Sitafloxacin fumarate crystal form a and its pharmaceutical use
A technology of sitafloxacin and fumarate, applied in the preparation of carboxylates, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of low stability and low dissolution rate of preparations, and achieve high and stable dissolution rate. The effect of high performance and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
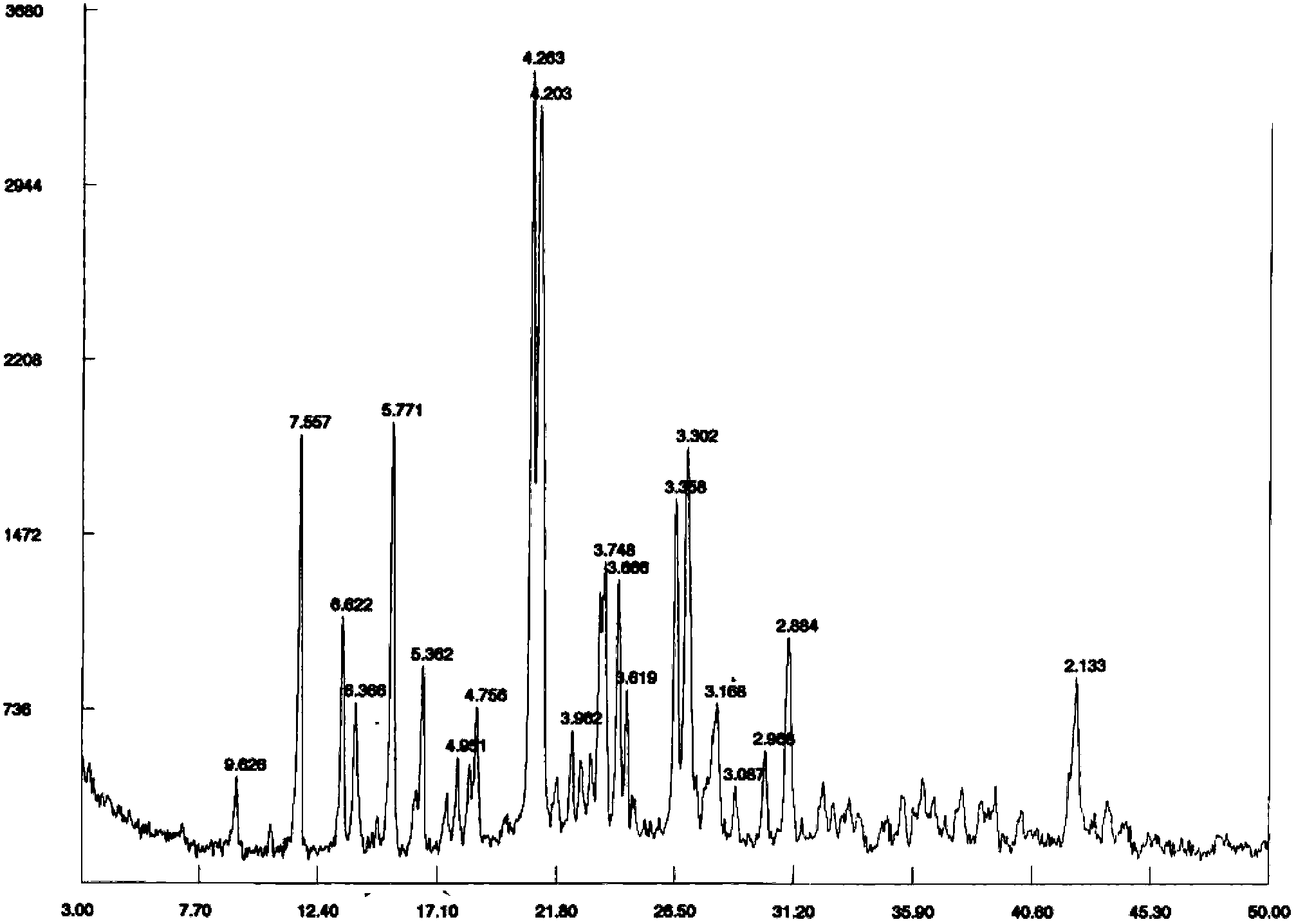
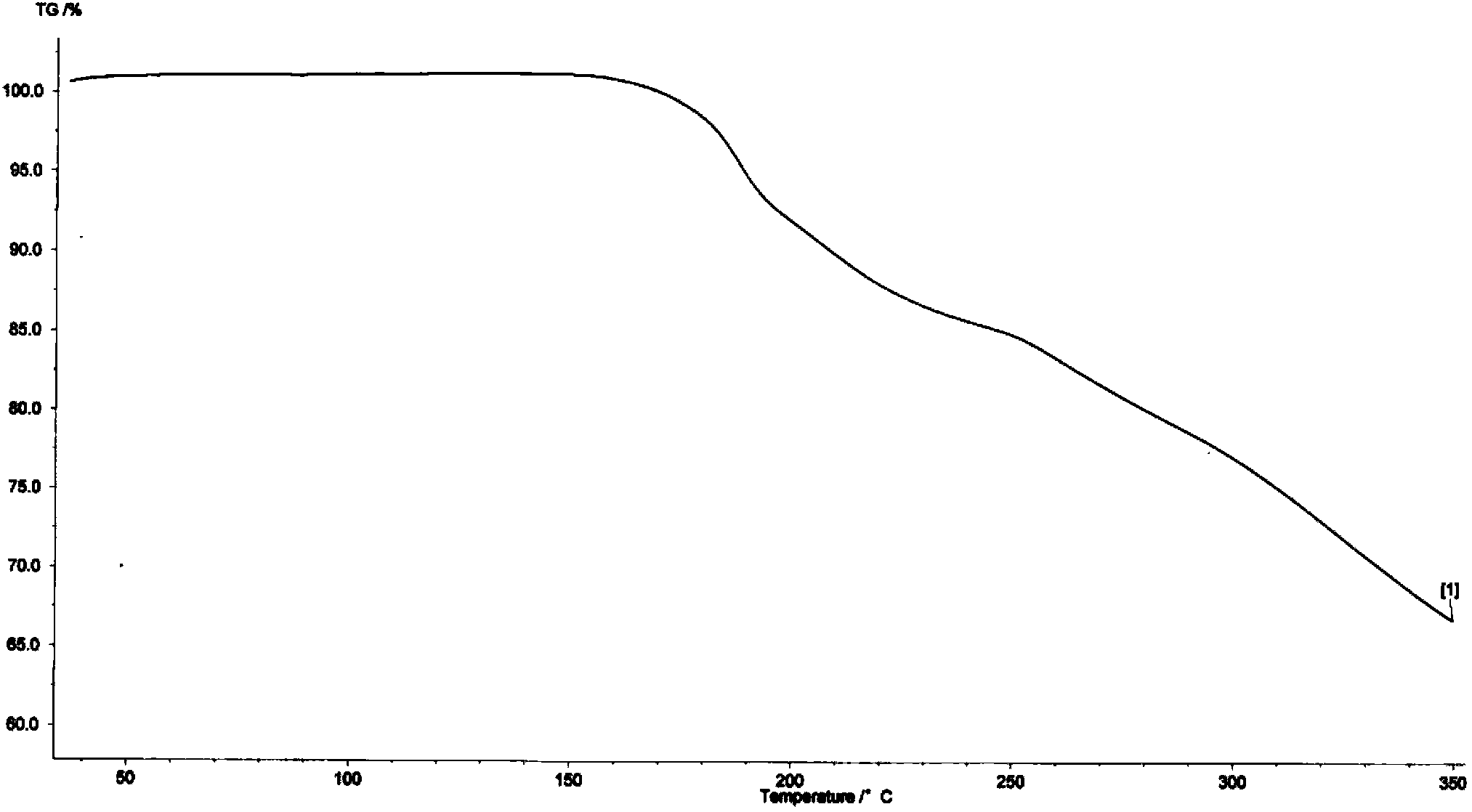
Image
Examples
Embodiment 1
[0033] Embodiment 1 Preparation of sitafloxacin fumarate crystal form A
[0034] Take 4.0g of sitafloxacin free base (containing 1.5 crystalline water, commercially available, Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd.) into a 1L three-necked bottle, add 200ml of acetone, stir and heat up to 55-60°C under reflux under dark conditions, After the solution was clear, it was filtered while it was hot, and the filtrate was reheated to reflux.
[0035] Dissolve 1.27g of fumaric acid in 44ml of acetone, add it dropwise to the reaction flask under reflux, and react under reflux for 1h after dropping, add 300ml of n-heptane under the condition of heat preservation, and precipitate a light yellow solid, lower it to room temperature, stir and crystallize for 1h, After filtering, the filtrate was washed with a small amount of n-heptane, and dried under vacuum at 35-40°C to obtain 4.75 g of a light yellow solid.
Embodiment 2
[0036] Embodiment 2 Preparation of sitafloxacin fumarate crystal form A
[0037] Take 4.0g of sitafloxacin free base (containing 1.5 crystal water, commercially available, Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd.) into a 1L three-necked bottle, add 200ml of methyl ethyl ketone, stir and heat up to 55-60°C under dark conditions, dissolve After clearing, filter while hot, and reheat the filtrate to reflux.
