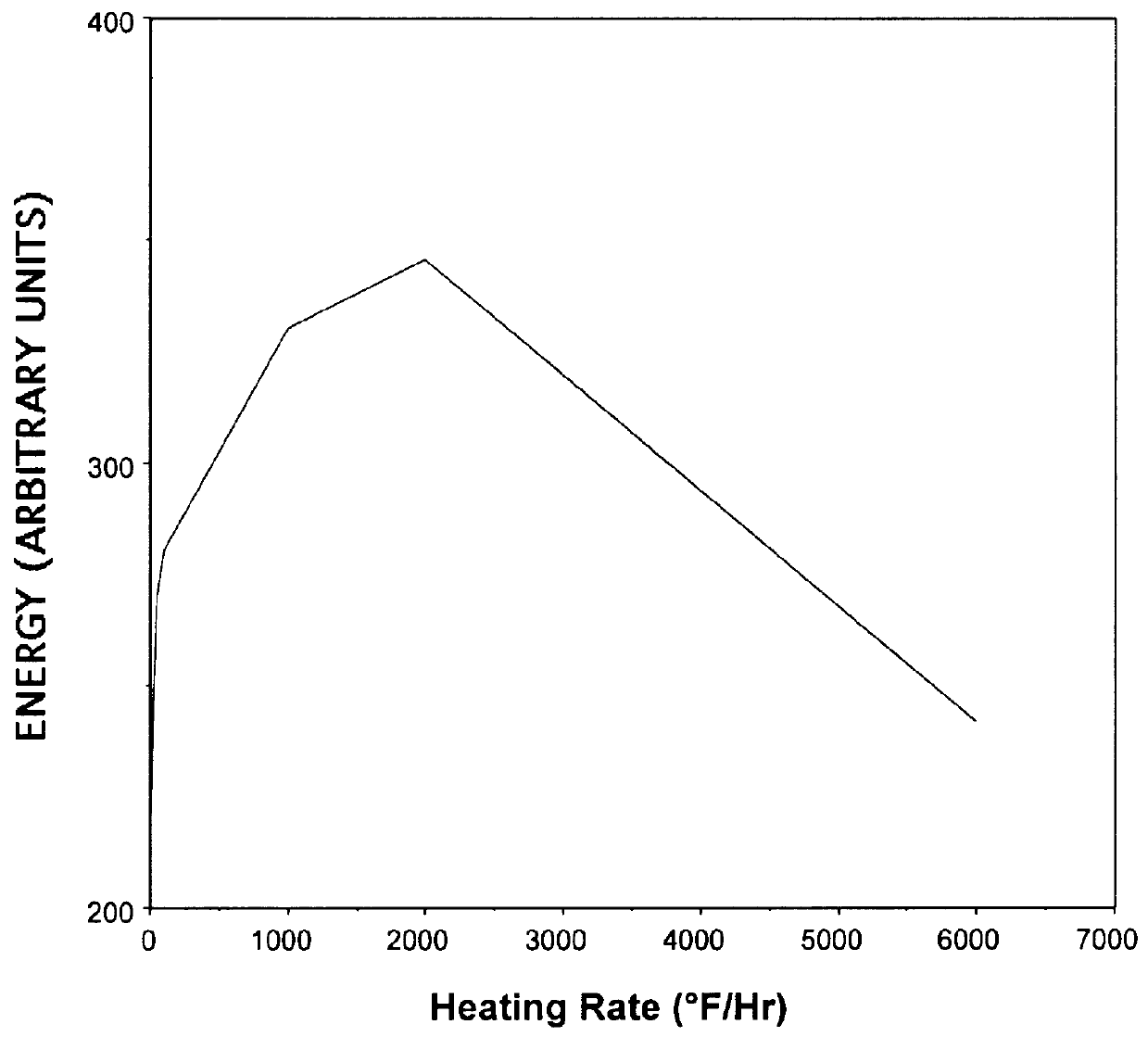
Munitions cook-off warning system
A process for indicating the likelihood of cook-off for energetic materials by calculating the heat flux and chemical venting relative to known critical parameters for the energetic materials. The process further may include an alarm.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Selectable effect warhead
ActiveUS20100282115A1Reduce impactSelectively eliminatedAmmunition projectilesProjectilesExplosive AgentsBlast effects
A munition includes a casing, the casing formed at least in part from a material comprising (i) a meltable or phase-changing material, and (ii) an energetic material; an explosive payload contained within the casing; and a fuze arrangement, the fuze arrangement comprising a main fuze configured and arranged to ignite the high explosive, and at least one secondary fuze configured and arranged to cause the casing material to melt or undergo a phase change. A method of selectively altering the mode of operation of a munition includes: forming a casing, the casing comprising a material comprising (i) a meltable or phase-changing material, and (ii) an energetic material; introducing an explosive payload into the casing; providing a fuze arrangement comprising a main fuse and at least one secondary fuze configured and arranged to cause the casing material to melt or undergo a phase change; and selectively activating the main fuze and the at least one secondary fuze in a manner that provided at least a first and a second mode of operation, the first mode of operation comprising blast coupled with fragmentation effects, and the second mode of operation comprising mainly blast effects.
