Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
116 results about "Absolute configuration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An absolute configuration refers to the spatial arrangement of the atoms of a chiral molecular entity (or group) and its stereochemical description e.g. R or S, referring to Rectus, or Sinister, respectively.
Novel C-glycosides, uses thereof
The present invention relates to novel C-glycoside compounds of given absolute configuration, to a process for synthesising them and to compositions containing them. The invention also relates to the cosmetic use of these C-glycoside compounds as agents to stimulate the synthesis of glycosaminoglycans containing a D-glucosamine and / or N-acetyl-D-glucosamine residue, advantageously hyaluronic acid, and / or of proteoglycans, advantageously proteoglycans containing hyaluronic acid, by fibroblasts and / or keratinocytes. The invention also relates to a cosmetic process for treating keratin materials using a composition containing at least one C-glycoside compound according to the invention.
Owner:LOREAL SA
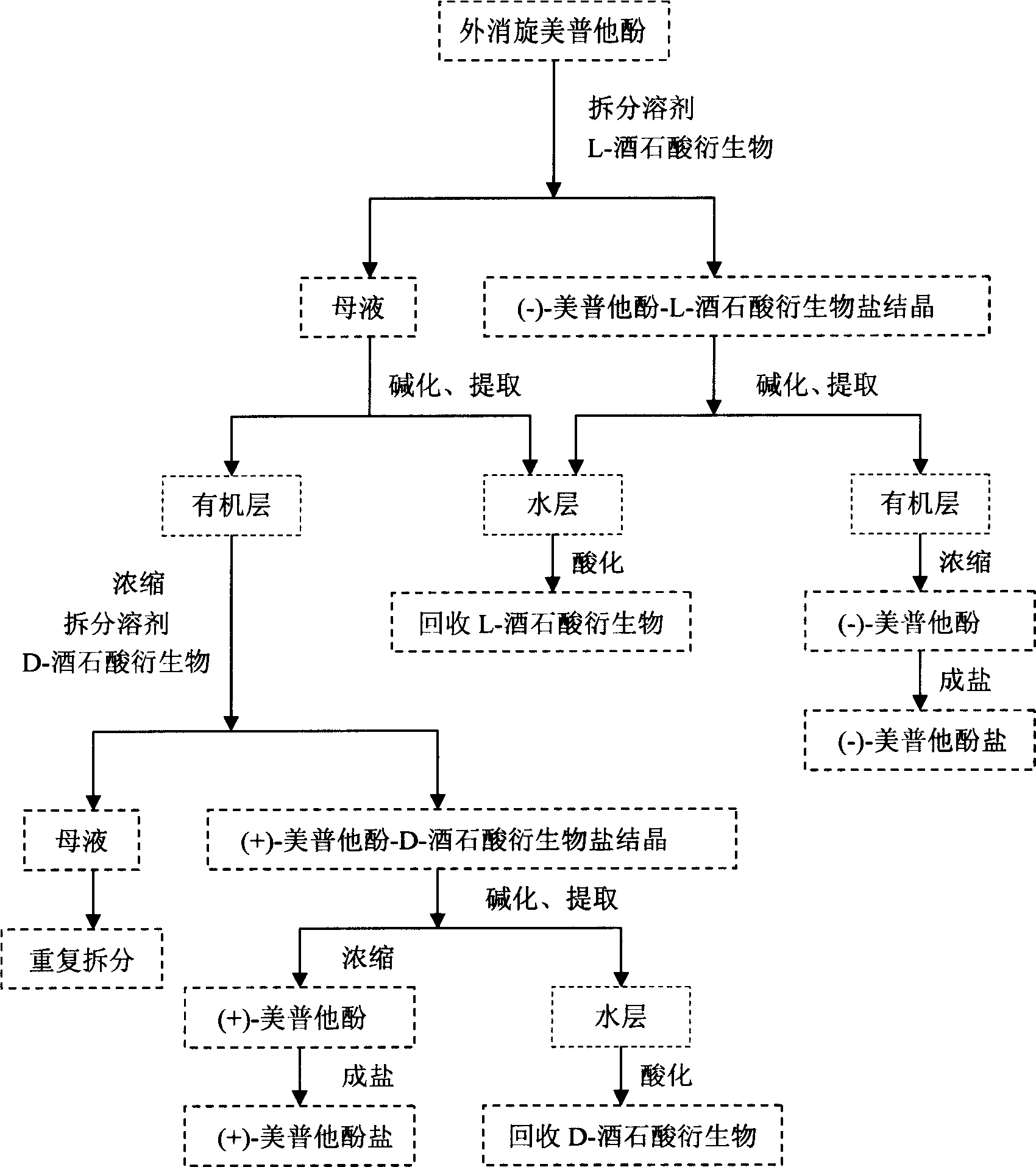
Optical-purity meptazinol orits salts, and preparing method
InactiveCN1850804APharmacologically activeNervous disorderOrganic chemistryAbsolute configurationSingle crystal
This invention belongs to pharmacy field, it relates optical pure mei pu ta fen and its preparation method and application. Racemation mei pu ta fen is splited by optical pure tartaric acid evolving object to get optical pure monomer, enantiomeric excess testing is done through capillary electrophoresis method, their e.e.valude are both excess 99 percent. Laevorotation and dextrorotation mei put a fen absolute configuration is decided by single crystal X-diffraction method as S and R. the R(+)mei pu ta fen has srong activity in analgesia activity testing, but S(-)mei pu ta fen has more stronger activity in acetylcholine esterase restrain activity testing, analgesic medicine and senile dementia medicine can be further prepared.
Owner:FUDAN UNIV
Method for analysis of reaction products
InactiveUS7045360B2Chemical analysis using catalysisAnalysis using chemical indicatorsAbsolute configurationReaction product
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
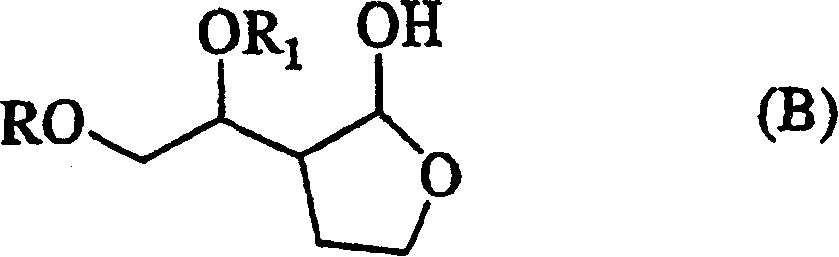
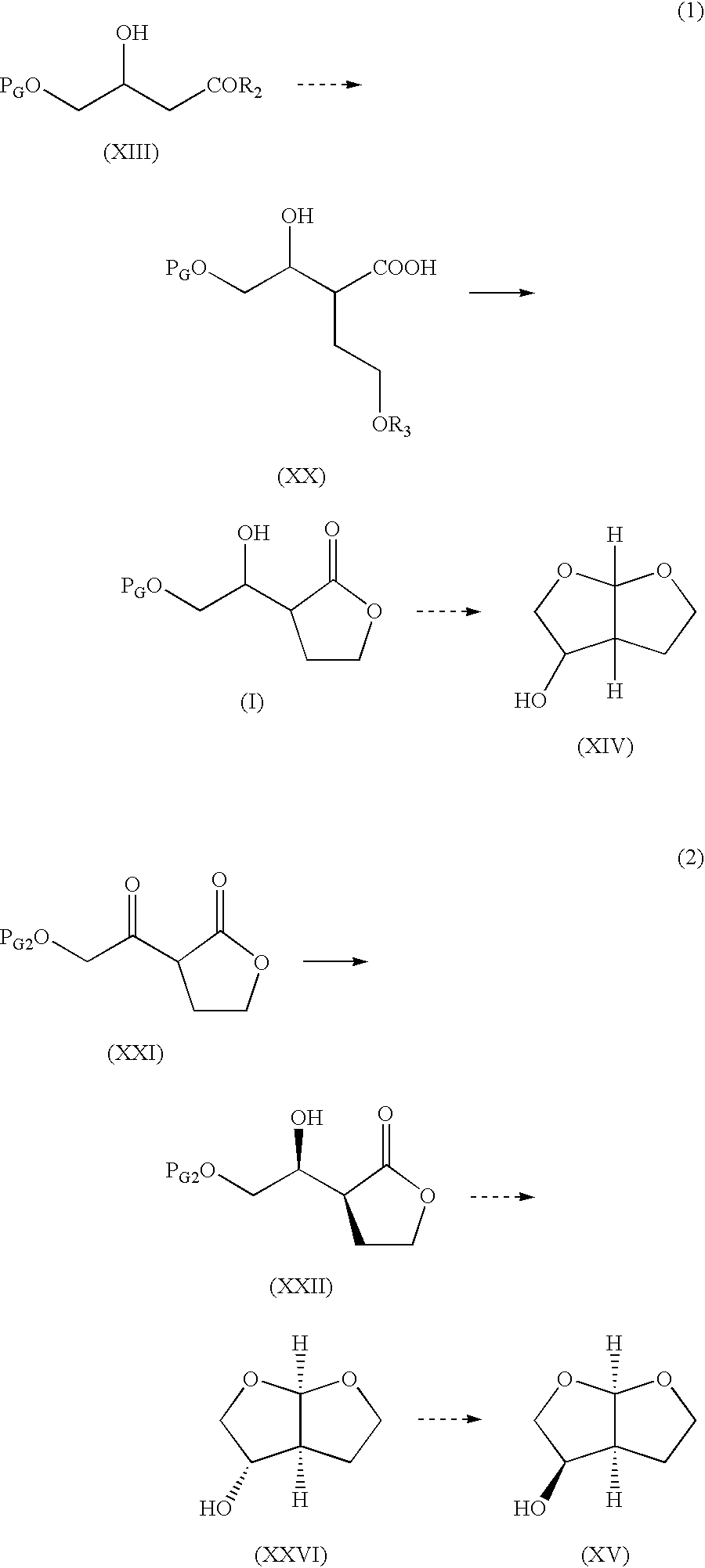
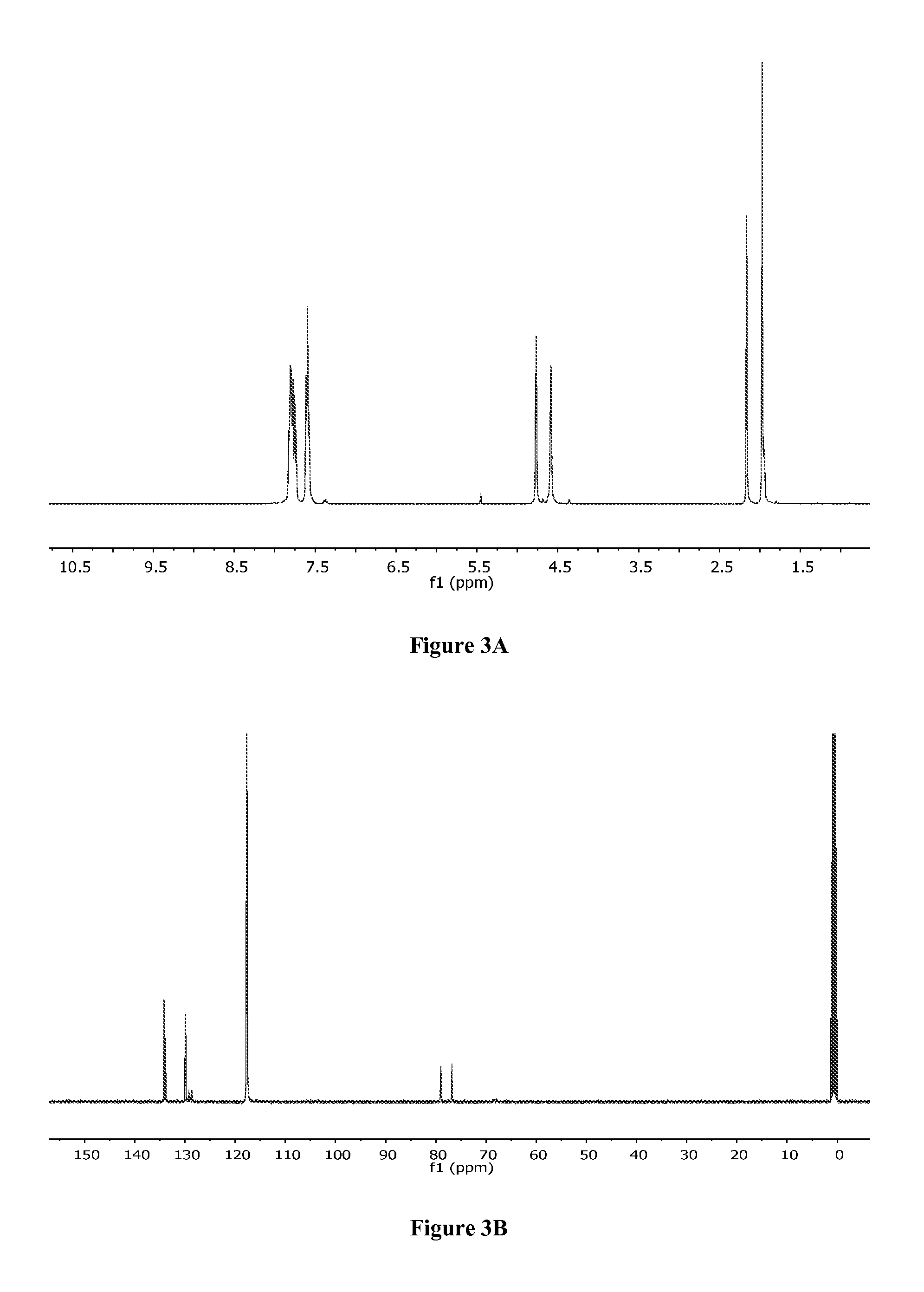
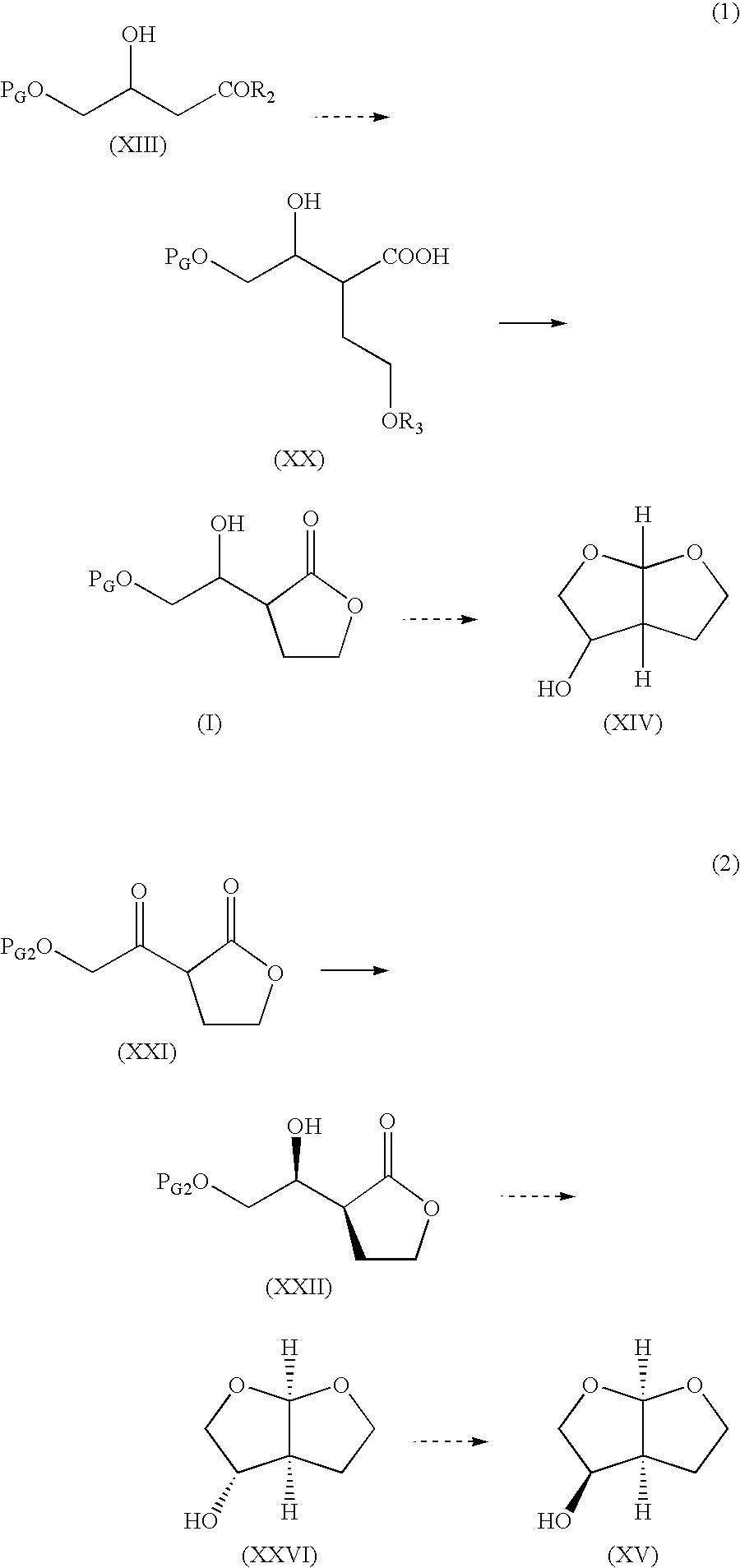
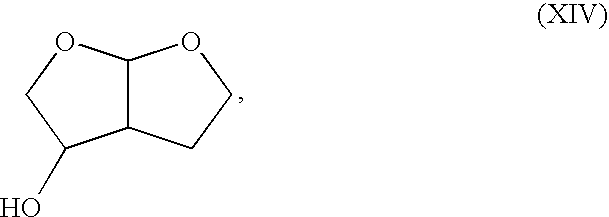
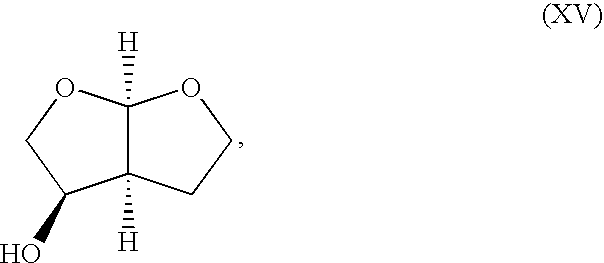
Production method of hexahydrofurofuranol derivative, intermediate therefor and production method thereof
InactiveCN1753898AEfficient preparationLow costOrganic chemistryAbsolute configurationIndustrial scale
A process for efficiently producing compound (XIV) being useful as a drug intermediate on an industrial scale at low cost without the need to use ozonization and highly toxic agents; and an intermediate for use in the process. In particular, a process for producing a compound having the absolute configuration of the formula (XV) or an enantiometric isomer thereof without the need to use means such as optical resolution; and an intermediate for use in the process. (1) Compound (XIV) is produced by deriving compound (I) from compound (XIII) as a starting material and sequentially performing introduction of a protective group, reduction and cyclization. Particularly, compound (XIV) is produced by deriving compound (I) through compound (XX) from compound (XIII) as a starting material. Compound having the absolute configuration of the formula (XV) or the like is produced in high stereoselectivity from optically active compound (XIII) as a starting material. (2) Compound (XXVI) is produced by deriving compound (XXII) from compound (XXI) as a starting material through stereoselective reduction thereof and sequentially performing introduction of a protective group, reduction and cyclization. Compound (XV) is produced by inverting hydroxyl of the compound (XXVI). (1) (2) wherein symbols are as defined in the description.
