Preparation method of sitafloxacin intermediate
A technology of sitafloxacin and intermediates, which is applied in the field of preparation of intermediates, can solve the problems of low product yield, cumbersome post-processing, many side reactions, etc., and achieves high solvent recovery rate, simple post-processing and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
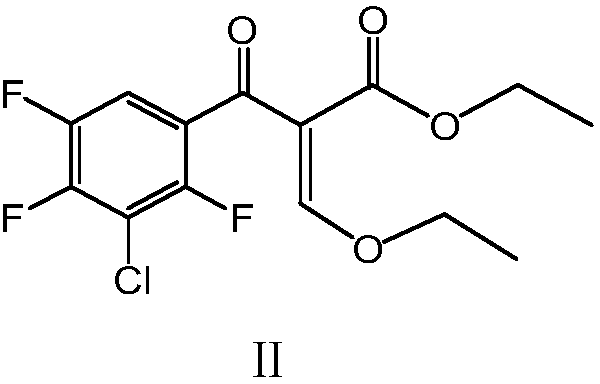
[0037] Add (21g, 0.10mol) 3-chloro-2,4,5-trifluorobenzoic acid, 210ml dichloromethane and 1ml DMF into a 500ml four-neck flask, stir until dissolved, cool down to 0-10°C, and slowly add (13.1g, 0.11mol) thionyl chloride, dropwise completed, transferred to room temperature for 3h reaction, dichloromethane was distilled off at 55-60°C under normal pressure, and excess thionyl chloride was distilled off under reduced pressure to obtain the intermediate Acyl chloride (IV), add 100ml of dichloromethane distilled out into the reaction bottle, stir, and make a solution for later use.
[0038] Add (23.4g, 0.105mol) ethyl 3-bromo-2-ethoxyacrylate (V) and 100ml dichloromethane into a 500ml reaction flask, stir evenly, cool down to 0-10°C, add (6.8g, 0.105mol ) zinc powder, stirred for 0.5h, slowly added dropwise the intermediate acid chloride (IV) solution, the dropwise addition was completed, transferred to react at room temperature for 2h, filtered, added 100ml of water in the filtrat...
Embodiment 2
[0041] Add (21g, 0.10mol) 3-chloro-2,4,5-trifluorobenzoic acid, 210ml dichloromethane and 1ml DMF into a 500ml four-neck flask, stir until dissolved, cool down to 0-10°C, and slowly add (13.1g, 0.11mol) thionyl chloride, after the dropwise addition was completed, it was transferred to room temperature to react for 4 hours to obtain the intermediate acid chloride (IV) solution, which was ready for use.
[0042] Add (23.4g, 0.105mol) ethyl 3-bromo-2-ethoxyacrylate (V) and 100ml dichloromethane into a 500ml reaction flask, stir evenly, cool down to 0-10°C, add (7.2g, 0.11mol ) zinc powder, stirred for 1h, slowly added dropwise the intermediate acid chloride (IV) solution, after the dropwise addition was completed, transferred to react at room temperature for 3h, filtered, added 150ml of water in the filtrate, stirred, left to stand, layered, separated the organic phase, added Dry over anhydrous sodium sulfate, filter, and recover dichloromethane from the filtrate under normal pre...
Embodiment 3
[0044] Add (21g, 0.10mol) 3-chloro-2,4,5-trifluorobenzoic acid, 210ml dichloromethane and 1ml DMF into a 500ml four-neck flask, stir until dissolved, cool down to 0-10°C, and slowly add (13.4g, 0.105mol) oxalyl chloride, after the dropwise addition was completed, it was transferred to room temperature and reacted for 3h to obtain the intermediate acid chloride (IV) solution, which was ready for use.
[0045] Add (23.4g, 0.105mol) ethyl 3-bromo-2-ethoxyacrylate (V) and 100ml dichloromethane into a 500ml reaction flask, stir evenly, cool down to 0-10°C, add (7.2g, 0.11mol ) iron powder, stirred for 1h, slowly added dropwise the intermediate acid chloride (IV) solution, after the dropwise addition was completed, transferred to react at room temperature for 2h, filtered, added 150ml of water in the filtrate, stirred, left to stand, layered, separated the organic phase, added Dry over anhydrous sodium sulfate, filter, first recover dichloromethane from the filtrate under normal press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com