Owner:LOCKHEED MARTIN CORP
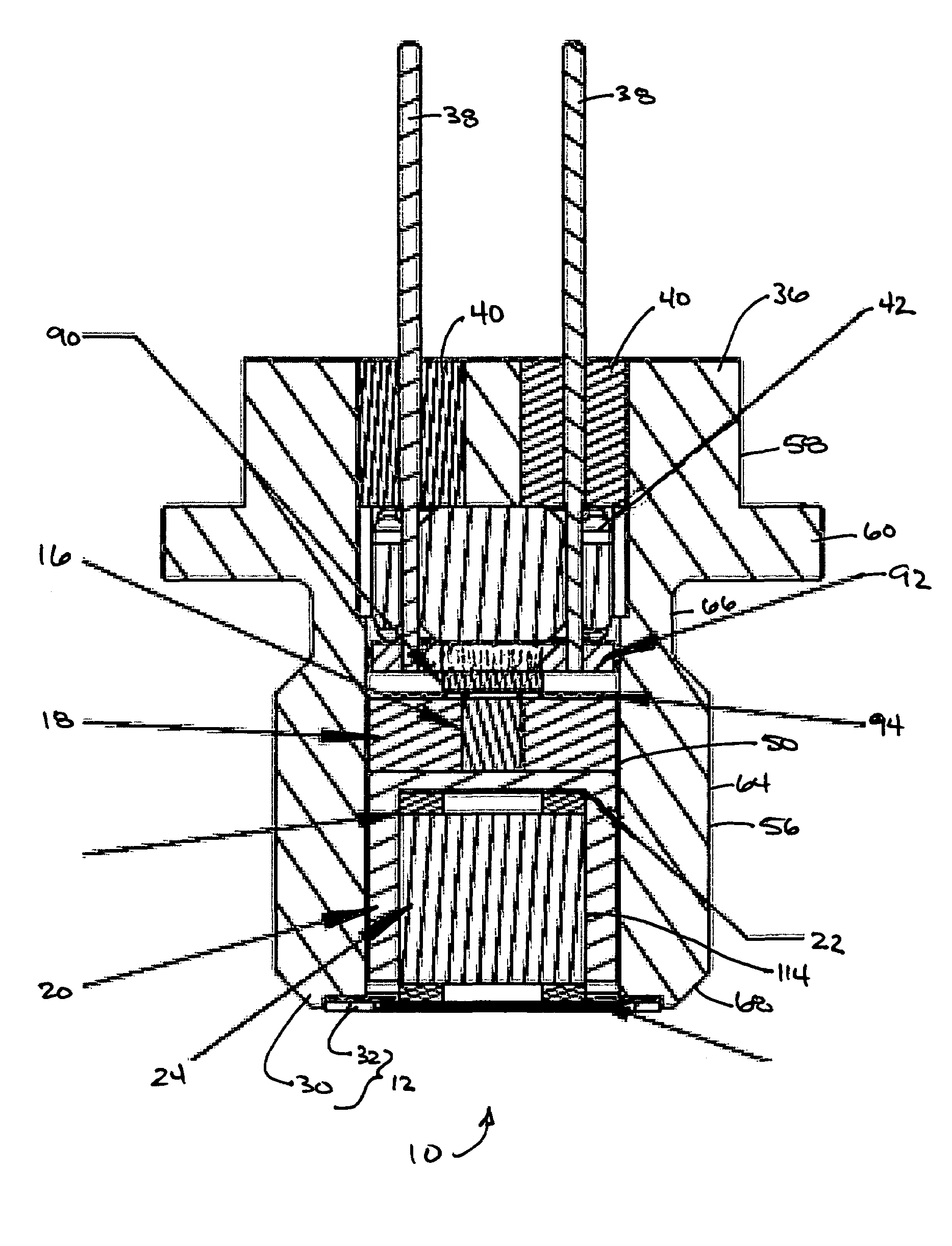
Rigid reactive cord and methods of use and manufacture
The present invention provides a rigid reactive cord such as a detonating cord (10) that includes a core (38) of an energetic material and a rigid non-metal sheath (40) disposed about the core. The cord (10) is sufficiently rigid so that it can be inserted through a material to be fractured or through passages formed therein. A method of using the rigid reactive cord for the removal of combustion residue from boiler tubes is also presented.
Owner:DYNO NOBEL INC
Preparation method of Al/W/PTFE energetic material
The invention relates to a preparation method of an Al / W / PTFE energetic material and belongs to the field of energetic materials. The preparation method orderly comprises raw material powder preparation, surface modification, mixed powder drying, compression molding and sintering molding. The preparation method utilizes conventional Al, W and PTFE powder as raw materials which are easily purchased and have a low cost. The preparation method has simple processes and a low cost and is suitable for batch production. Compared with the