Owner:SUMITOMO CHEM CO LTD
Production method of hexahydrofurofuranol derivative, intermediate therefor and production method thereof
InactiveUS6867321B2Resolution problemEfficiently and economicallyBiocideOrganic chemistryAbsolute configurationEnantiomer
The present invention provides a method for producing compound (XIV) useful as an intermediate for pharmaceutical agents efficiently and economically on an industrial scale without using ozone oxidation and highly toxic reagent, and an intermediate used for this method. Particularly, the present invention provides a method for producing a compound having an absolute configuration represented by the formula (XV) and an enantiomer thereof without using a technique such as optical resolution and the like, and an intermediate used for this method.(1) Compound (XIII) as a starting material is led to compound (I), and after introducing a protecting group, subjected to reduction and cyclization to give compound (XIV). Particularly, compound (XIII) as a material is led to compound (I) via compound (XX) to produce compound (XIV). Using an optically active compound (XIII) as a starting material, a compound having an absolute configuration represented by the formula (XV) and the like are produced highly stereoselectively. (2) Compound (XXI) as a starting material is stereoselectively reduced to give compound (XXII), and by introduction of a protecting group, reduction and cyclization, compound (XXVI) is obtained, and by inverting hydroxyl group, compound (XV) is produced. wherein each symbol is as defined in the specification.
Owner:SUMITOMO CHEM CO LTD
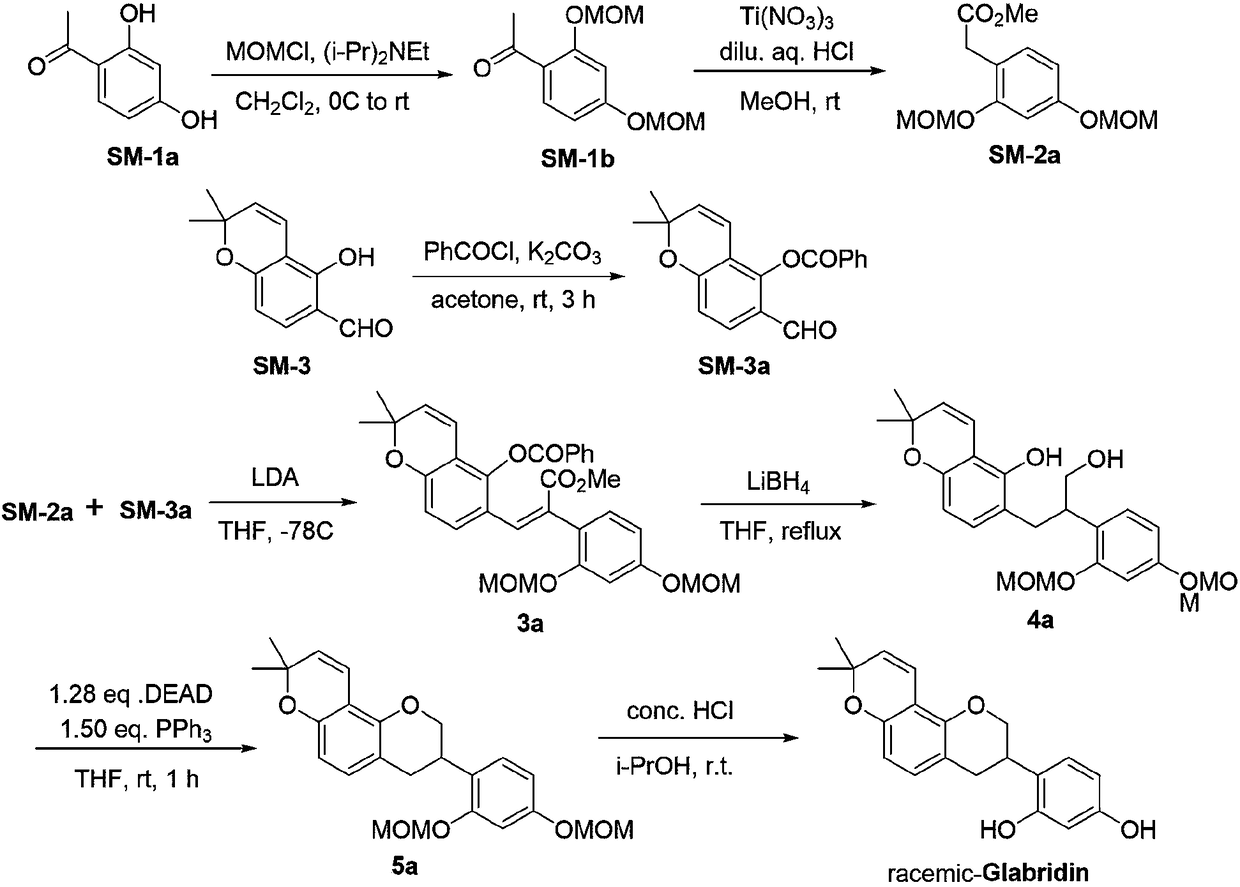
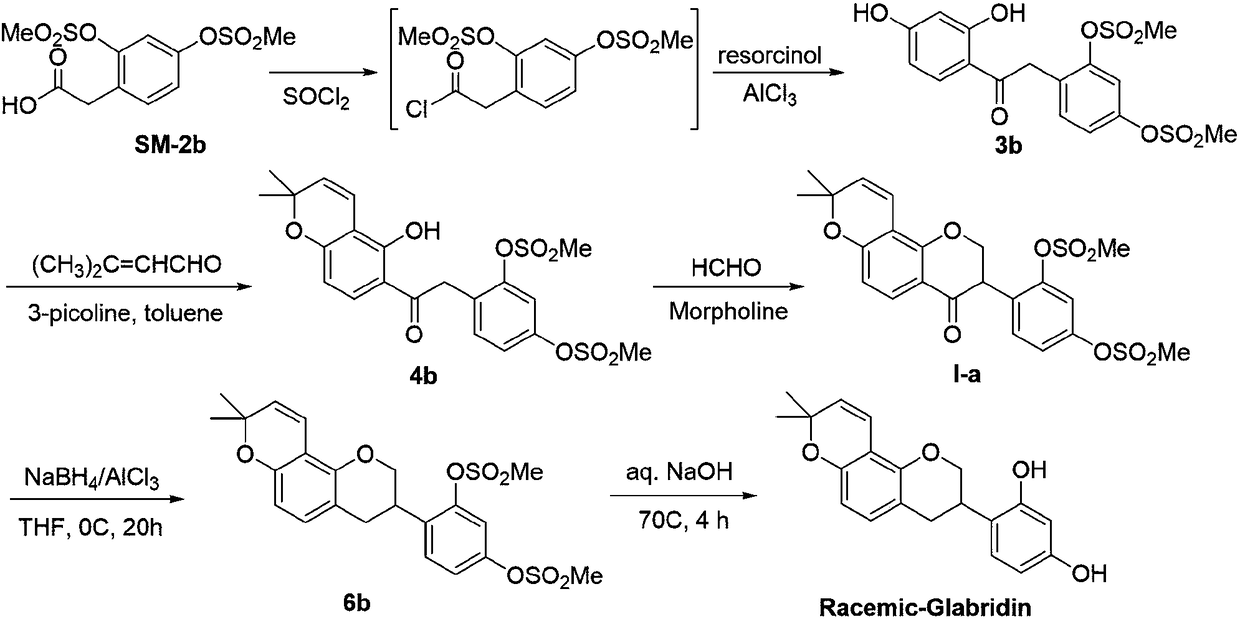
Method for asymmetrically synthesizing glabridin with optical purity under catalysis of ruthenium compound
ActiveCN108440553AHigh yieldHigh stereoselectivityOrganic chemistry methodsBulk chemical productionAbsolute configurationTrifluoroacetic acid
The invention relates to a method for asymmetrically synthesizing glabridin with optical purity under catalysis of a ruthenium compound. The method comprises the following steps: 1) taking isoflavoneprotected by a protection group as a raw material and carrying out dynamic kinetic asymmetric hydrogen transfer reaction under the catalysis effect of a ruthenium trichloride compound and the action of an acid-alkali buffering system to obtain chiral isoflavol with an absolute configuration being (3R, 4R); 2) removing hydroxyl of the chiral isoflavol under the action of triethylsilane and trifluoroacetic acid to obtain a product with an absolute configuration being (R); 3) removing a protection group of the product with the configuration being (R) in step 2) under an acidic or alkaline condition to obtain the glabridin with the configuration (R) and the optical purity. The method provided by the invention can be used for synthesizing the glabridin with the optical purity in a high-yield and high-stereoselectivity manner; the obtained product is completely the same as that of the glabridin extracted from glycyrrhiza glabra and can be used for replacing the glabridin derived from naturalplants to be industrially applied.
Owner:烟台六谛医药科技有限公司 +2
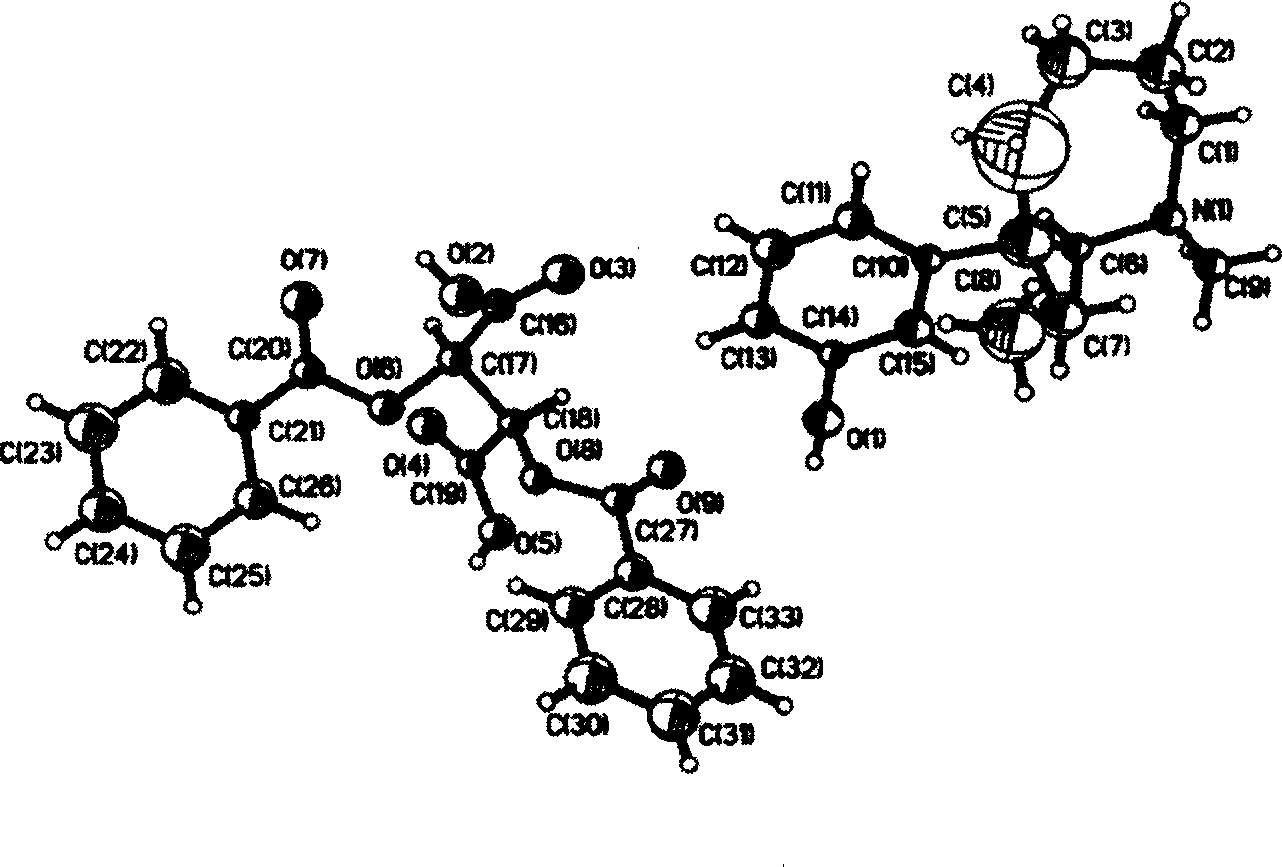
Oriented synthesis and crystal structure of 21(S) argatroban, and preparation for monohydrate thereof
InactiveCN101235031AEasy to operateLow costOrganic chemistryBlood disorderAbsolute configurationSynthesis methods
The invention relates to a 21(S) argatroban rational synthesis method, a corresponding crystal structure and a hydrate preparation method, wherein the rational synthesis uses (3S)-1, 2, 3, 4-tetrahydrochysene-3-methyl-8-quinolinesulfonyl chloride as raw material to prepare single diastereoisomer 21(S) argatroban, and uses single crystal and polycrystalline powder X diffraction method to determine the absolute configuration as 21(S) and the crystal systems I, II, and the 21(S) argatroban is soluble in hot water, via controlling the cooling speed, a 21(S) argatroban hydrate is prepared. Animal tests prove that the anticoagulant function of the 21(S) argatroban hydrate is 2-3 powers of 21(R) argatroban hydrate.
Owner:TIANJIN WEIJIE TECH
FG-4592 single crystal and preparation method thereof
InactiveCN106187888AIn-depth understanding of the mechanism of action that exerts its efficacyOrganic chemistry methodsProtein targetSpace group
The invention discloses an FG-4592 single crystal. The single crystal X-ray diffraction pattern of the FG-4592 single crystal is characterized in that the crystal belongs to a triclinic system, the space group is P (please see the formula in the specification), the cell parameter a is equal to 8.5830 (17), the cell parameter b is equal to 9.2790 (19), and the cell parameter c is equal to 11.359 (2) (please see the formula in the specification); alpha is equal to 99.16 (3) degrees, beta is equal to 108.36 (3) degrees, gamma is equal to 102.16 (3) degrees, the cell size is 814.2 (3)-<3> (please see the formula in the specification), and the number Z of molecules in a cell is equal to 2. The invention further discloses a method for preparing the FG-4592 single crystal. A simple solvent and a sample are matched to obtain the single crystal of FG-4592 fast, and the absolute configuration of the compound is obtained through a single crystal XRD. The crystal form of the sample can be determined through single crystal X-ray powder diffraction pattern simulation. The single crystal of FG-4592 is prepared, the absolute configuration and crystal form of the molecules can be obtained, the combination mode of the molecules and target protein (PHD) can be simulated through a molecular docking means, and the medicine effect achieving action mechanism of the single crystal is deeply known.
Owner:JIANGSU DEYUAN PHARMA
Azide and preparation method thereof
ActiveCN103304535AEasy to prepareThe reaction conditions are mild and easy to controlOrganic chemistryAbsolute configurationAzide
The invention discloses an azide and a preparation method thereof. The azide (I) can be used as a key raw material for preparing medicines with a 1,2,3-triazole structure, such as ticagrelor. The preparation method for the azide comprises the following step of: performing azidation reaction on an amino compound (II) and an azidation reagent (III) to obtain the azide (I). The preparation method is moderate in conditions, safe and environmentally-friendly, high in chemical yield, and especially capable of keeping the original absolute configuration and chiral purity.
Owner:徐州飞云泡沫制品有限责任公司
Inula wissmannian extract and preparation thereof and application in preparation of antitumor drug
The invention provides an inula wissmannian extract and preparation thereof and application in preparation of an antitumor drug. The extract is gemma alkane type sesquiterpene lactone inula wissmannian lactone methyl, ethyl, propyl, butyl and amyl or sheepear inula herb lactone. The extract has the following chemical structural formula and absolute configuration that: due to the toxicity killing function experiment of inula wissmannian lactone methyl, ethyl, propyl, butyl and amyl or sheepear inula herb lactone on human hepatoma cells HepG2, human prostatic cancer cells PC-3, human gastric carcinoma cells MGC-803, human leukemia cells K562, human nasopharynx cancer cells KB and normal human hepatocytes LO2, the result shows that the gemma alkane type sesquiterpene lactone has strong tumor cell killing function, and proliferation of tumor cells can be inhibited, so that the tumor cells are killed. The animal in-vivo experiment proves that the inula wissmannian plant extract and monomeric compounds have the anti-tumor effect and can serve as active ingredients for preparing the antitumor drug. A novel cancer treatment medicine is provided clinically, and high clinical application values and social benefits are generated.
Owner:SHANGHAI JIAO TONG UNIV
Inula wissmannian extracts, preparation for same, and application of extracts in preparation of anti-inflammatory medicine
Owner:SHANGHAI JIAO TONG UNIV
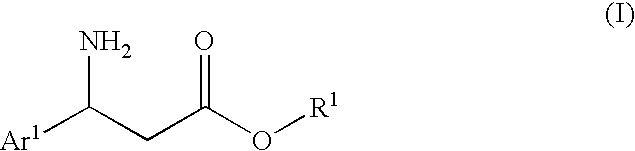
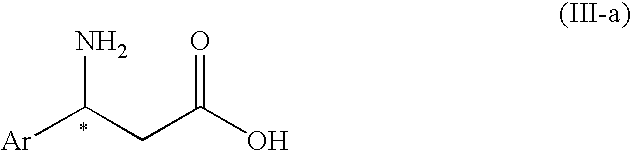
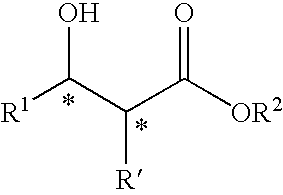
3-Amino-3-arylpropionic acid n-alkyl esters, process for production thereof, and process for production of optically active 3-amino-3-arylpropionic acids and esters of the antipodes thereto
The present invention is to provide an n-alkyl 3-amino-3-arylpropionate represented by the formula (I): wherein Ar1 represents an aryl group which may have a substituent(s), provided that a phenyl group and 4-methoxyphenyl group are excluded, R1 represents an n-propyl group or an n-butyl group, and a process for preparing the same, and its optically active compound and an optically active (S or R)-3-amino-3-arylpropionic acid represented by the formula (III-a): wherein Ar represents an aryl group which may have a substituent(s), and * represents an asymmetric carbon, and a process for preparing an optically active n-alkyl (R or S)-3-amino-3-arylpropionate represented by the formula (IV-a): wherein Ar and R1 have the same meanings as defined above, * represents an asymmetric carbon, provided that it has a reverse absolute configuration to the compound of the formula (III-a).
