Use of aluminum in perforating and stimulating a subterranean formation and other engineering applications
a technology of engineering applications and perforation, applied in the field of use of aluminum, can solve the problems of crushing the perforation zone and initializing a multitude of cracks, and achieve the effect of enhancing mechanical effects and increasing energy outpu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
by Detonation of an HE / Al Mixture
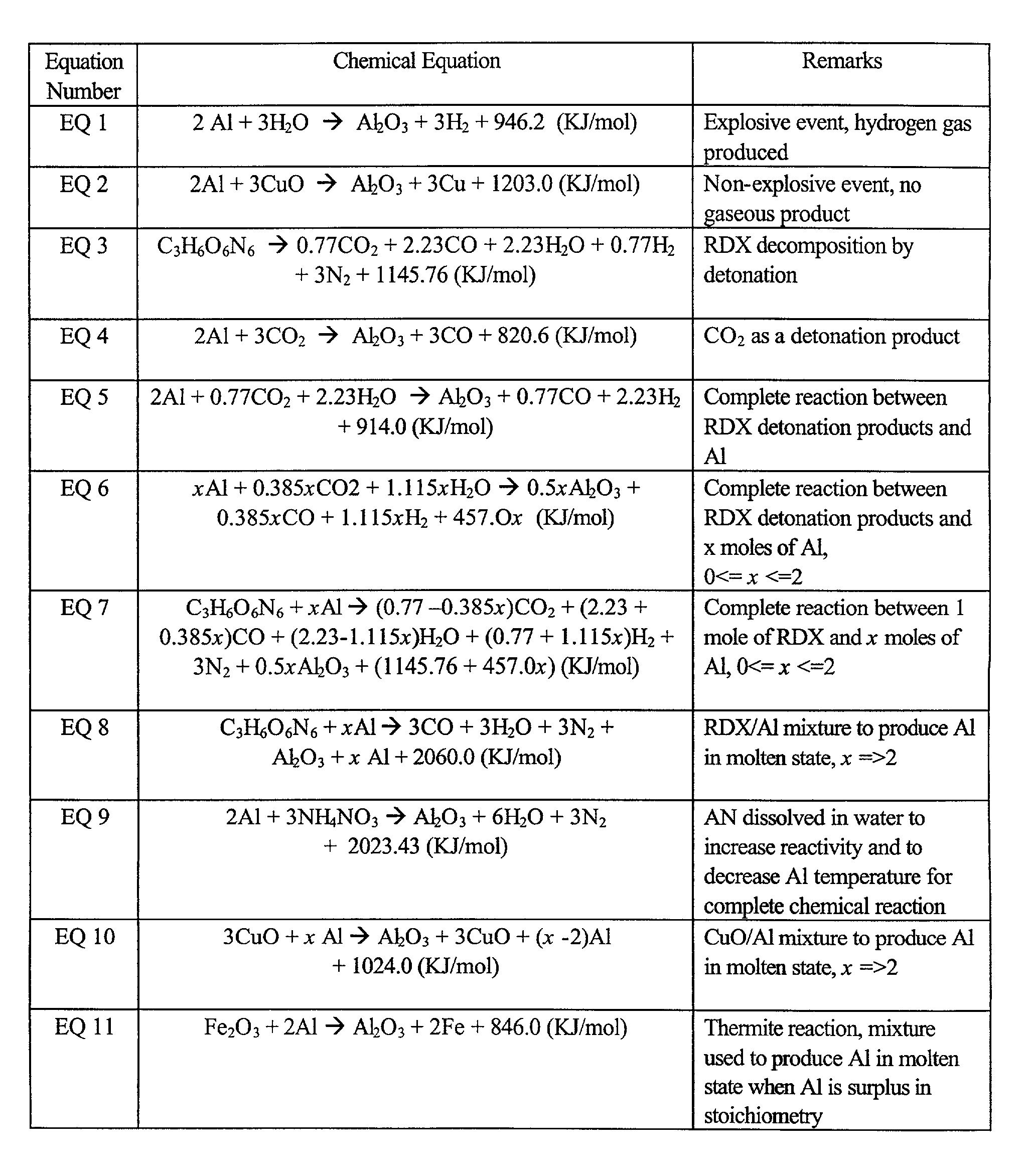
[0078]When aluminum powder is mixed with a high explosive, upon detonation of the mixture there are two energy sources to heat the reaction products to a high temperature. One is the detonation heat, or the heat released by the detonation decomposition of the high explosive itself; the other is that from the reactions between the detonation products of the said high explosive and the aluminum powder. The high explosive used in the mixture is not necessarily rich in oxygen. As a matter of fact, for some commonly used high explosives like RDX, HMX and TNT, they have negative values in oxygen balance.