traditional Al / PTFE energetic material (Al: 26.4wt% and PTFE: 73.6wt%), the Al / W / PTFE energetic material obtained by the preparation method has higher compressive strength, density and reaction threshold. When a mass fraction of W of the energetic material is 60%, compared with the traditional Al / PTFE energetic material, the Al / W / PTFE energetic material improves density by 88%, dynamic strength limit by 21% and a reaction threshold by 24%.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
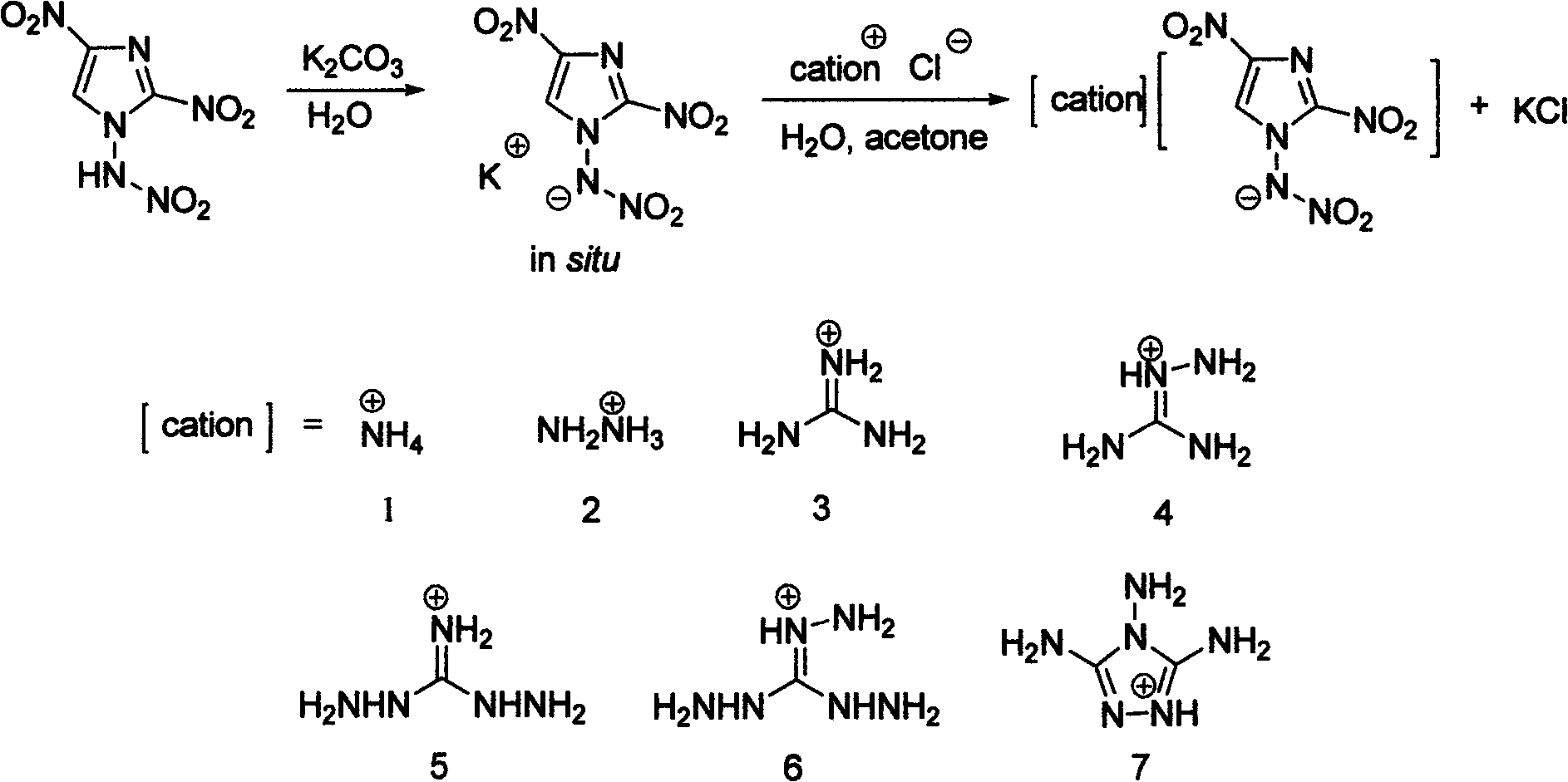
Energetic ion salts of 1-nitramine-2, 4-dimetridazloe and preparation method thereof
InactiveCN103483264AExcellent detonation speed and pressure performanceThe synthesis method is simpleOrganic chemistryOrganic compound preparationNitroimidazoleHydrazine compound
The invention discloses energetic ion salts of 1-nitramine-2, 4-dimetridazloe and a preparation method thereof, and belongs to the technical field of energetic materials. The synthetic method is as follows: dissolving the 1-nitramine-2, 4-dimetridazloe in deionized water to obtain a pale yellow clear liquid, adding with stirring 0.5 time molar equivalent of potassium carbonate at room temperature for in-situ generation of 1-nitramine-2, 4-dimetridazloe potassium salt, then adding one time molar equivalent of ammonium chloride, hydrazine hydrochloride, guanidine hydrochloride, monoaminoguanidine hydrochloride, diaminoguanidine hydrochloride, triaminoguanidine hydrochloride and 3, 4, 5-triamino-1, 2, 4-triazole hydrochloride, stirring to precipitate a pale yellow solid precipitate, after about 1 hour of reaction, filtering the pale yellow precipitate, further recrystallizing a coarse product by use of an acetone and diethyl ether mixed solvent to obtain a pure product. The synthetic method of the invention is simple, mild in condition and high in yield, and is environmental friendly due to using of the deionized water as a solvent. The density of involved seven salts is 1.70-1.93g cm<-3>, the detonation velocity calculated by EXPLO software is between 8370 and 9209 m s<-1>, the detonation pressure is between 29.3 and 40.5 GPa, the actually measured impact sensitivity is 4-40J, the detonation performance is excellent, and the energetic ion salts are potential energetic materials.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com