Owner:UBE IND LTD
Puerarin monocrystal and preparation method thereof
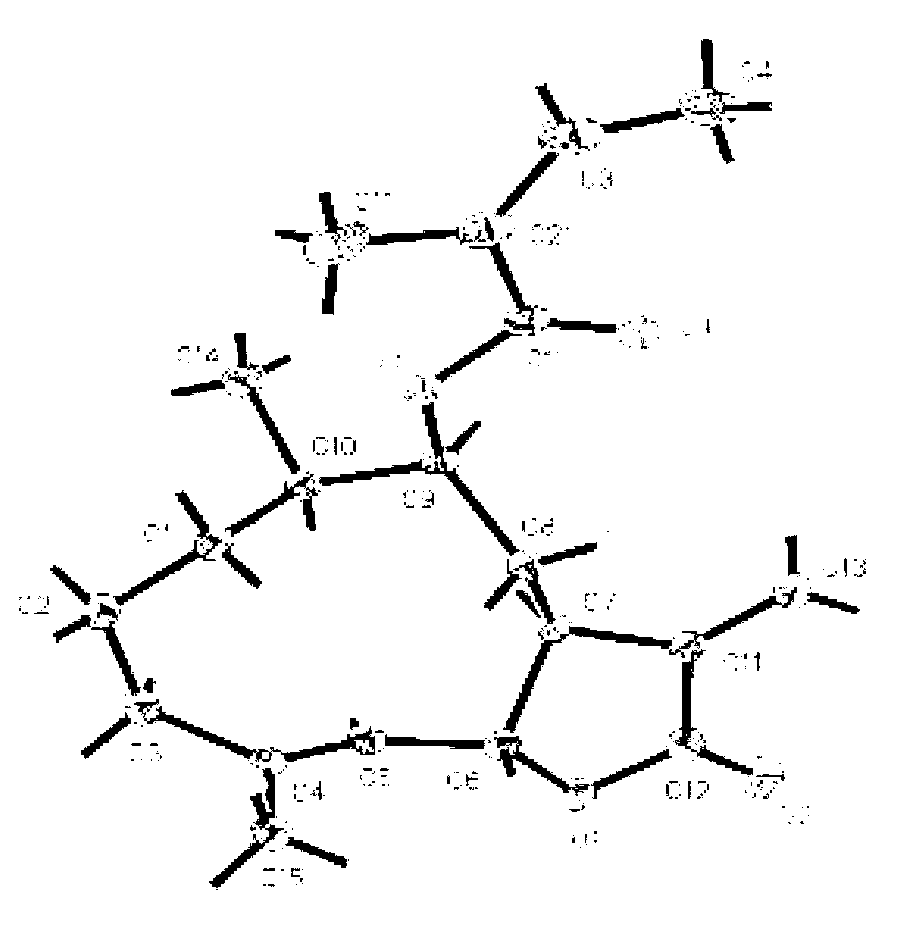
The invention relates to a puerarin monocrystal and a preparation method thereof. The puerarin monocrystal is named crystal form A, the CCDC number is 708830, the crystal structure is asymmetrical, each crystal cell contains two asymmetrical molecules, i.e. molecule a and molecule b, each of which carries a molecular crystal water, and moreover, the binding sites of the crystal waters are different. The crystal water of the molecule a and a hydroxyl group on a benzene ring carry out hydrogen bonding, the crystal water of the molecule b and a carbonyl group on a flavone mother nucleus carry out hydrogen bonding, the hydroxymethyl group of the glucose of the molecule a is at the position of an equatorial bond and perpendicular to a ring plane, the oxygen atom of the hydroxymethyl group is in a saccharide ring plane, the hydroxymethyl group of the glucose of the molecule b is at the position of an axial bond and perpendicular to a ring plane, the hydrogen on the rest pyranose carboatomic ring is an axial bond, the hydroxyl group is positioned at an equatorial bond, and the molecular formulas of the molecule a and the molecule b are the same, i.e. C21H20O9.H2O. The absolute configuration of the puerarin monocrystal is an asymmetrical isomer. The melting point of the puerarin monocrystal is increased, and the heat stability is remarkably enhanced compared with powder. The average purity of the puerarin crystal form A reaches 99.8 percent, higher than 99.1 percent of average purity of bulk pharmaceutical chemicals, and the quality is remarkably increased.
Owner:张幸国
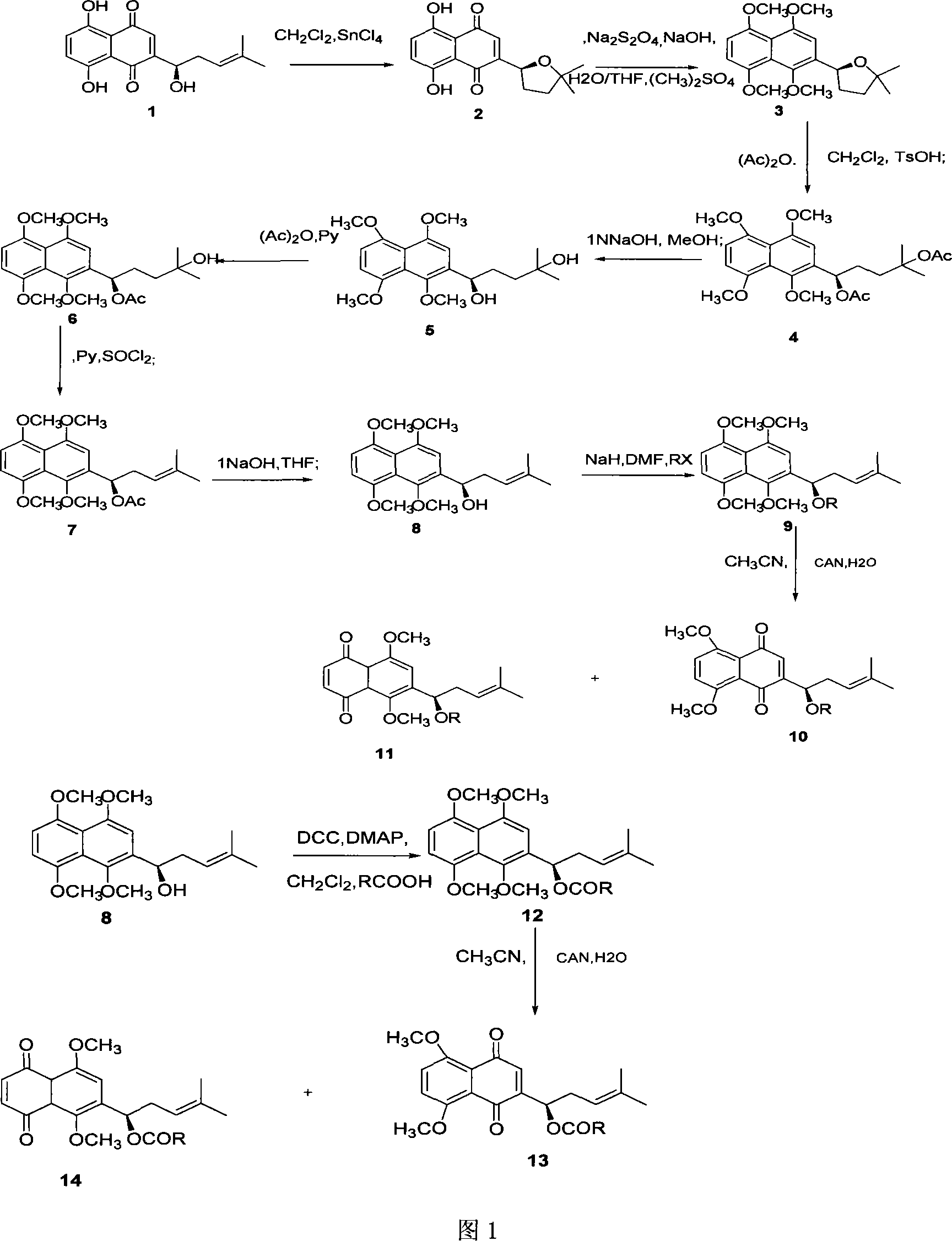
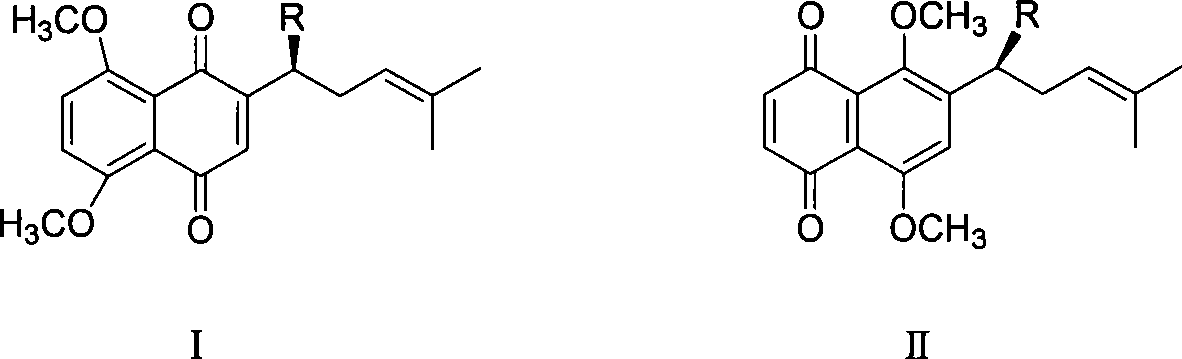
Method for synthesizing alkannin dimethyl ether derivative
InactiveCN101139287AOrganic compound preparationCarboxylic acid esters preparationAbsolute configurationSide chain
The present invention is a synthesis method of the shikonin dimethyl ester derivative in the pharmaceutical and chemical technological field. The synthesis method of the second-level side-chain isomer and the sixth-level side-chain isomer of the shikonin dimethyl ester of the present invention uses the shikonin as the raw material; the shikonin is first cyclized; then the cyclized shikomin is in the tetramethyl and ring-opening reaction; in the process, the absolute configuration of the shikonin is maintained; then the esterification or etherification is done; at last, the methyls are selectively doffed to get the target compound. The reagent in each step of the reaction is easy to get; the conditions are mild; the collection rates of the reaction all surpass 65 percent; the total collection rate is 33.2 percent (calculated by the shikonin). The reaction process has no racemic reaction.
Owner:SHANGHAI JIAO TONG UNIV
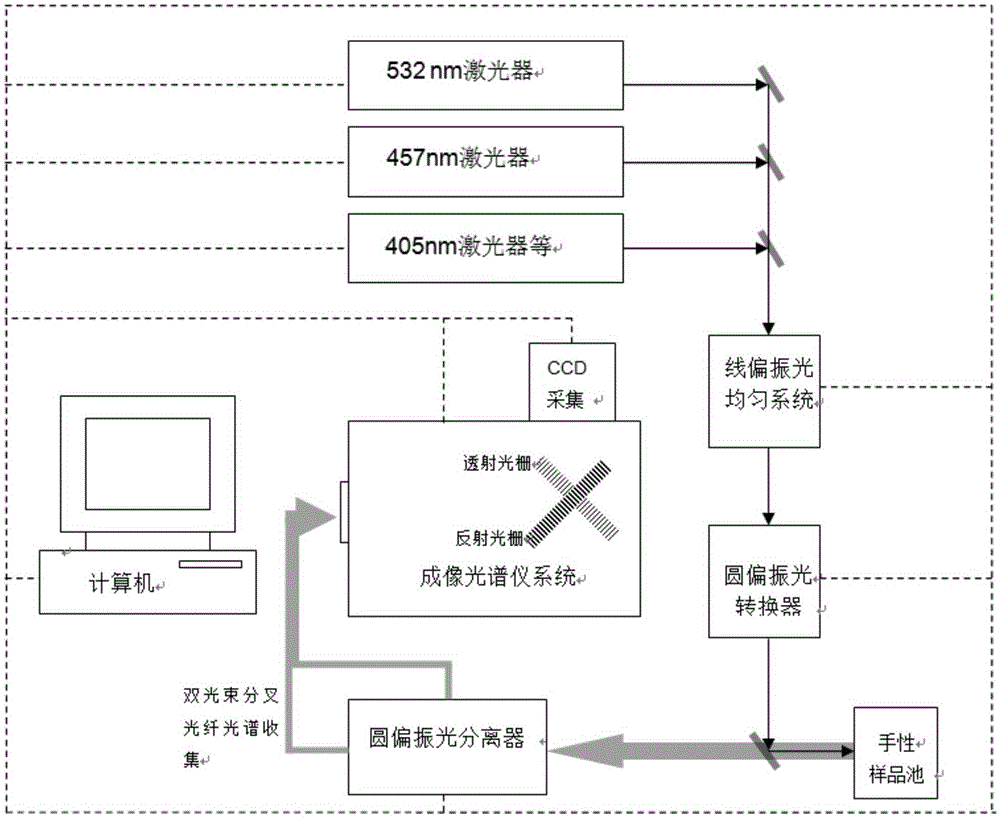
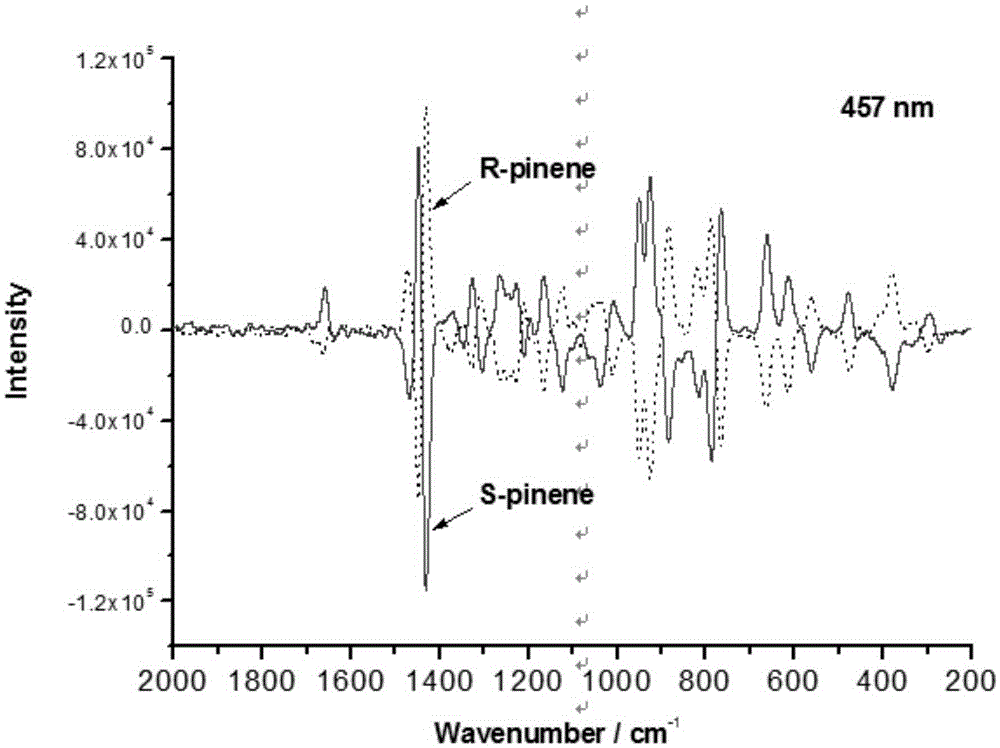
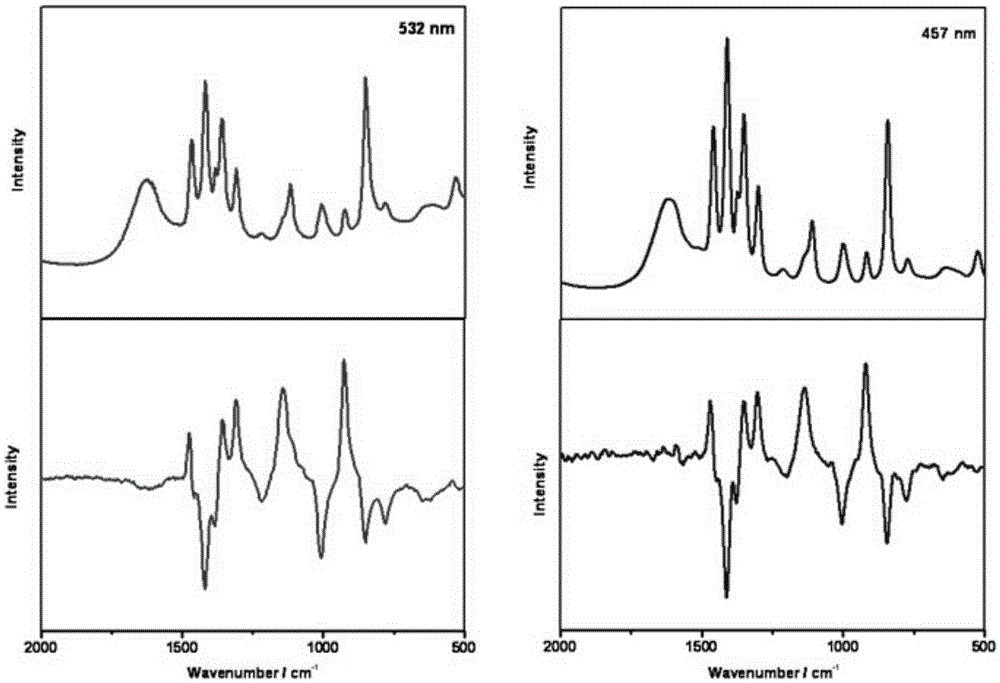
Short wavelength laser chiral Raman spectrometer
The invention relates to a short wavelength laser chiral Raman spectrometer, which comprises a laser excitation light source having a wavelength of 325-532 nm, a linearly polarized light uniformization system, a circularly polarized light converter, a sample pool, a circularly polarized light separator, double light beam forking optical fiber, a Rayleigh line filter, an imaging spectrometer, a short-wavelength, wide-range and sensitive charge coupled device (CCD), and a computer. According to the present invention, the short wavelength laser chiral Raman spectrometer is used for the determination of the chiral absolute configuration of chiral molecules and biomolecules, and is the powerful tool for the absolute configuration and conformation analysis of the chiral molecules in the fields of chemistry, biologly, and medicine.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
C-glycosides, uses thereof
Owner:LOREAL SA
Process for producing optically active flurbiprofen
InactiveUS20060135617A1High optical purityEfficient productionPreparation from carboxylic acid saltsBiocideOrganic solventAbsolute configuration
The present invention provides a method for producing optically active flurbiprofen. The method of the present invention includes mixing racemic flurbiprofen and (S)- or (R)-3-methyl-2-phenylbutylamine in an organic solvent to produce a diastereomeric salt; and treating the diastereomeric salt with an acid in a second solvent. In the method of the present invention, flurbiprofen having a desired absolute configuration can be obtained very efficiently without repeating the procedure for optical resolution a plurality of times.