[0038] Dissolve 1.27g of fumaric acid in 44ml of methyl ethyl ketone and add it dropwise to the reaction flask under reflux. After the dropwise reaction, reflux for 50min. Add 300ml of n-hexane under heat preservation conditions to precipitate a light yellow solid. Cool down to room temperature, stir and crystallize for 1h, and filter , The filtrate was washed with a small amount of n-heptane, and dried under vacuum at 35-40°C to obtain 4.69 g of a pale yellow solid.
Embodiment 3
[0039] Embodiment 3 Preparation of sitafloxacin fumarate crystal form A
[0040] Take 8.0g sitafloxacin free base (containing 1.5 crystal water, commercially available, Beijing Zhongshuo Pharmaceutical Technology Development Co., Ltd.) into a 1L three-necked bottle, add 200ml of dichloromethane and anhydrous methanol mixed solvent (dichloromethane and The volume ratio of anhydrous methanol is 1:1), stirring and raising the temperature to 40-45°C and reflux under the condition of avoiding light. After dissolving, filter while it is hot, and reheat the filtrate to reflux.
[0041]Dissolve 2.12g of fumaric acid in 44ml of a mixed solvent of dichloromethane and anhydrous methanol (the volume ratio of dichloromethane to anhydrous methanol is 1:1), and add it dropwise into the reaction flask under reflux, and react under reflux for 1h after dropping. Add 300ml of n-heptane under the condition of heat preservation, and a light yellow solid precipitates out. Cool down to room temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com