Sitafloxacin dihydrate crystal, and preparation method and composition tablet thereof
A technology of sitafloxacin and dihydrate, applied in the field of medicine, can solve the problems of inability to obtain antibacterial effect, slow dissolution, insoluble in water, etc., and achieves high tablet dissolution rate and bioavailability, simple process and stable good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Preparation of sitafloxacin dihydrate crystal form
[0034] 1) Dissolve 1 kg of sitafloxacin crude product in 3 kg of absolute ethanol, 2 kg of anhydrous acetone and 5 kg of deionized water, and heat to reflux to obtain solution A;
[0035] 2) Cool down to 40°C-50°C, add 25g of activated carbon, stir for 30 minutes to obtain solution B, cool down to 20-25°C for crystallization for 1 hour, and grow crystal for 3 hours;
[0036] 3) Rinse with 2kg of absolute ethanol to obtain a white solid;
[0037] 4) Put the solid obtained in step 3) in a vacuum drying oven, vacuumize the system, and dry at 30° C. to constant weight to obtain the crystal form of sitafloxacin dihydrate.
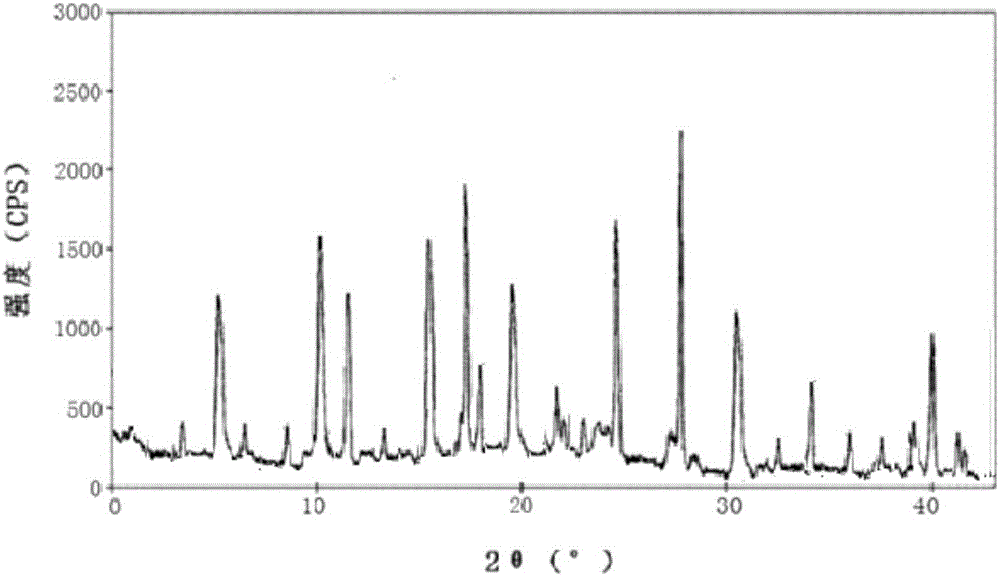
[0038] The X-ray powder diffraction spectrogram (see figure 1 ) at 2θ of 3.7°, 5.3°, 10.3°, 11.5°, 15.3°, 16.8°, 17.7°, 19.8°, 21.7°, 24.8°, 27.6°, 30.6°, 34.1°, and 39.8° .
[0039] Elemental analysis:
[0040] Measured values: C51.21%, H4.98%, Cl7.97%, F8.51%, N9.45%, O17.95%.
[0...
Embodiment 2
[0044] Embodiment 2: Preparation of sitafloxacin dihydrate crystal form
[0045] 1) Dissolve 1 kg of sitafloxacin crude product in 8 kg of absolute ethanol, 5 kg of anhydrous acetone and 10 kg of deionized water, and heat to reflux to obtain solution A;
[0046] 2) Cool down to 40°C-50°C, add 25g of activated carbon, stir for 30 minutes, filter to obtain solution B, cool down to 20-25°C for crystallization for 1 hour, and grow crystals for 6 hours;
[0047] 3) Rinse with 3kg of absolute ethanol to obtain a white solid;
[0048] 4) Put the solid obtained in step 3) in a vacuum drying oven, vacuumize the system, and dry at 40° C. to constant weight to obtain the sitafloxacin dihydrate crystal form.
[0049] According to the XPRD data, the obtained crystal form is consistent with the crystal form in Example 1. The thermogravimetric analysis spectrum obtained by using the PEPyrisDiamondTG thermogravimetric analyzer of Perkin-Elmer Company of the United States is consistent with ...
Embodiment 3
[0050]Embodiment 3: Preparation of sitafloxacin dihydrate crystal form
[0051] 1) Dissolve 1 kg of sitafloxacin crude product in 5 kg of absolute ethanol, 3 kg of anhydrous acetone and 20 kg of deionized water, and heat to reflux to obtain solution A;
[0052] 2) Cool down to 40°C-50°C, add 25g of activated carbon, stir for 30 minutes, filter to obtain solution B, cool down to 20-25°C for crystallization for 1 hour, and grow crystals for 12 hours;
[0053] 3) Rinse with 5kg of absolute ethanol to obtain a white solid;
[0054] 4) The solid obtained in step 3) is placed in a vacuum drying oven, the system is evacuated, and dried at 50° C. to constant weight to obtain the crystal form of sitafloxacin dihydrate.
[0055] According to the XPRD data, the obtained crystal form is consistent with the crystal form in Example 1. The thermogravimetric analysis spectrum obtained by using the PEPyrisDiamondTG thermogravimetric analyzer of Perkin-Elmer Company of the United States is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com