Owner:NAGASE & COMPANY
Chromone dipolymer derivative as well as preparation method and application thereof
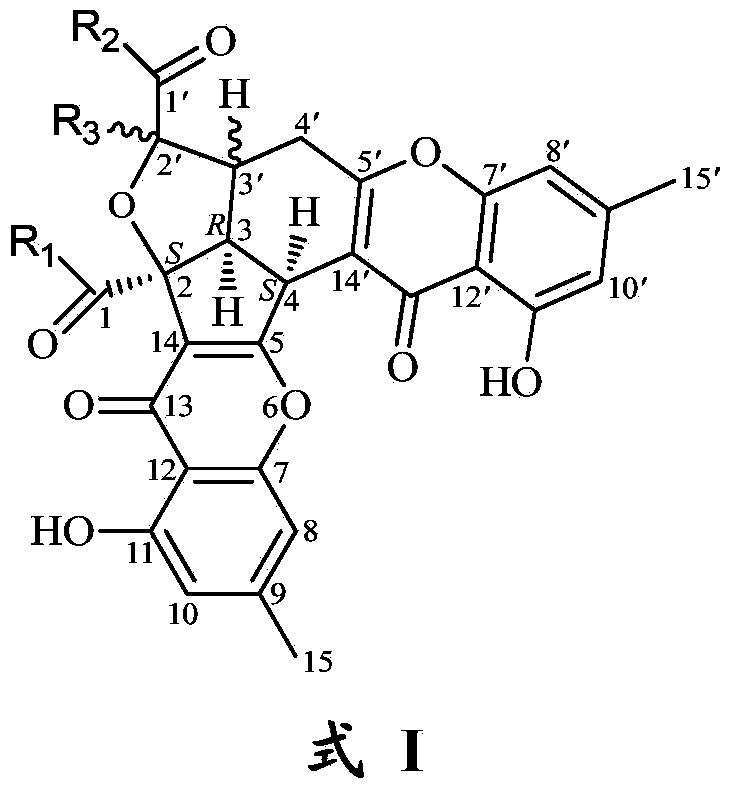
InactiveCN103992333AEfficient killingPrevent proliferationOrganic active ingredientsFungiAbsolute configurationChromone
The invention belongs to the field of medical chemical engineering and relates to a chromone dipolymer derivative as well as a preparation method and application thereof. Specifically, the invention relates to a compound with formula I, wherein S and R respectively represent absolute configurations of corresponding marking carbon atoms; R1-R3 independently represent various substituent groups, respectively; R3 and H connected by a wavey line can orientate alpha or beta. The invention further relates to penicillium purpurogenum of the compound with the formula I. Experiments prove that the compound with the formula I has good anti-tumor activity, can be used for preparing biological reagents such as a tumor cell killing agent, a tumor cell proliferation inhibitor and an anti-tumor agent, and further can be used for preparing anti-tumor and anti-cancer drugs. The formula I is as shown in the specification.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Transition metal complex having diphosphine compound as ligand
ActiveUS7135582B2Efficiently obtainedOrganic compound preparationGroup 5/15 element organic compoundsAbsolute configurationDiphosphines
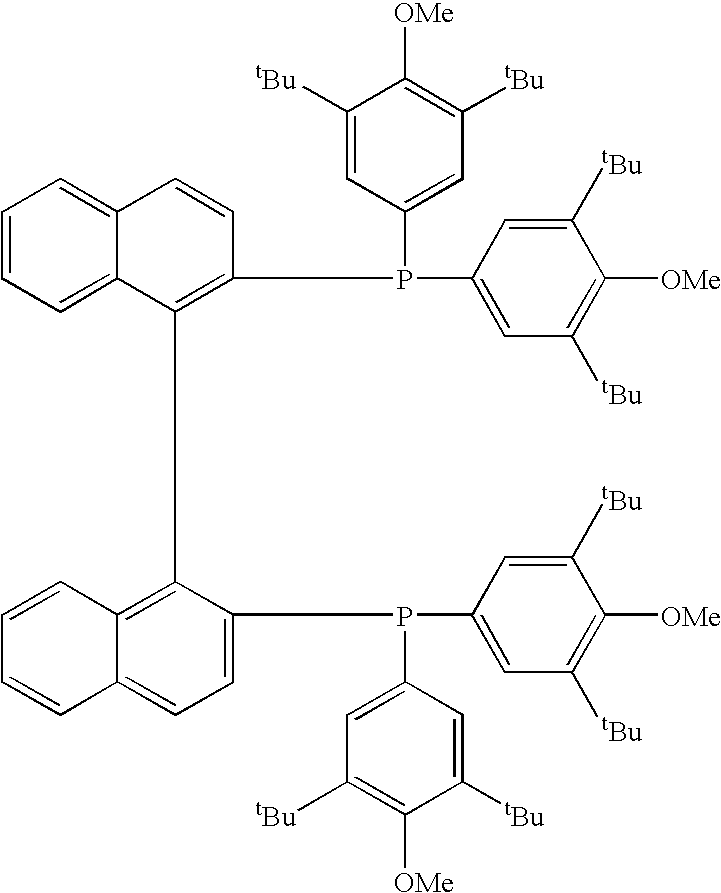
A transition metal complex having 2,2′-bis[bis(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-1,1′-binaphthyl as a ligand. The presence of the transition metal complex in the reaction system of an asymmetric reaction system allows the preparation of an objective compound having an objective absolute configuration with improved efficiency.
Owner:SPERA PHARMA INC
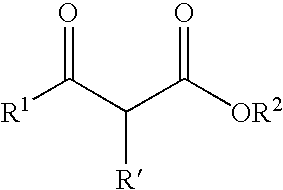
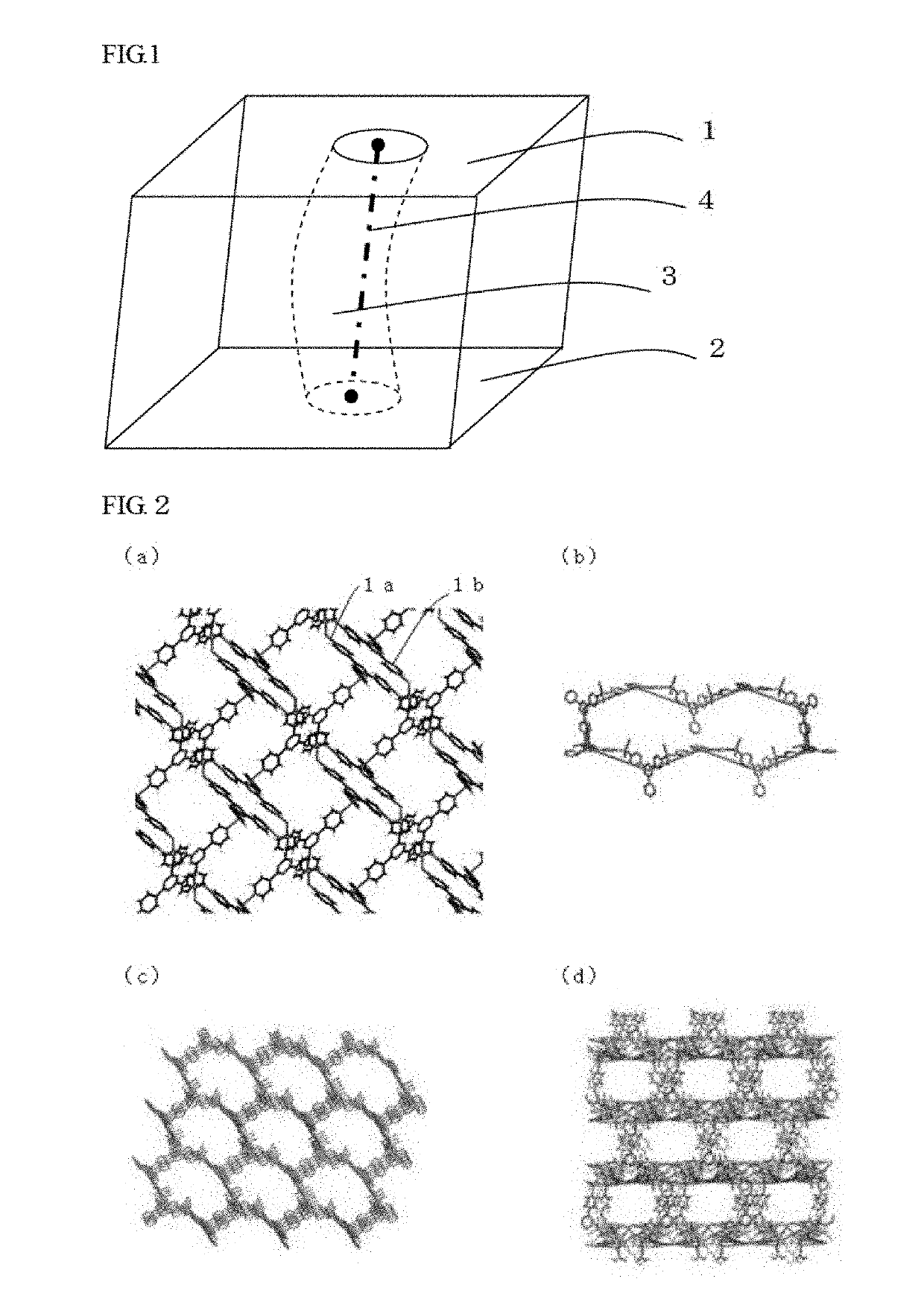
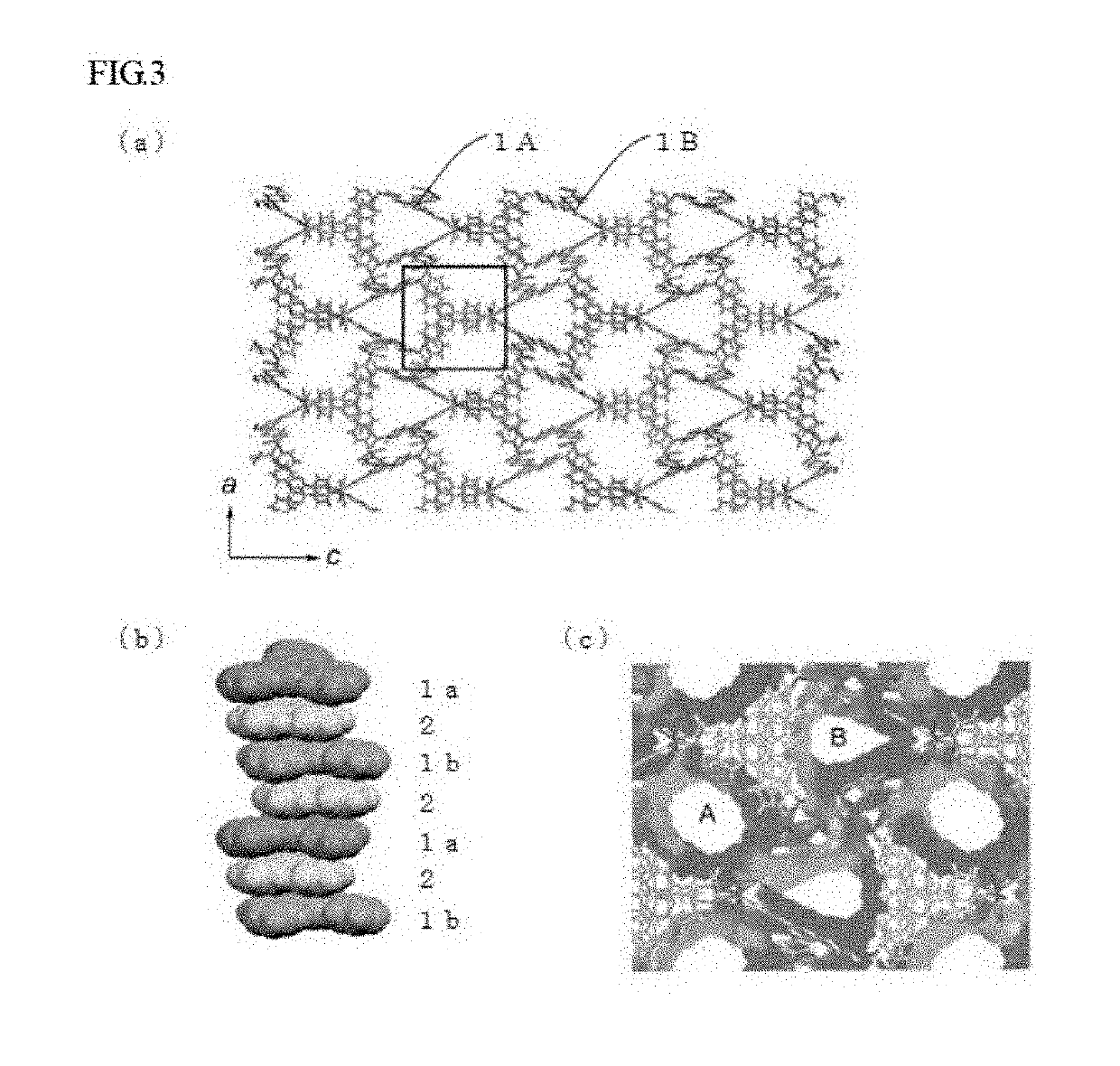
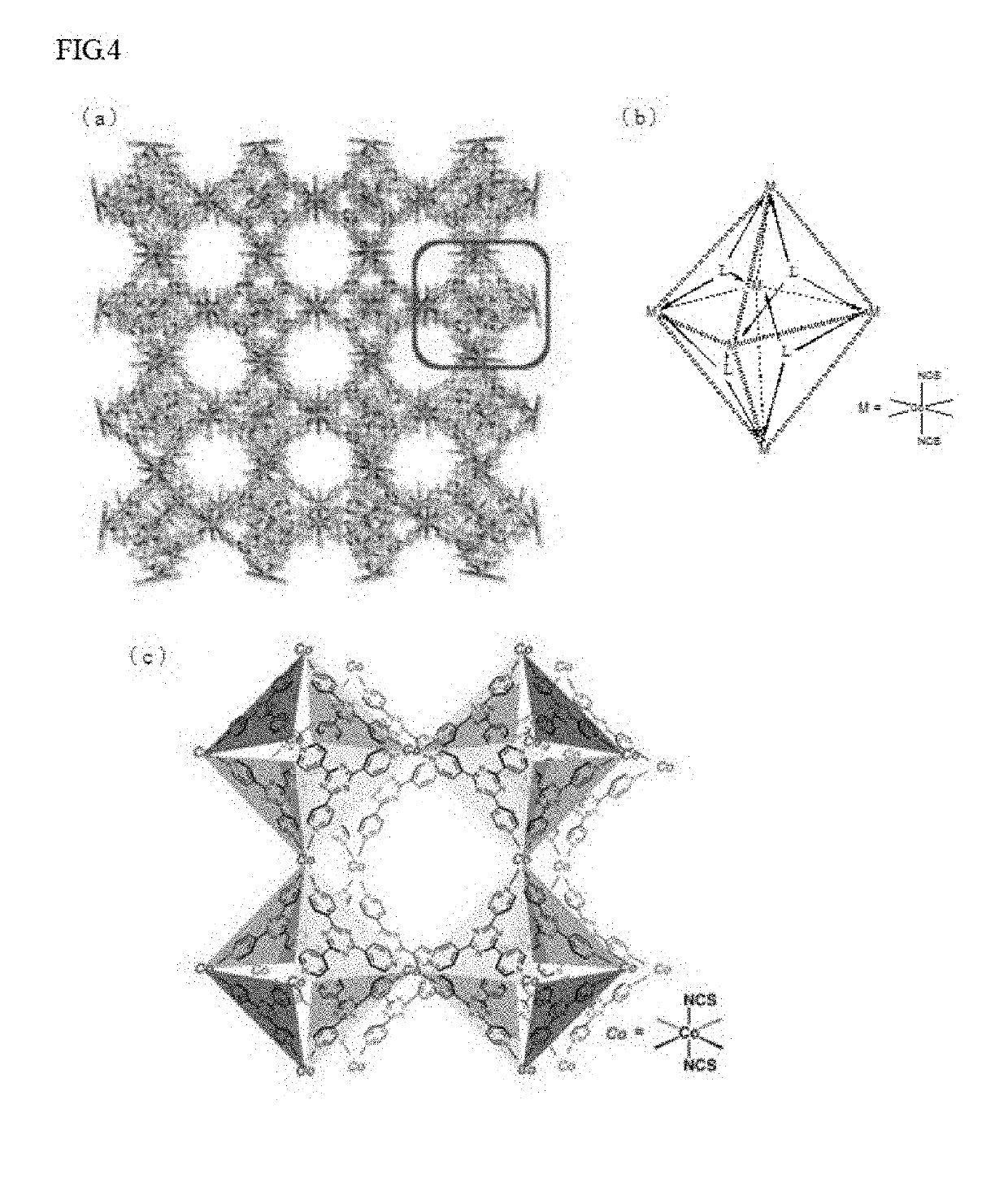
Method of preparing sample for crystal structure analysis, method of determining absolute configuration of chiral compound, and polynuclear metal complex monocrystal
ActiveUS10309035B2Used to determinePolycrystalline material growthMaterial analysis using wave/particle radiationAbsolute configurationCrystal structure
Method for preparing a crystal structure analysis sample for determining an absolute configuration of a chiral compound includes bringing a single crystal of a porous compound into contact with a solvent solution that contains a chiral compound, the single crystal of the porous compound including a three-dimensional framework, and either or both of pores and voids that are defined by the three-dimensional framework, and are three-dimensionally arranged in an ordered manner, the three-dimensional framework being formed by one molecular chain or two or more molecular chains, or formed by one molecular chain or two or more molecular chains, and a framework-forming compound, and comprising a chiral substituent of which the absolute configuration is known, the crystal structure analysis sample having a structure in which molecules of the chiral compound are arranged in either or both of the pores and the voids of the single crystal in an ordered manner.