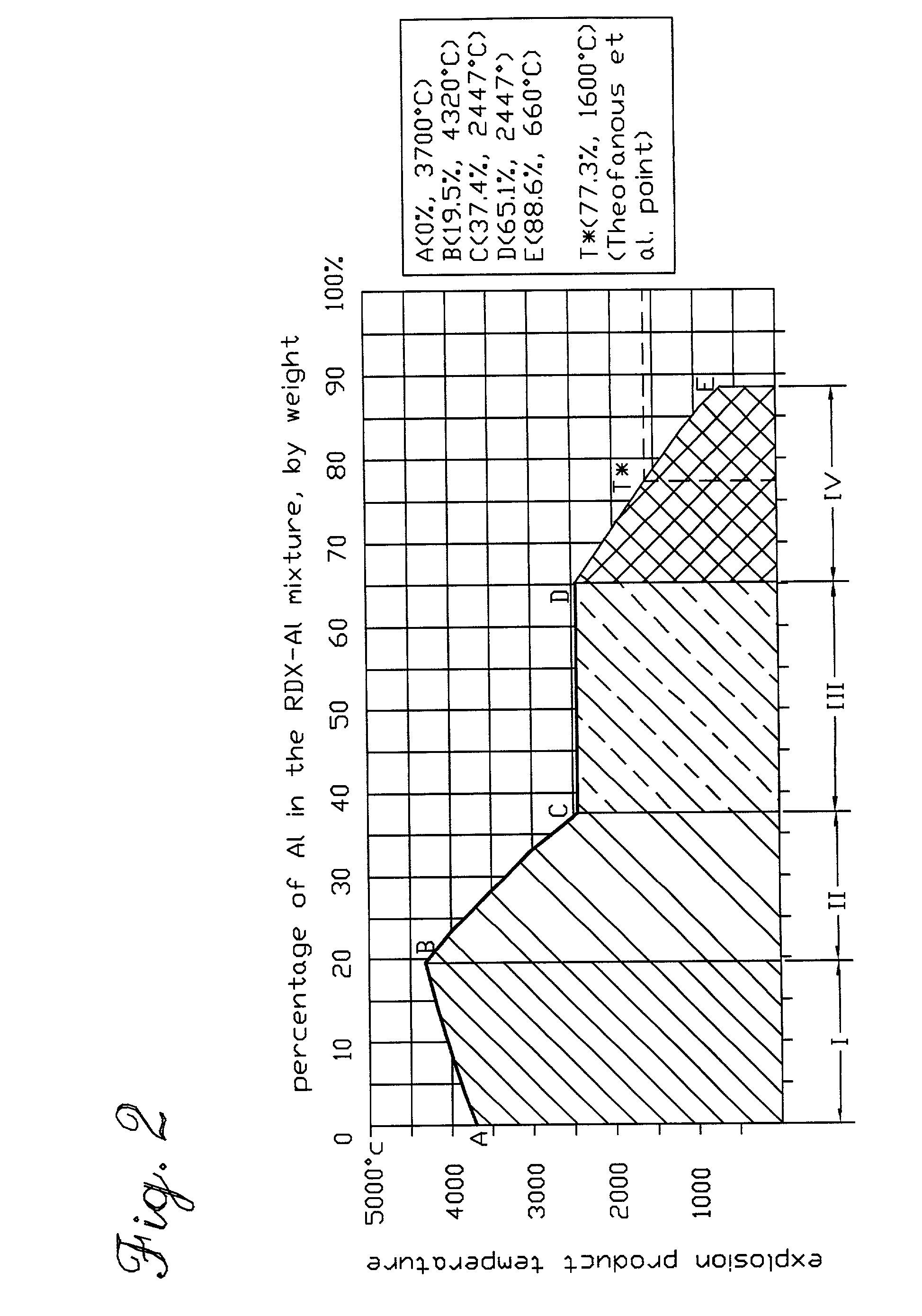
[0079]For high explosives, the temperature of its detonation products is normally in the order of 3000˜4000° C. In terms of heat generated by the detonation of explosives and the heat needed to melt aluminum, the heat of detonation for typical high explosives is in the order of 4˜6 KJ / gram and that the heat needed to melt 1 gram of aluminum is only 0.396 KJ...
embodiment 2
by Combustion or Detonation of an Oxidizer / Al Mixture
[0093]In the second embodiment of the of the present invention to produce Al in molten state, aluminum (preferably in powder form) is mixed with commonly used oxygen carrying reagents and aluminum is surplus in stoichiometry in the mixture. The oxygen carrying reagents, here generally referred as oxidizers, can be a metal oxide, a chlorate, perchlorate or nitrates that are compatible with aluminum powder, or even water or water solution of the said chlorate, perchlorate and nitrate. When such a mixture is used, the thermal energy to heat the reaction products along with the surplus aluminum may come from one or two sources depending on the oxidizer actually used and also the properties of the mixture (detonable or not). If the mixture is not detonable, the thermal energy released from the combustion reaction between aluminum and the oxidizer is the only energy source to heat the reaction products along with the surplus aluminum to...
embodiment 3
by Shocking / Heating Al
[0112]In addition to the two embodiments of chemical methods to produce molten aluminum described, there is still a third embodiment, namely the shock wave along with reaction products heating method. In this method, the aluminum material can be either in solid form, or be compacted aluminum powder. Often the shock wave alone from the detonation of an explosive charge may not have enough energy to melt aluminum, but if the aluminum material comes in contact with the explosive charge, the high temperature detonation products along with the said shock heating will put the aluminum material well above its melting point. Consequently, typical uses of this method can be to make shaped charge liners, cases, charge carriers completely or partly with aluminum. Then upon detonation of the explosive charge, the liner material projected into a perforation, the shaped charge case and carrier heated and broken in a well bore, can all be forced to interact with water and cau...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com