Owner:THE UNIV OF TOKYO
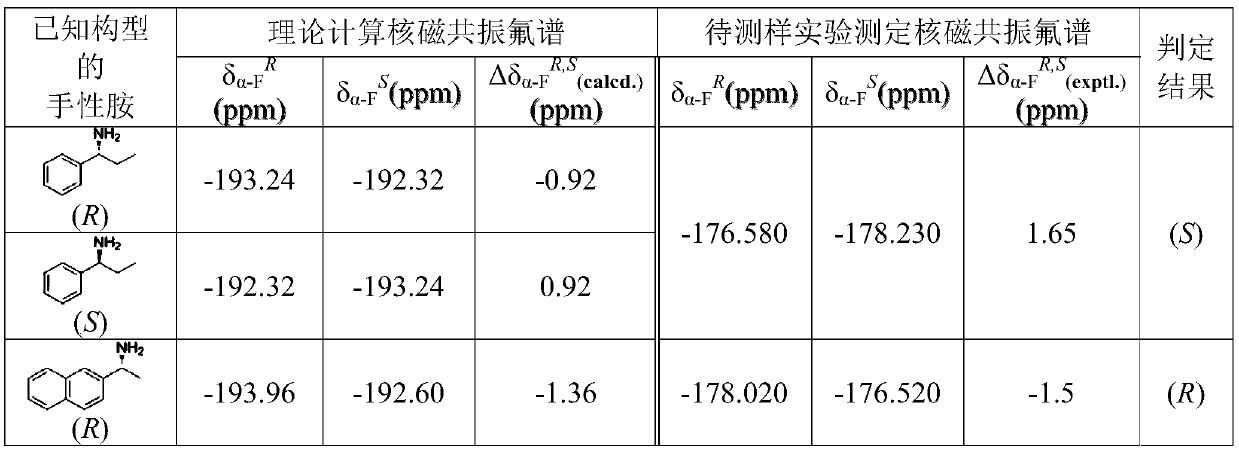
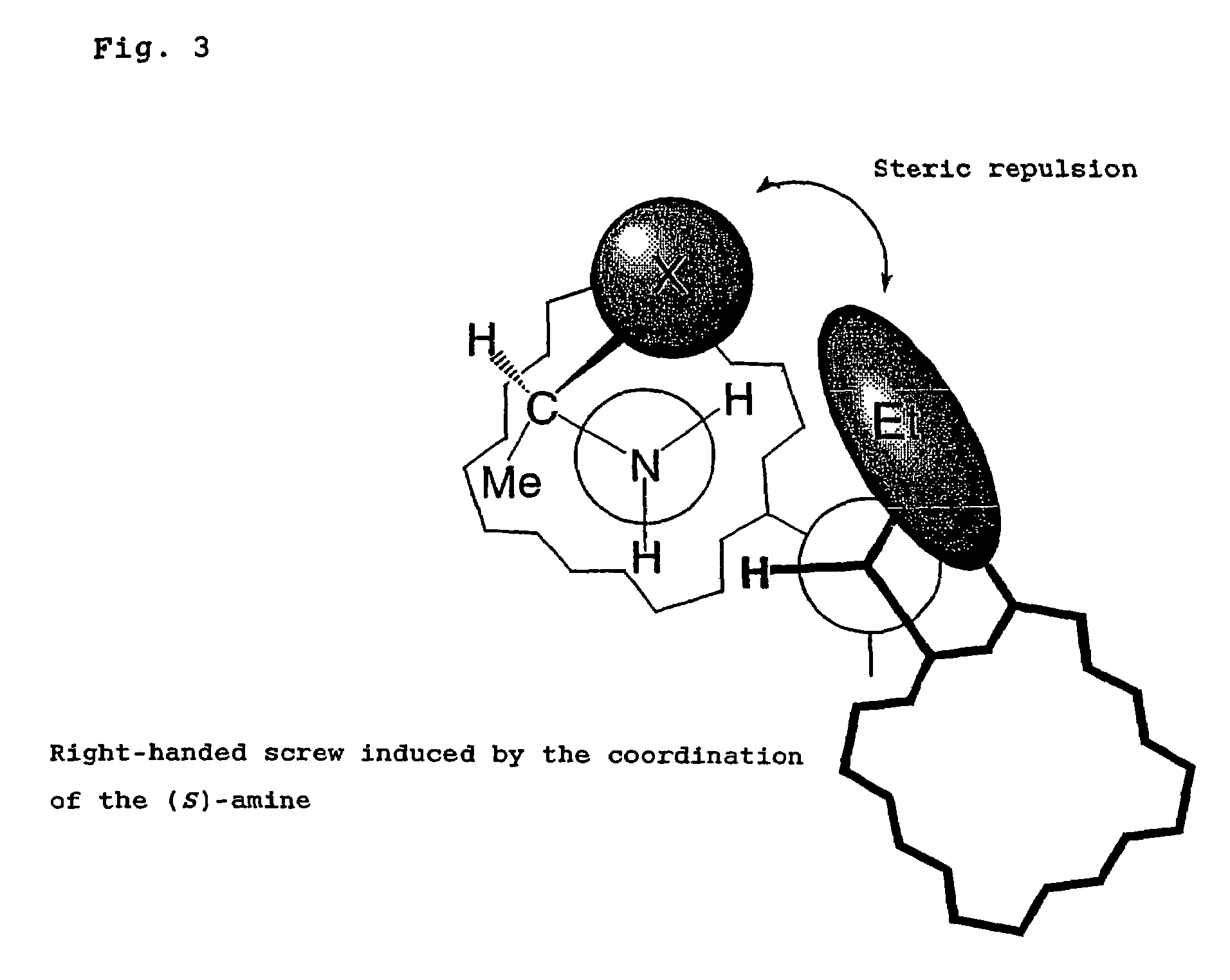
Method for calculating and determining absolute configuration of chiral amines according to nuclear magnetic resonance fluorine spectrum theory
InactiveCN110047558AMolecular entity identificationInstrumentsNMR - Nuclear magnetic resonanceAbsolute configuration
The invention discloses a method for calculating and determining absolute configuration of chiral amines according to nuclear magnetic resonance fluorine spectrum theory. The main features of the method for determining the absolute configuration of chiral amines are: obtaining theoretically calculating two configurations of a diastereomeric amide compound having the most stable conformation through a theoretical calculation fluorine spectrum method, theoretically calculating the fluorine spectral chemical shift difference DeltadeltaAlpha-FR, S, and then using the nuclear magnetic resonance fluorine spectrum to obtain the chemical shift difference DeltadeltaAlpha-FR, S of the diastereomer amide compound, by comparing the DeltadeltaAlpha-FR, S positive and negative signs obtained by the twomethods, accurately determining the absolute configuration of the sample to be tested. The method is applicable to the determination of the absolute configuration of chiral amines, amino alcohols, amino acid esters and various chiral compounds. The method is simple, easy to operate and high in accuracy. The method is a simple and efficient new method for determining absolute configuration.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
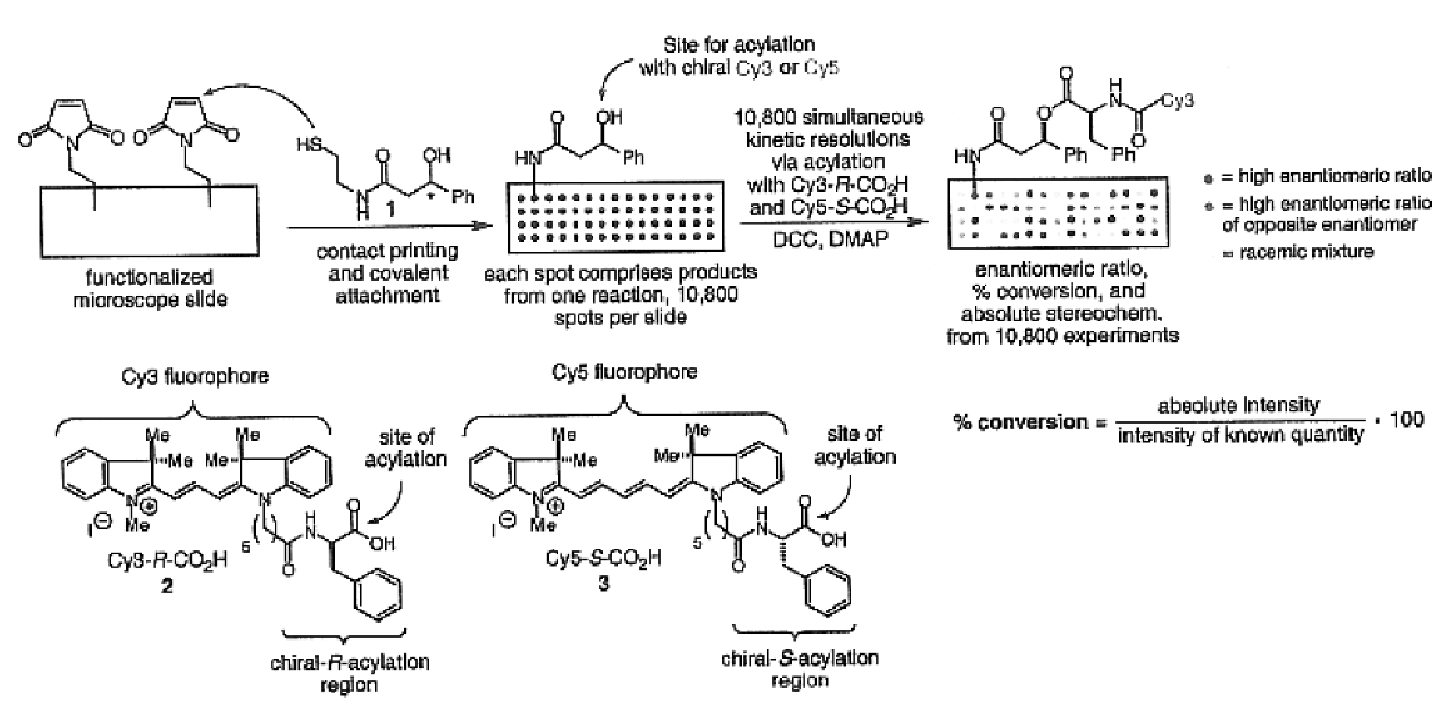
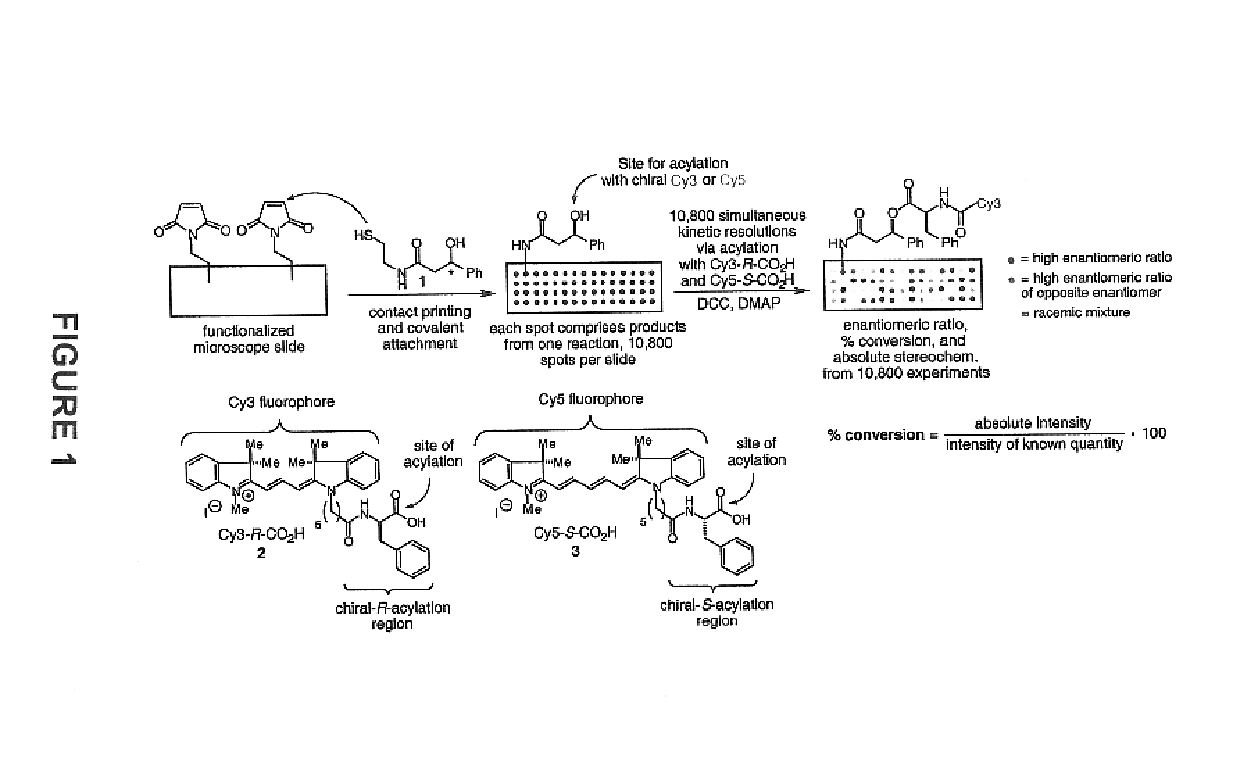
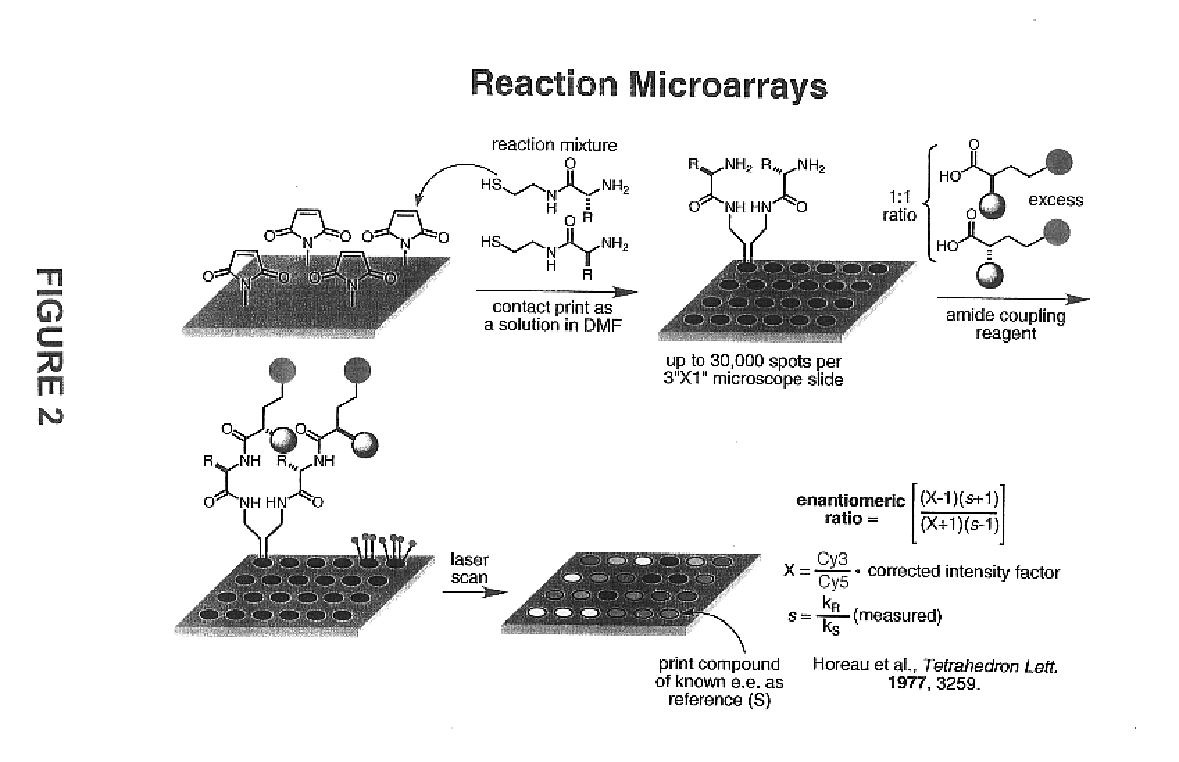
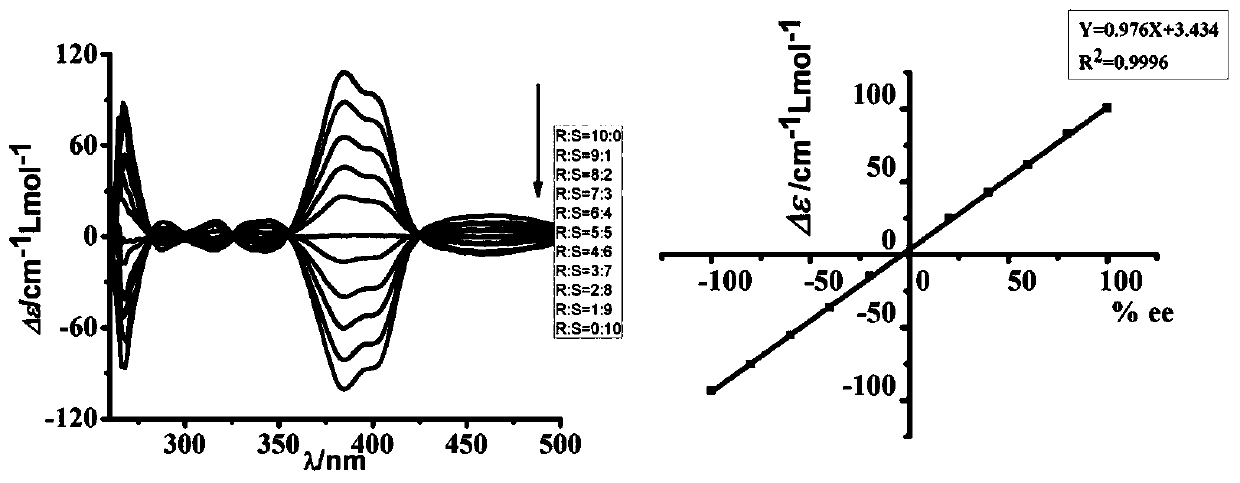
Determining stereoisomeric excess, concentration and absolute configuration
ActiveUS20160011156A1Rapid and accurate ee detectionHigh-throughput ee screening applicationChemical analysis using catalysisMaterial analysis by optical meansAbsolute configurationAnalyte
The present invention is directed to analytical methods for determining the concentration, and / or stereoisomeric excess, and / or absolute configuration of chiral analytes in a sample.
Owner:GEORGETOWN UNIV
Production method of hexahydrofurofuranol derivative, intermediate therefor and production method thereof
InactiveUS7468448B2Efficiently and economicallyResolution problemOrganic chemistryBulk chemical productionAbsolute configurationEnantiomer
The present invention provides a method for producing compound (XIV) useful as an intermediate for pharmaceutical agents efficiently and economically on an industrial scale without using ozone oxidation and highly toxic reagent, and an intermediate used for this method. Particularly, the present invention provides a method for producing a compound having an absolute configuration represented by the formula (XV) and an enantiomer thereof without using a technique such as optical resolution and the like, and an intermediate used for this method.(1) Compound (XIII) as a starting material is led to compound (I), and after introducing a protecting group, subjected to reduction and cyclization to give compound (XIV). Particularly, compound (XIII) as a material is led to compound (I) via compound (XX) to produce compound (XIV). Using an optically active compound (XIII) as a starting material, a compound having an absolute configuration represented by the formula (XV) and the like are produced highly stereoselectively.(2) Compound (XXI) as a starting material is stereoselectively reduced to give compound (XXII), and by introduction of a protecting group, reduction and cyclization, compound (XXVI) is obtained, and by inverting hydroxyl group, compound (XV) is produced.wherein each symbol is as defined in the specification.
Owner:SUMITOMO CHEM CO LTD
Reagent for determining the absolute configuration of chiral compound and determination method
InactiveUS7648841B2Material analysis by observing effect on chemical indicatorAnalysis by material excitationDimerAlkaline earth metal
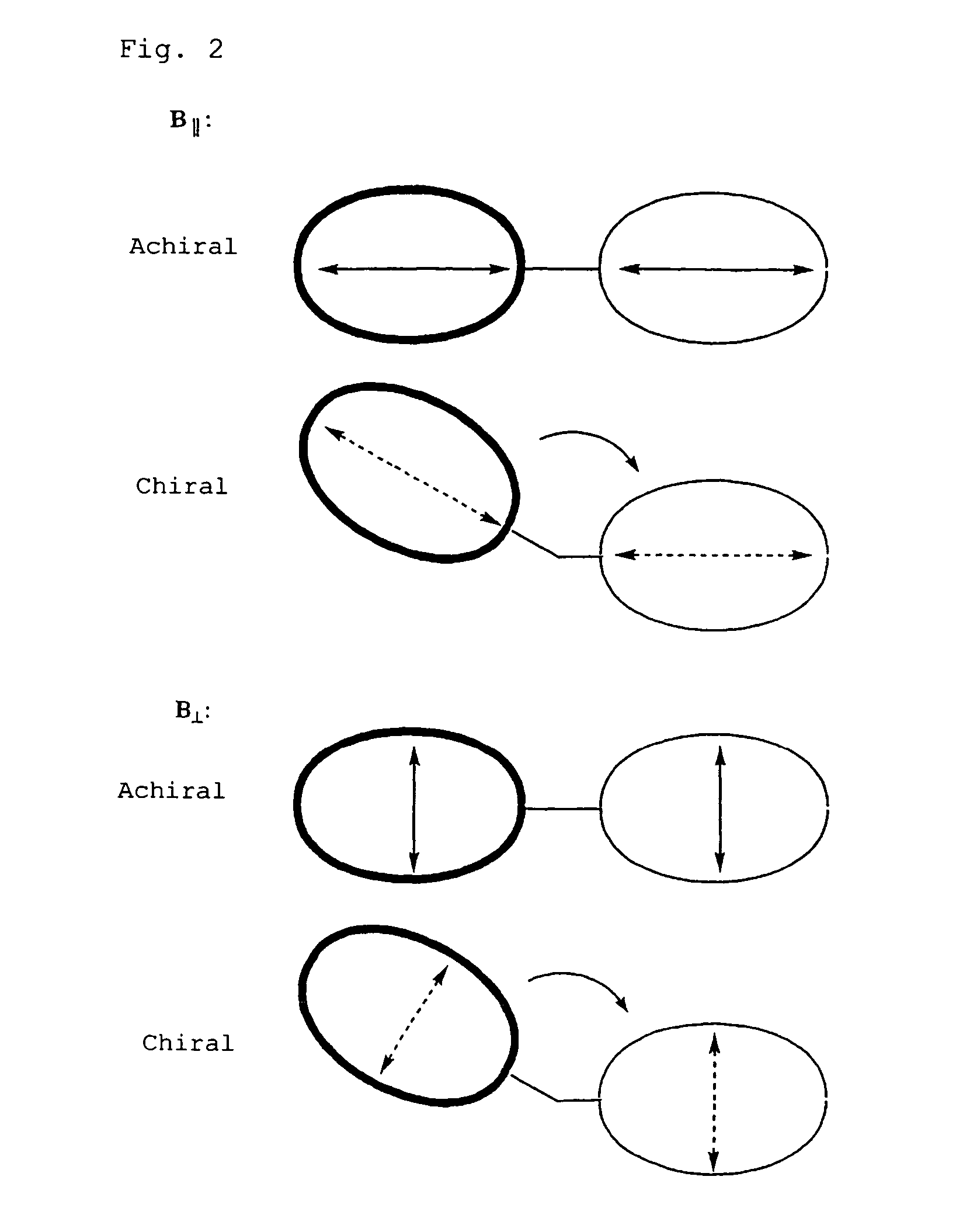
The present invention relates to a reagent for determining the absolute configuration of a chiral compound containing as an active ingredient a metalloporphyrin dimer, wherein the metalloporphyrin dimer has an alkaline-earth metal ion as a central metal and has a carbon chain-crosslinked structure in which at least one of the two porphyrin rings has a substituent bulkier than methyl at least one of the second carbon atoms from a carbon atom bonded to the crosslinking carbon chain along the outer periphery of the porphyrin ring and a method for determining the absolute configuration of an asymmetric carbon atom of the chiral compound using the reagent.
Owner:JAPAN SCI & TECH CORP
Fluorinated quinolylamide foldamer, preparation method, chirality recognition method and application
ActiveCN109705036AOrganic chemistryAnalysis by subjecting material to chemical reactionAbsolute configurationAlcohol
The invention discloses a fluorinated quinolylamide foldamer, a preparation method, a chirality recognition method of the fluorinated quinolylamide foldamer and an application of the fluorinated quinolylamide foldamer. The fluorinated quinolylamide foldamer has a structural formula represented by a formula (1) shown in the description, wherein R1 and R4 represent alkoxy of any length and may be the same or different, X is an O or N atom, R2 is an electron withdrawing group, for example nitro or cyano, R3 is alkyl of any length, and n is a random positive integer. The fluorinated quinolylamidefoldamer disclosed by the invention can be applied to the recognition and ee value determination of absolute configurations such as chiral amines, chiral diamines, chiral amine alcohols and chiral amino-acid esters. According to the fluorinated quinolylamide foldamer, the preparation method, the chirality recognition method and the application, a chiral group is linked to the quinolylamide foldamer in a covalent bond mode in a manner that amino attacks the fluorinated quinolylamide foldamer under alkaline conditions, so that chirality is transferred to the quinolylamide foldamer, and absoluteconfigurations and ee values of chiral guest molecules are detected according to CD signals of corresponding quinolylamide foldamers.
Owner:WUYI UNIV +1
Environment-friendly oil purification equipment
InactiveCN107648926AGuaranteed heightSimple structureFatty-oils/fats refiningFiltration circuitsAbsolute configurationSlag
A clean oil environmental protection equipment, its box is fixed with an upper partition and a sealing plate in the horizontal direction to divide the box into a filter room, a clean oil room and an oil storage room; The swallowing hole of the plate is fixed with an oil suction pipe closely connected with the telescopic pipe, a filter residue box is installed in the filter chamber, a clean oil float floats on the oil surface in the clean oil chamber, a filter screen is fixed at the oil suction hole, and the oil suction pipe passes through There is a drain switch at the bottom of the oil cleaning room, an oil pump for delivering edible oil is installed in the oil storage room, and an observation window is arranged on the box body. The structure of the clean oil environmental protection equipment is simple and applicable. It adopts the method of combining filtration and sedimentation treatment to realize the separation of oil and residue and oil and water at the same time in one piece of equipment, and the effect of clean oil is good. It does not produce pollution and does not use energy. The use cost is low; the edible oil purified by the clean oil environmental protection equipment is clean and hygienic, has good market prospects for industrialization, high commercial value, and is very convenient to manufacture and use.
Owner:褚圣海
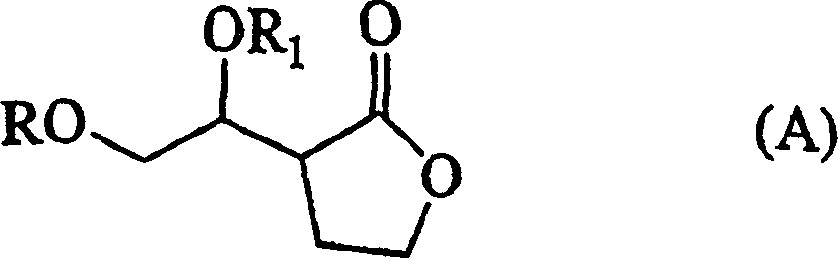
New pyrethroid compound and preparation method and application
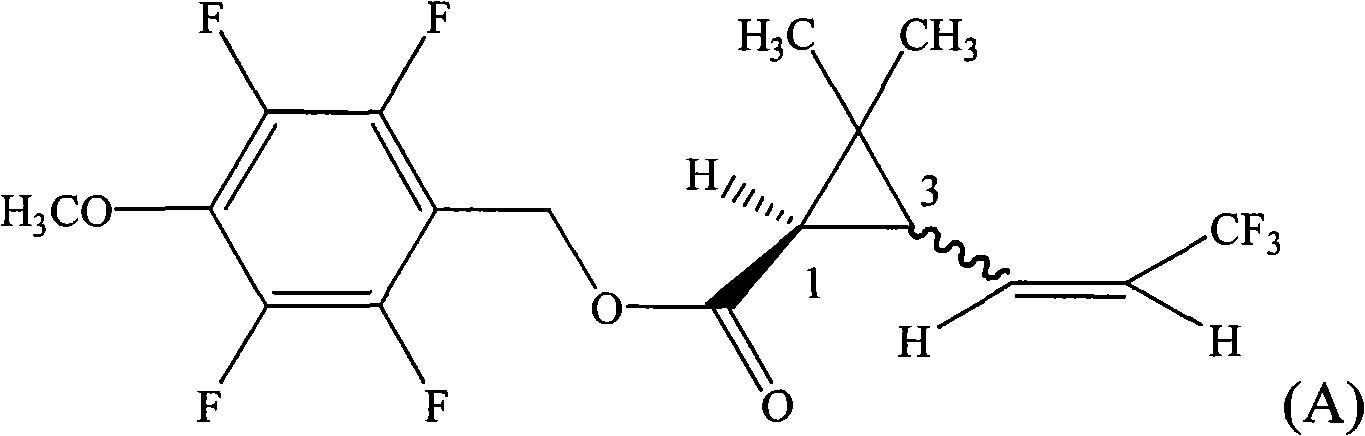
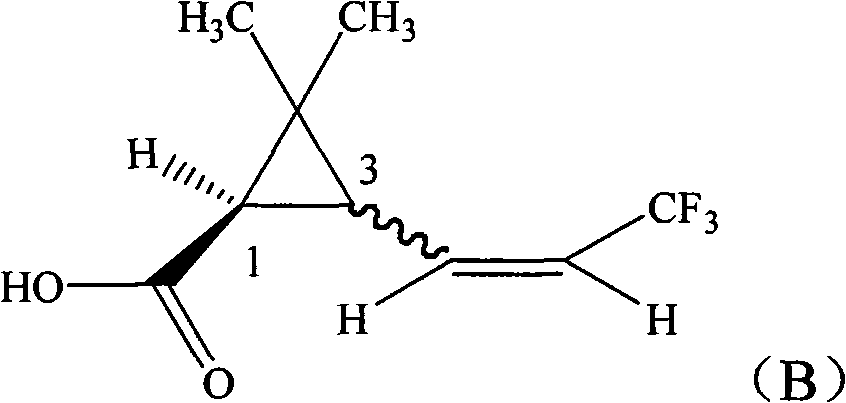
ActiveCN101665433AHigh activityEasy to solveBiocideOrganic compound preparationAbsolute configurationChemical compound
The invention provides a pyrethroid compound which is a stereoisomer of 2,3,5,6-tetrafluoro-4-methoxybenzyl-3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropane carboxylate. The compound is characterized in that the structure of the compound is shown in formula (A), wherein the carbon-carbon double bond in the carboxylic acid part of the formula (A) is Z configuration, the absolute configuration of the 1-site of cyclopropane is R configuration; the compound is 2,3,5,6-tetrafluoro-4-methoxybenzyl-1R-(Z)-3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropane carboxylate. The pyrethroid compound has high activity and significant effect for controlling the pestiferous pests. The invention also provides a preparation method and an application of the pyrethroid compound.
Owner:JIANGSU YANGNONG CHEM +1
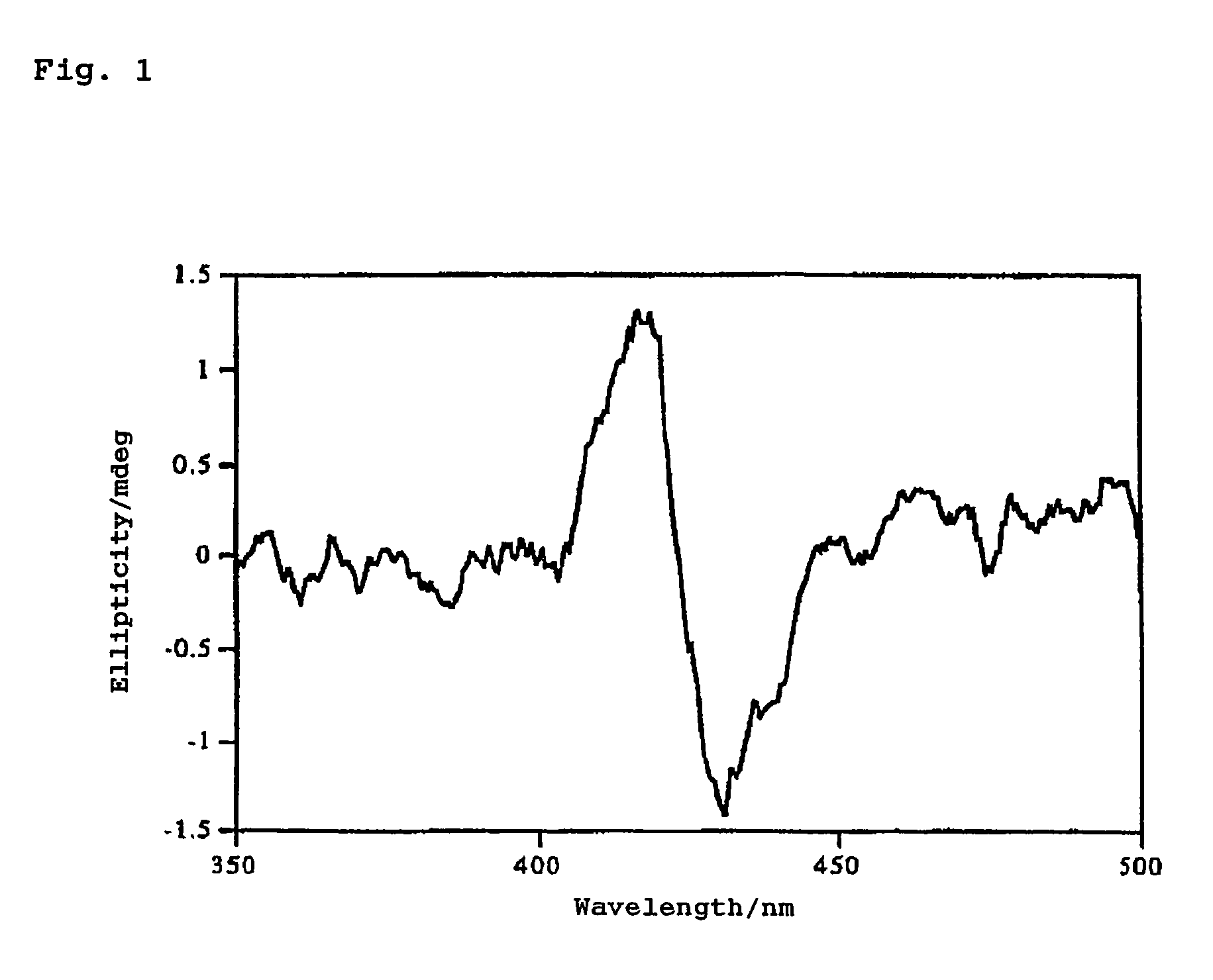
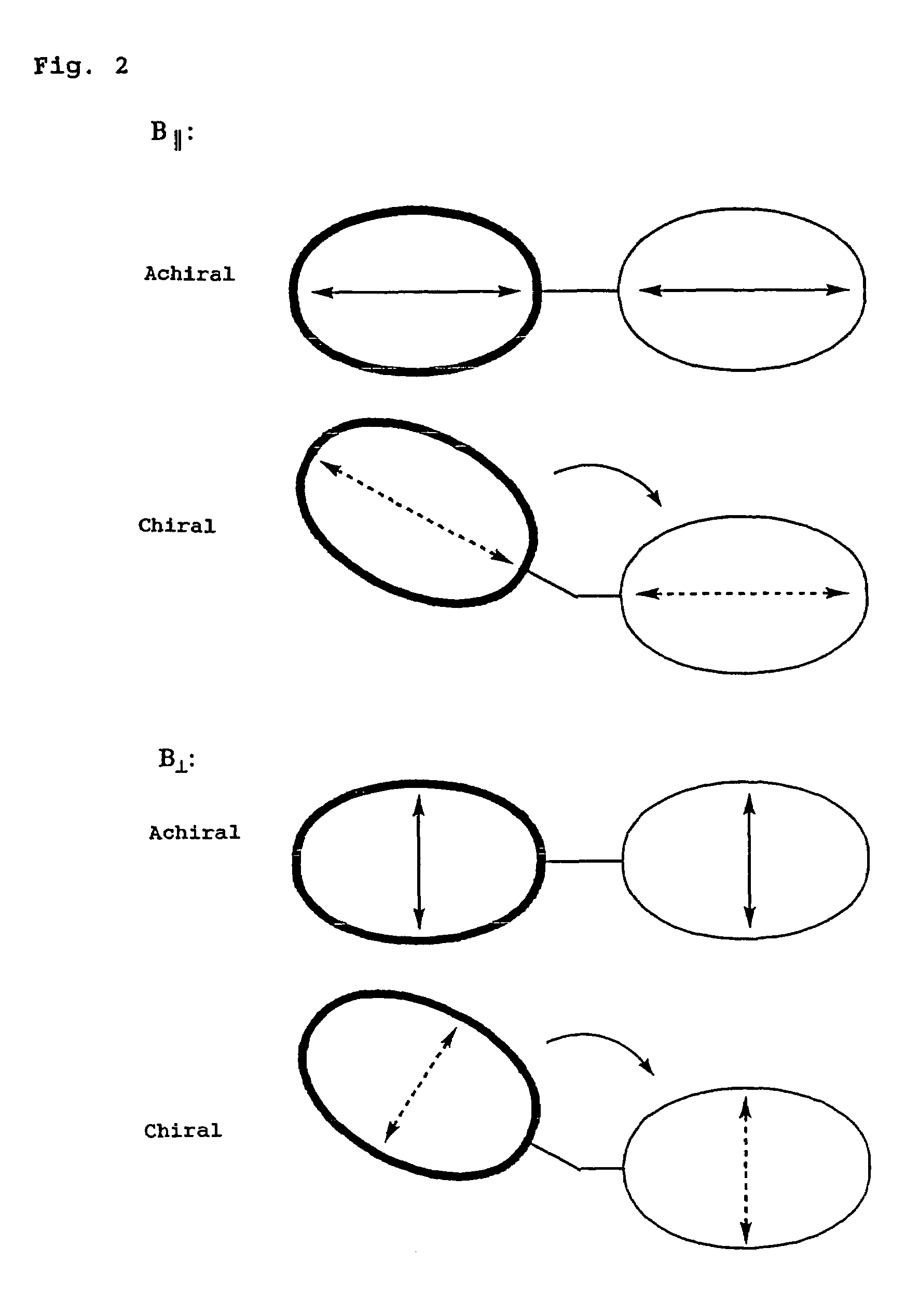
Method for determination of absolute configuration of chiral compounds
InactiveUS7736902B2Sure easyHigh sensitivityOrganic chemistryAnalysis by material excitationDimerAbsolute configuration
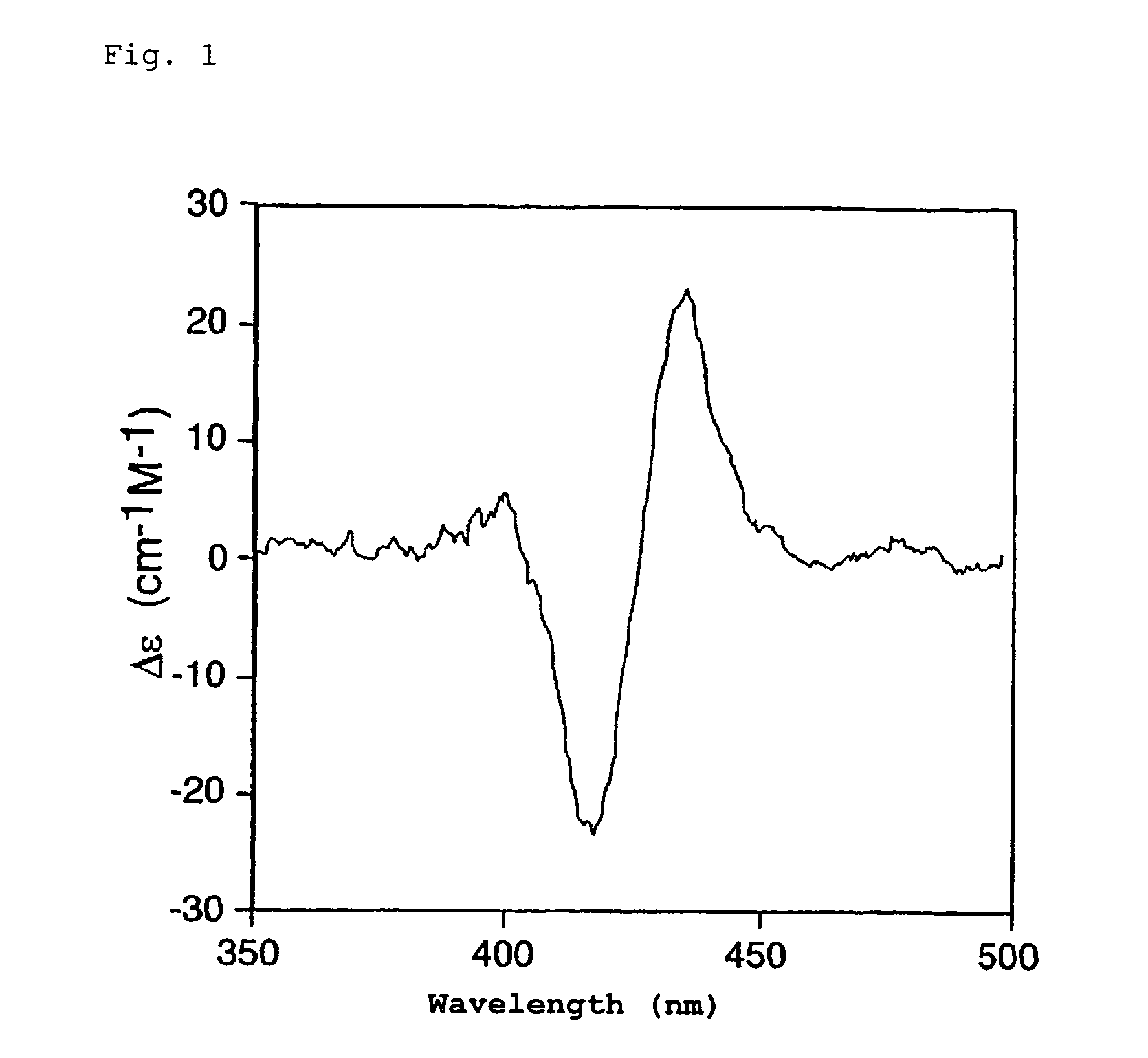
The present invention provides a simple, highly sensitive, and highly precise method for determining the absolute configuration of a chiral compound such as a diamine or an amino alcohol.The present invention is directed to a method for determining the absolute configuration of an asymmetric carbon of a chiral compound on the basis of the sign of the Cotton effect by analyzing a solution containing the chiral compound and a porphyrin dimer by circular dichroism spectroscopy.
Owner:JAPAN SCI & TECH CORP
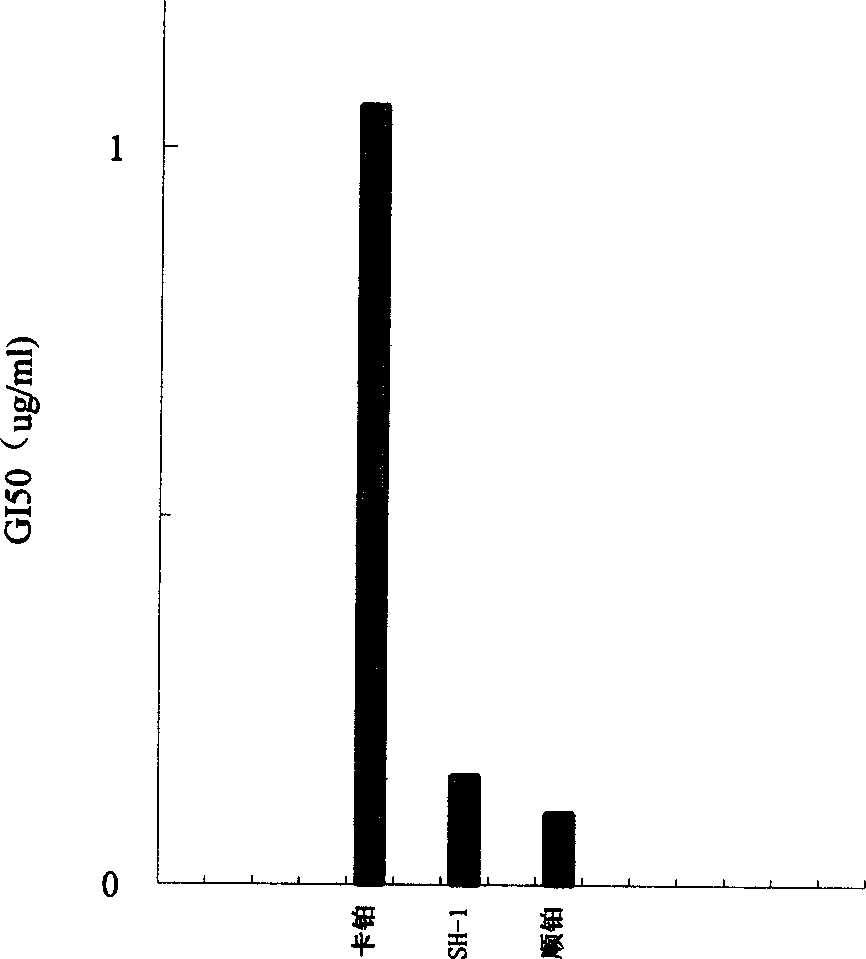
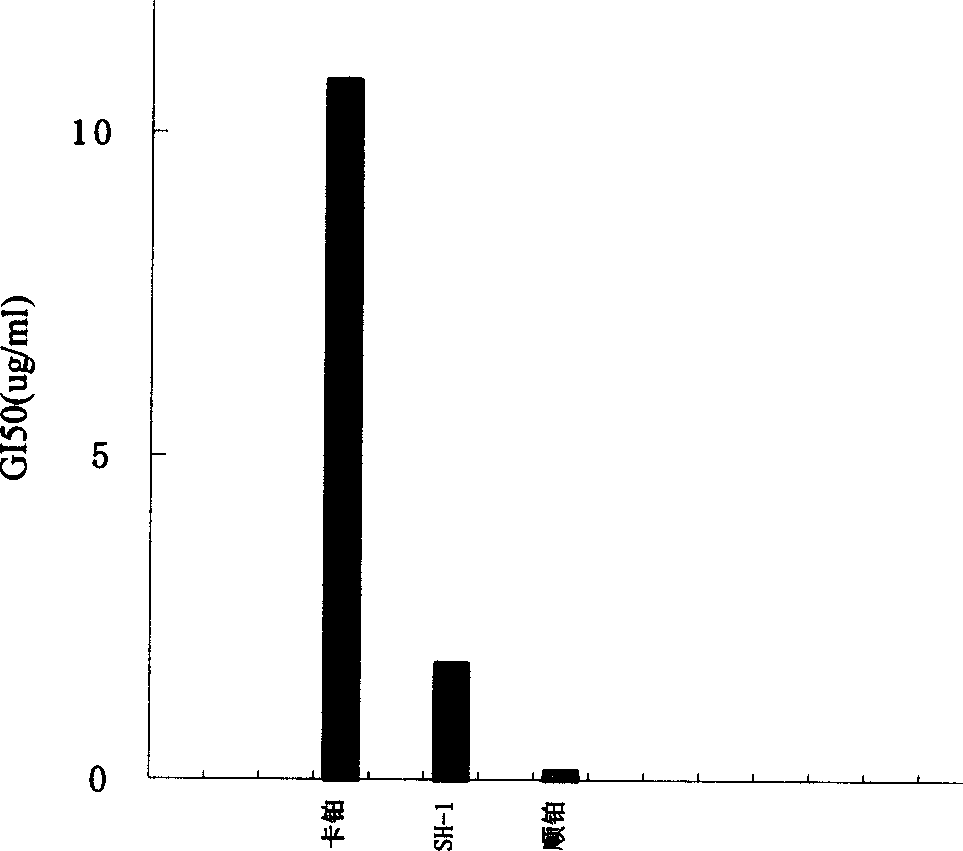
Anti-tumor platinum (II) complex
InactiveCN1569862AGroup 8/9/10/18 element organic compoundsAntineoplastic agentsMethoxyacetic acidAbsolute configuration
The invention discloses a group of platinum (II) coordinated complex having effective anticancer activity, which is represented by the structural formula (1) disclosed in the specification, wherein R groups are the same, which are hydrogen atoms or C1-5 alkyl, the cyclohexane diamine is 1,2-trans-cyclohexane diamine, R3 and R4 in formula (3) can be identical or different, which are hydrogen atom or C1-5 alkyl, or can connect with a carbon atom to form a cycloalkyl.
Owner:NANJING UNIV
Resolution of 1-cyclohexyl-2-(5H-imidazole[5,1-a]isobenzazole)ethyl-1-ketone
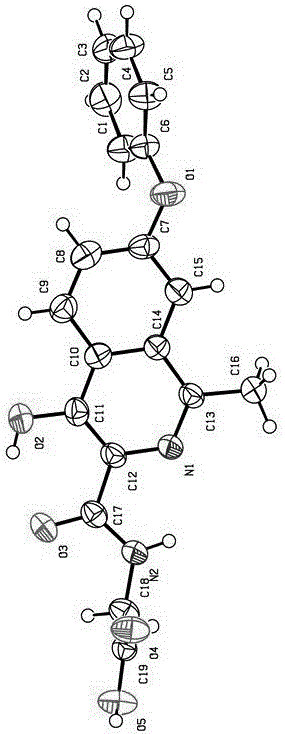
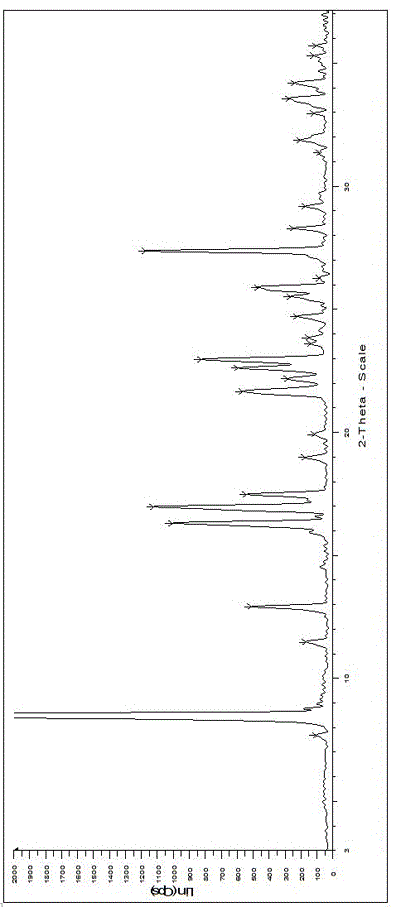
ActiveCN108239091AEasy to recycleReduce pollutionOrganic chemistry methodsAbsolute configurationAlcohol
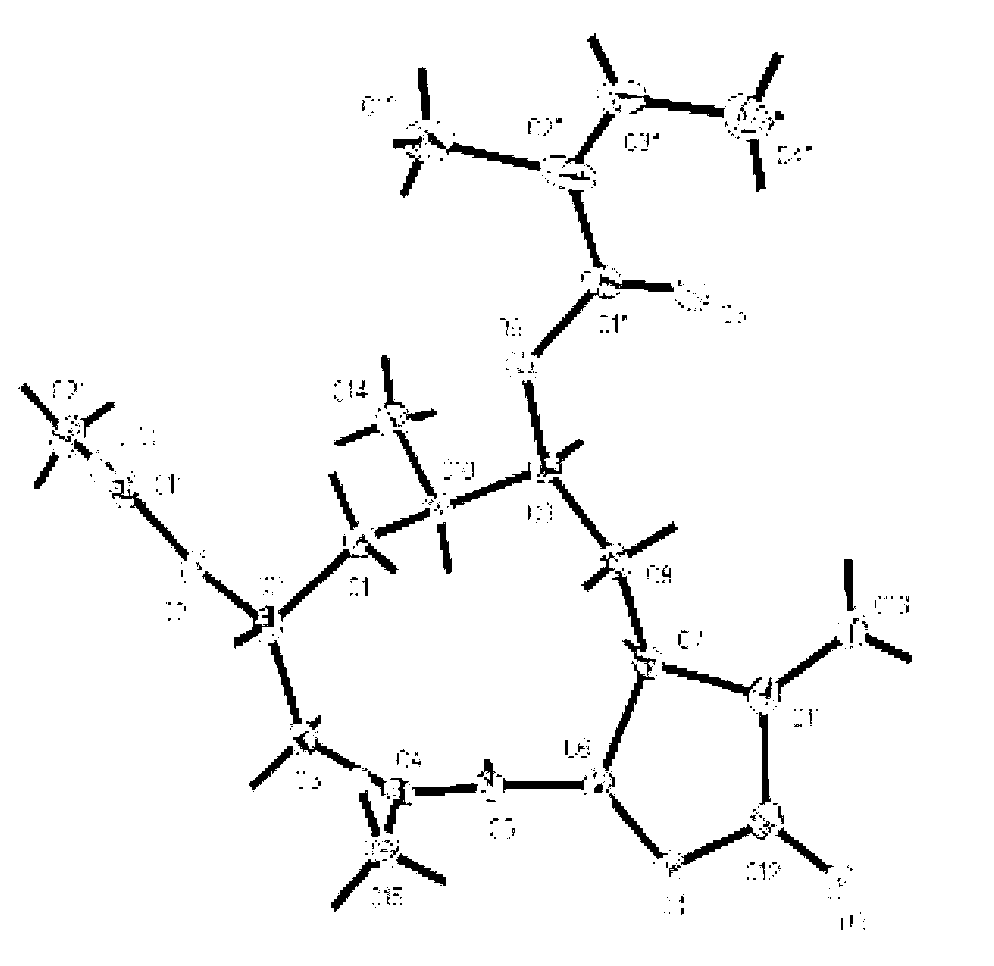
The invention provides a method for obtaining a pair of optical isomers by resolving racemic 1-cyclohexyl-2-(5H-imidazole[5,1-a]isobenzazole)ethyl-1-ketone (rac-1) shown in a formula rac-1. The methodcomprises the following steps: by taking L-di-p-methyl benzoyl tartrate or a hydrate thereof as a resolving agent, resolving in a mixed solvent system, thus obtaining a compound of (S)-1 and L-di-p-methyl benzoyl tartrate; enabling the compound to be free in water or alcoholic solution to obtain (S)-1, and classifying absolute configuration of a sample by comparing theoretic electronic circular dichroism with the practical electronic circular dichroism; and by virtue of the same steps, taking D-di-p-methyl benzoyl tartrate or a hydrate thereof as the resolving agent, and obtaining (R)-1. Themethod provided by the invention is simple to operate and high in resolution efficiency, the resolving agent is easy to recycle, and environmental pollution is low, so that the method is applicable tolarge-scale preparation and industrial production. (The formula rac-1 is described in the specification).
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


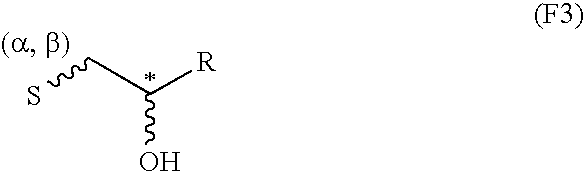




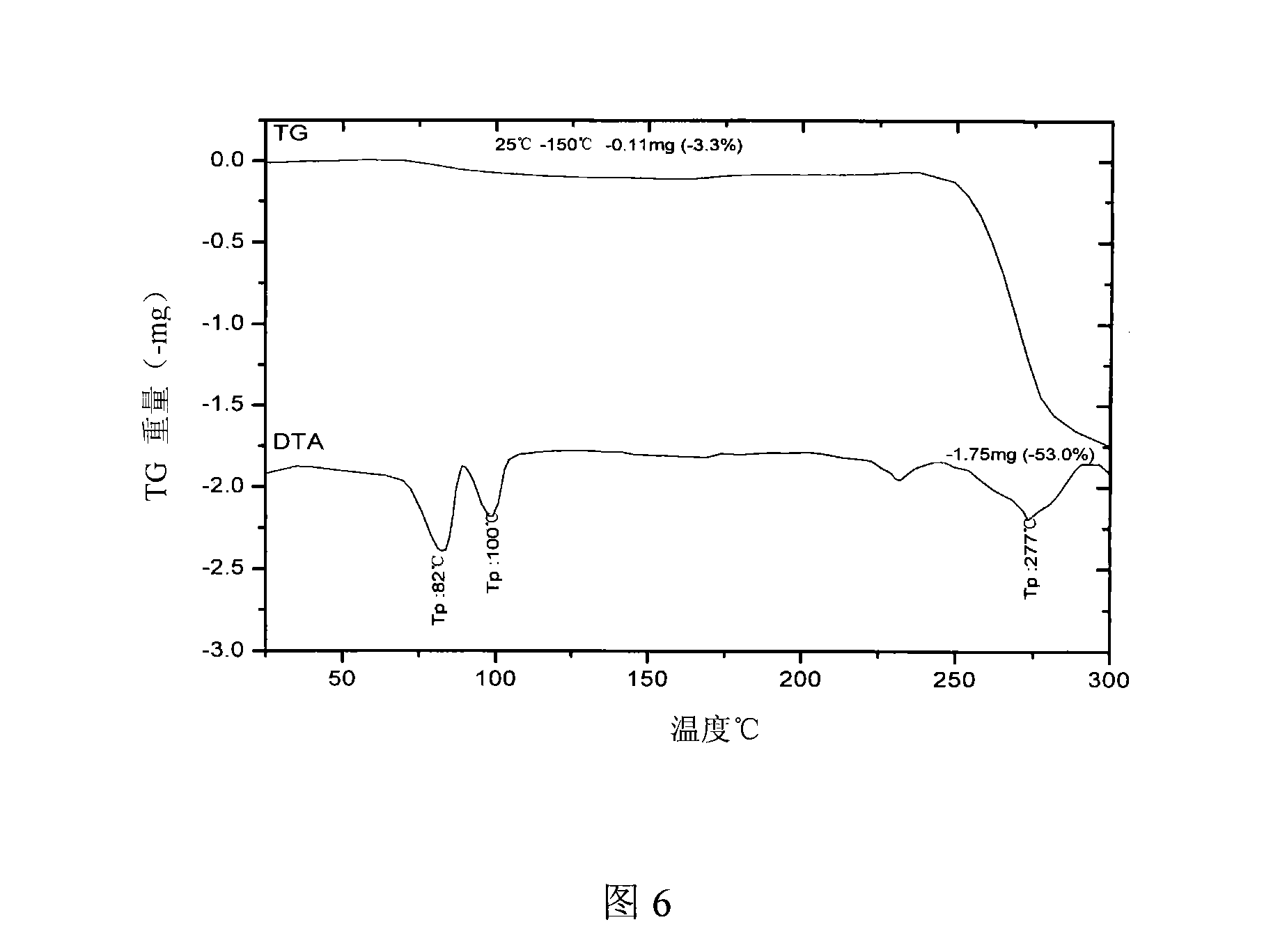
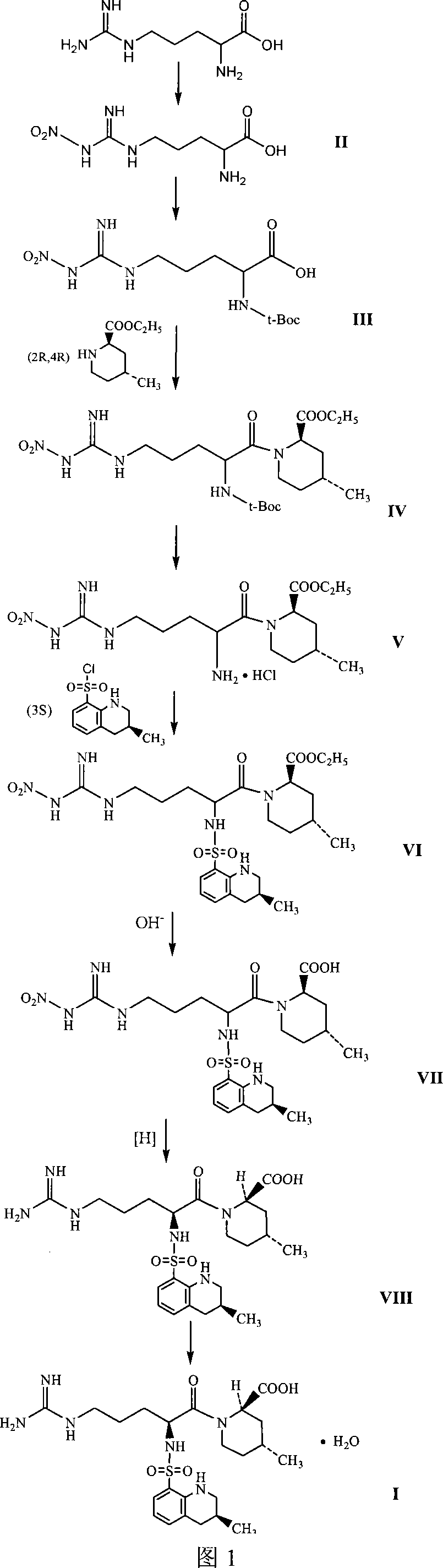
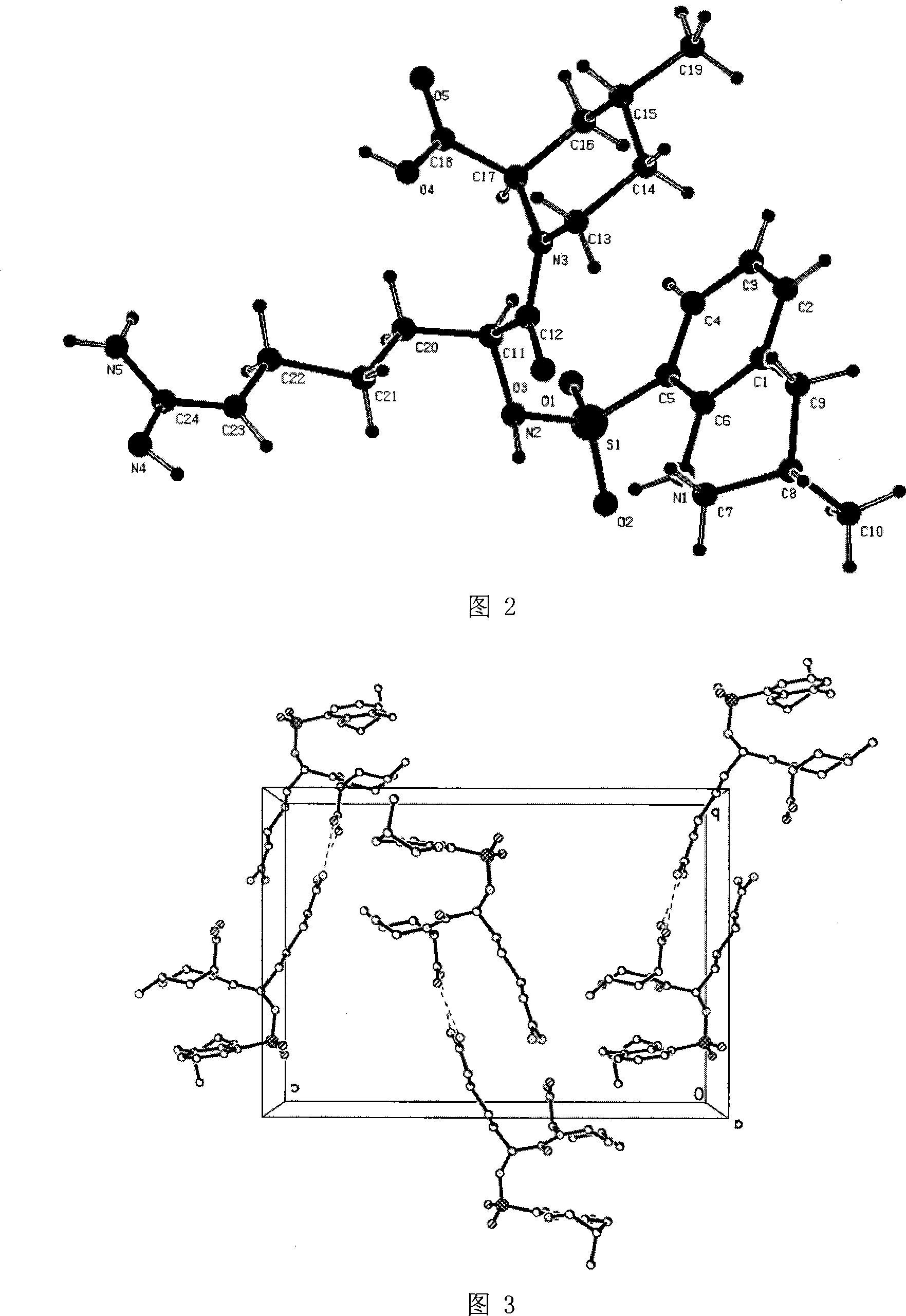
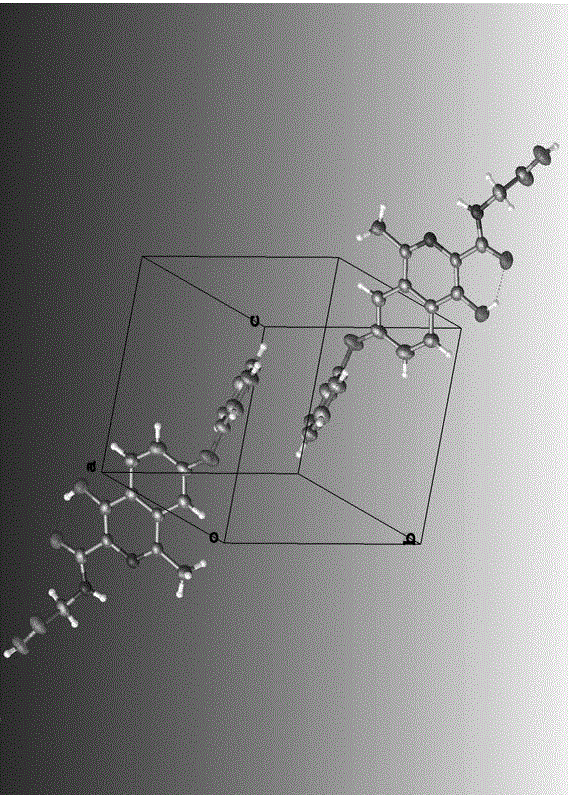

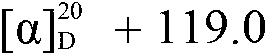

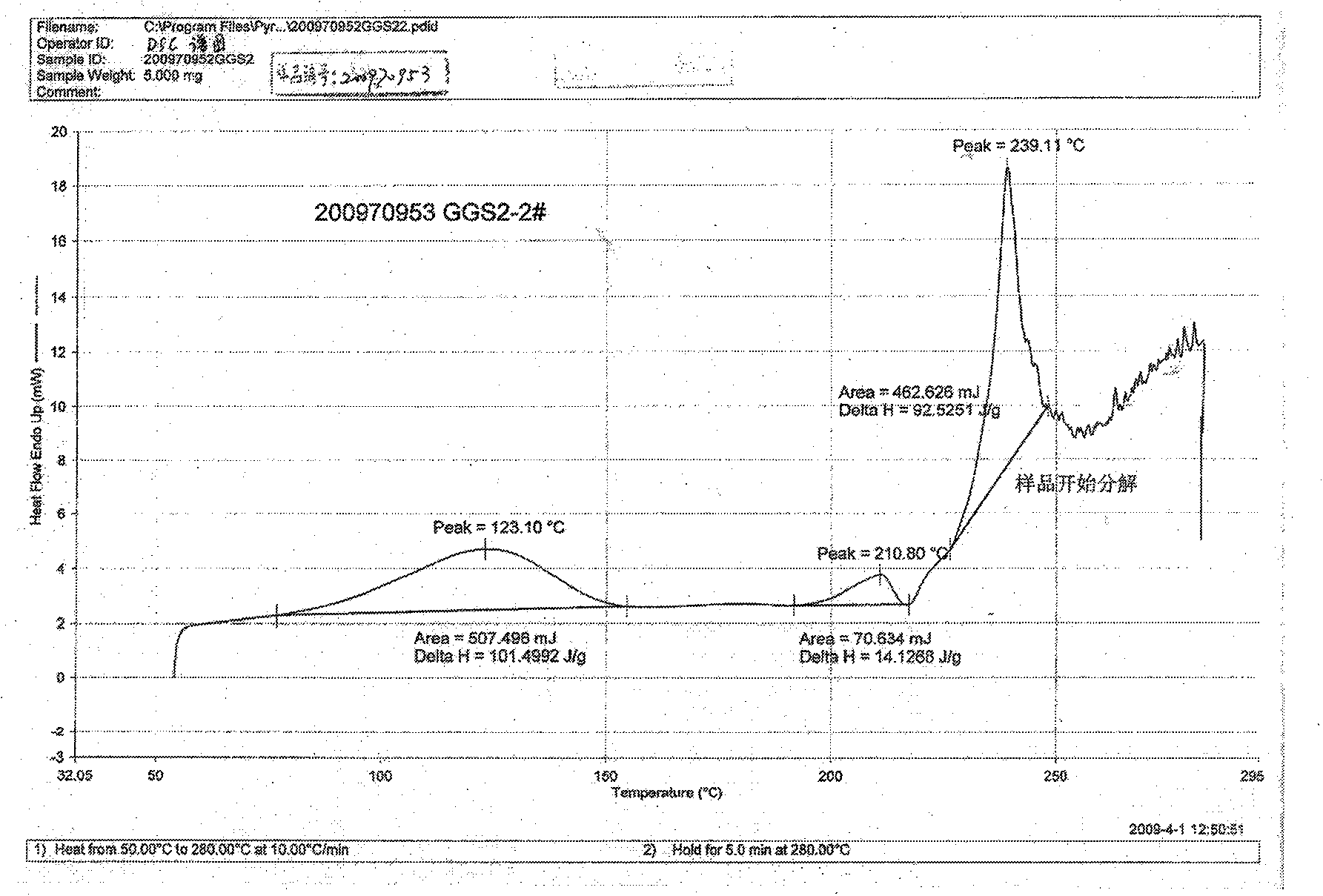
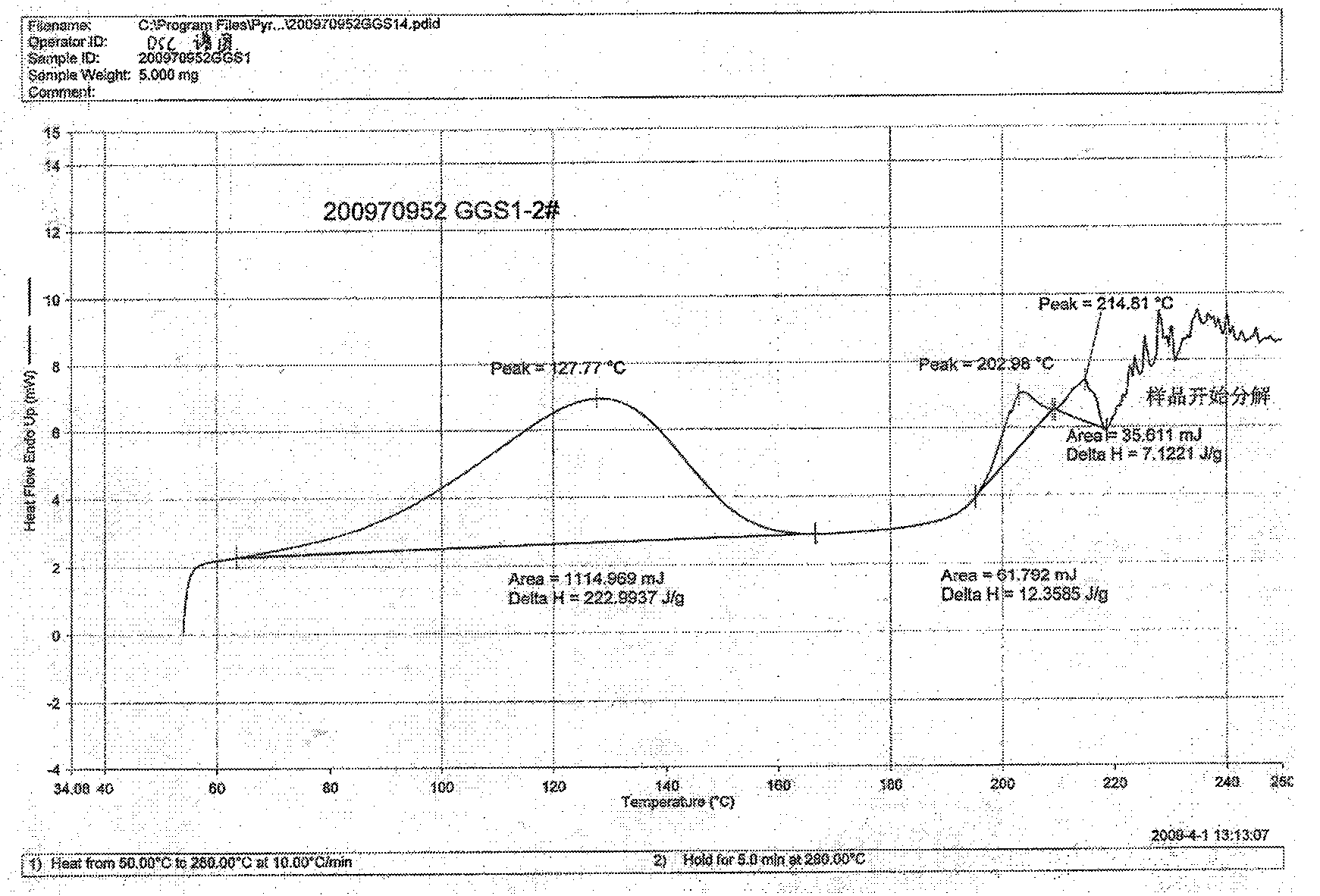
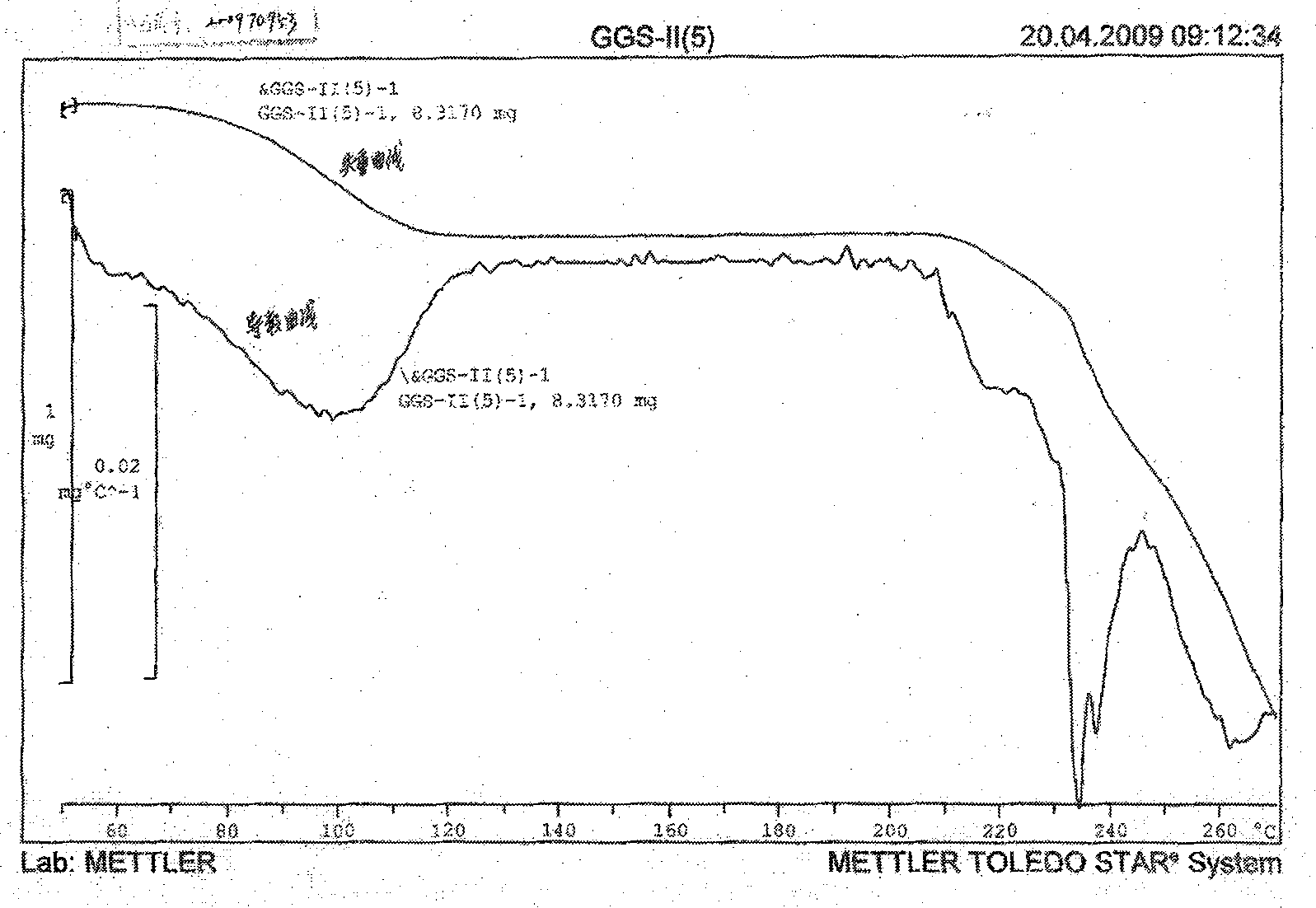


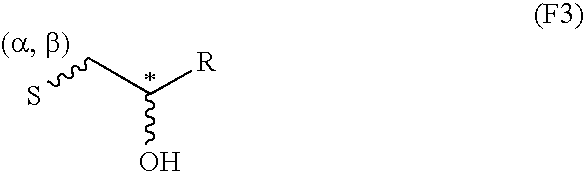









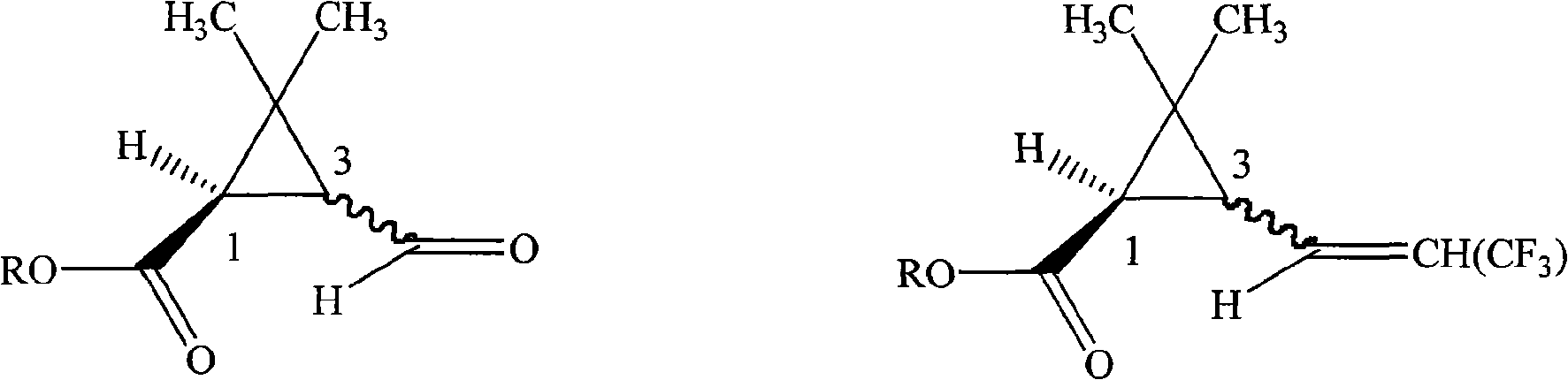
![Resolution of 1-cyclohexyl-2-(5H-imidazole[5,1-a]isobenzazole)ethyl-1-ketone Resolution of 1-cyclohexyl-2-(5H-imidazole[5,1-a]isobenzazole)ethyl-1-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e9eb3762-dbb1-4ee4-b9bc-43c06e76828f/HDA0001191099210000011.png)
![Resolution of 1-cyclohexyl-2-(5H-imidazole[5,1-a]isobenzazole)ethyl-1-ketone Resolution of 1-cyclohexyl-2-(5H-imidazole[5,1-a]isobenzazole)ethyl-1-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e9eb3762-dbb1-4ee4-b9bc-43c06e76828f/HDA0001191099210000012.png)
![Resolution of 1-cyclohexyl-2-(5H-imidazole[5,1-a]isobenzazole)ethyl-1-ketone Resolution of 1-cyclohexyl-2-(5H-imidazole[5,1-a]isobenzazole)ethyl-1-ketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e9eb3762-dbb1-4ee4-b9bc-43c06e76828f/HDA0001191099210000021.png)